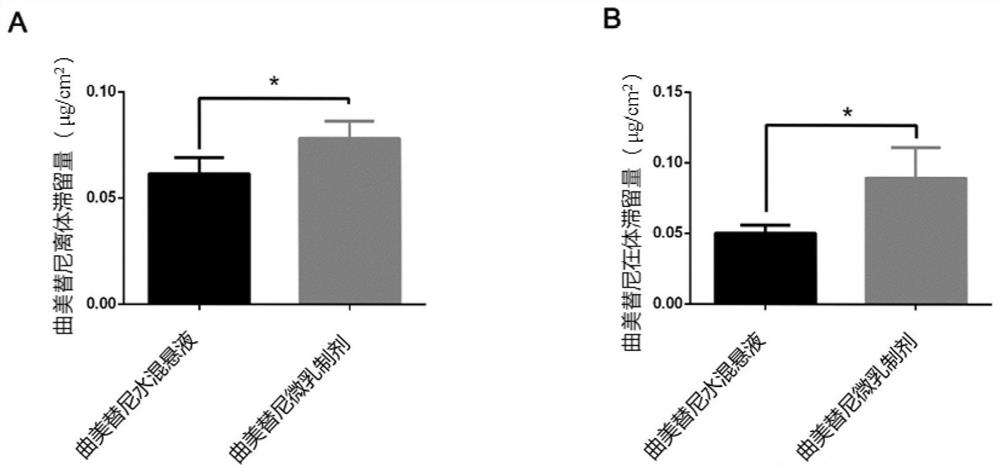
Trametinib microemulsion and application thereof
A technology of trametinib and microemulsion, which is applied to trametinib microemulsion and its application field, can solve the problems of lack of topical treatment preparations, insoluble trametinib, etc., and achieves an efficient, concise and controllable preparation process, and increases the Effect of patient compliance, avoidance of hepatic first-pass effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
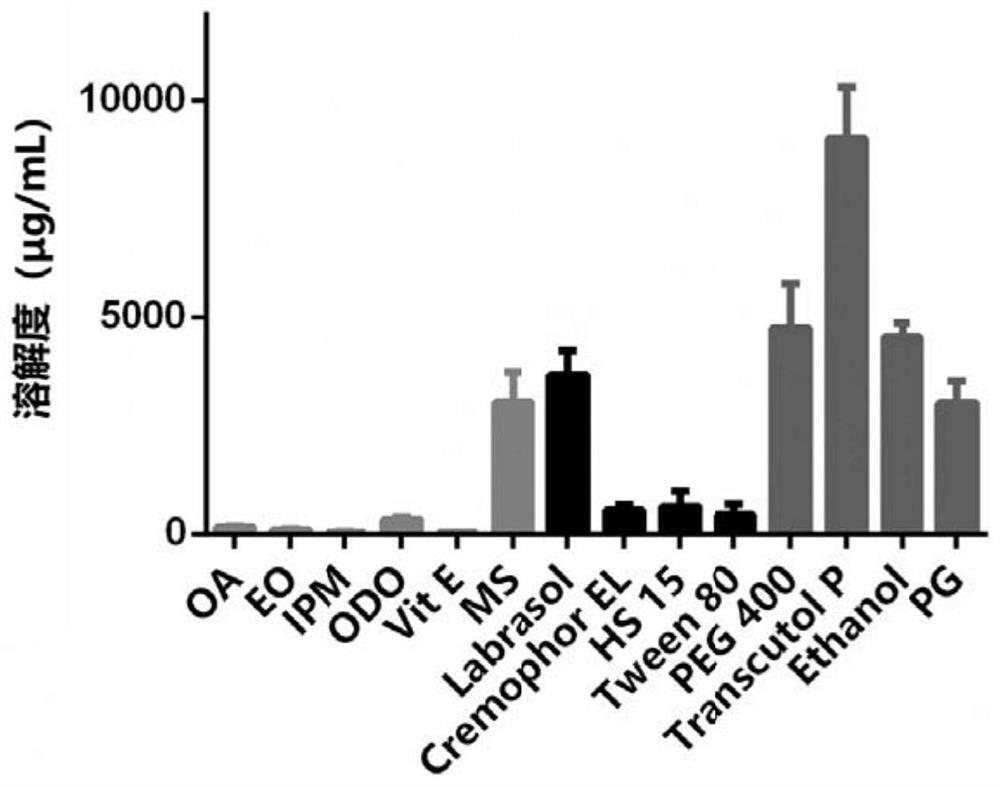
[0040] Embodiment 1 trametinib is in the mensuration of saturation solubility of different oil phases, surfactant, co-surfactant
[0041] Trametinib was added into different oil phases, surfactants and co-surfactants to investigate the solubility. Through the determination of saturation solubility, the optional prescription ingredients are determined for the next step of screening.
[0042] Experimental Materials:
[0043] Test items: oleic acid (OA), ethyl oleate (EO), isopropyl myristate (IPM), caprylic capric acid glyceride (ODO), vitamin E (Vit E), methyl salicylate ( MS), caprylic capric acid polyethylene glycol glyceride (Labrasol), polyoxyethylene castor oil EL (Cremophor EL), polyethylene glycol-15 hydroxystearate (SolutolHS15), Tween 80 (Tween 80), Polyethylene Glycol 400 (PEG 400), Diethylene Glycol Monoethyl Ether (Transcutol P), Ethanol (Ethanol), 1,2-Propanediol (PG).
[0044] Experimental method: Add excess trametinib to different oil phases, surfactants, and ...
Embodiment 2
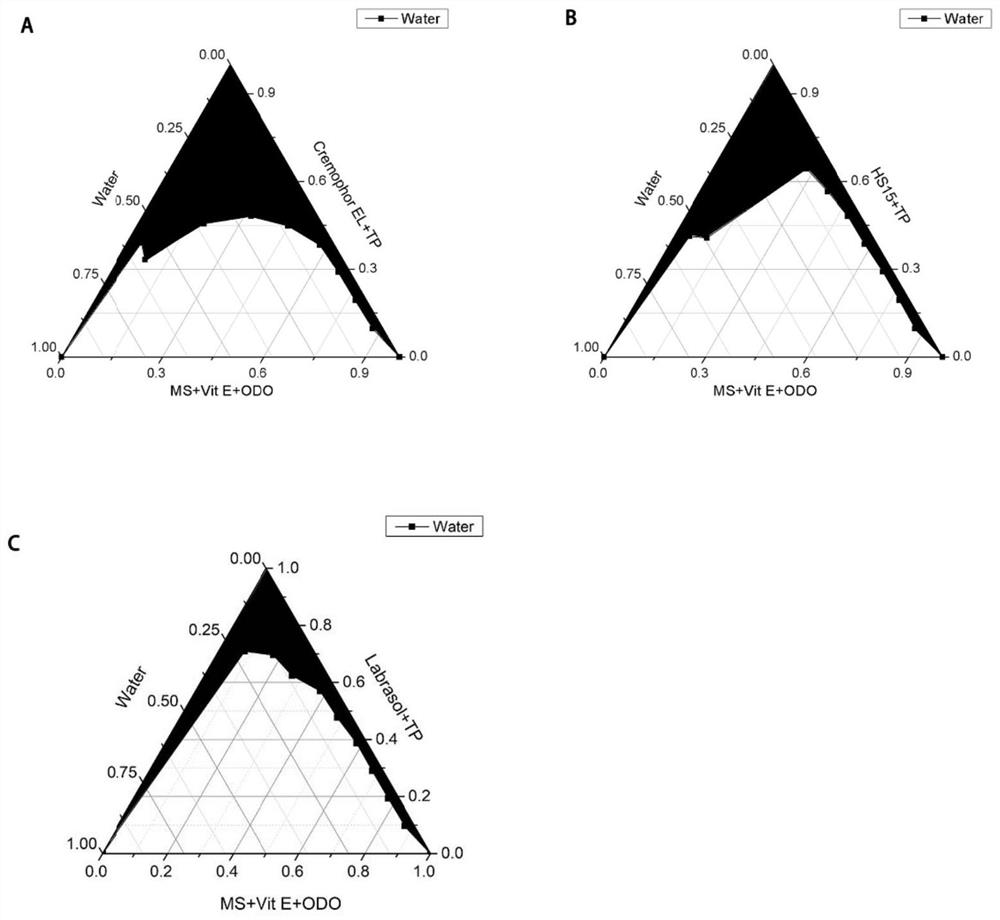
[0046] The screening of embodiment 2 surfactants
[0047] The fixed oil phase is a mixture of caprylic capric acid glyceride, methyl salicylate, and vitamin E, and the co-surfactant is Transcutol P. Microemulsions are prepared by adding different surfactants, and the pseudo-ternary phase diagram is drawn. The best surfactant is determined by drawing the pseudo-ternary phase diagram.
[0048] Experimental Materials:
[0049] Test items: caprylic capric acid polyethylene glycol glyceride (Labrasol), polyoxyethylene castor oil EL (CremophorEL), polyethylene glycol-15 hydroxystearate (Solutol HS15).
[0050] Experimental method: the mixture of glyceryl capricate, methyl salicylate, and vitamin E was used as the oil phase, and Transcutol P was used as the co-surfactant to prepare microemulsions with Labrasol, Cremophor EL, and HS15 as surfactants. According to the titration The content of each component at the end point and draw the pseudo ternary phase diagram. Compare the area...
Embodiment 3
[0053] Embodiment 3 A kind of preparation of microemulsion
[0054] The microemulsion of the present embodiment comprises the following raw materials by mass percentage:
[0055]
[0056]
[0057] Preparation method of microemulsion:
[0058] A. According to the formula ratio, at room temperature, methyl salicylate, vitamin E and caprylic acid glyceride are mixed to form a transparent and clear solution;
[0059] B. Add Transcutol P to the solution described in A according to the formula ratio;
[0060] C. According to the formula ratio, add Cremophor EL to the solution described in B, and form a clear and transparent solution through a magnetic stirrer;
[0061] D. According to the formula ratio, at room temperature, slowly add water dropwise to the solution described in C, and pass it through a magnetic stirrer until a uniform, stable, transparent and clear solution is formed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com