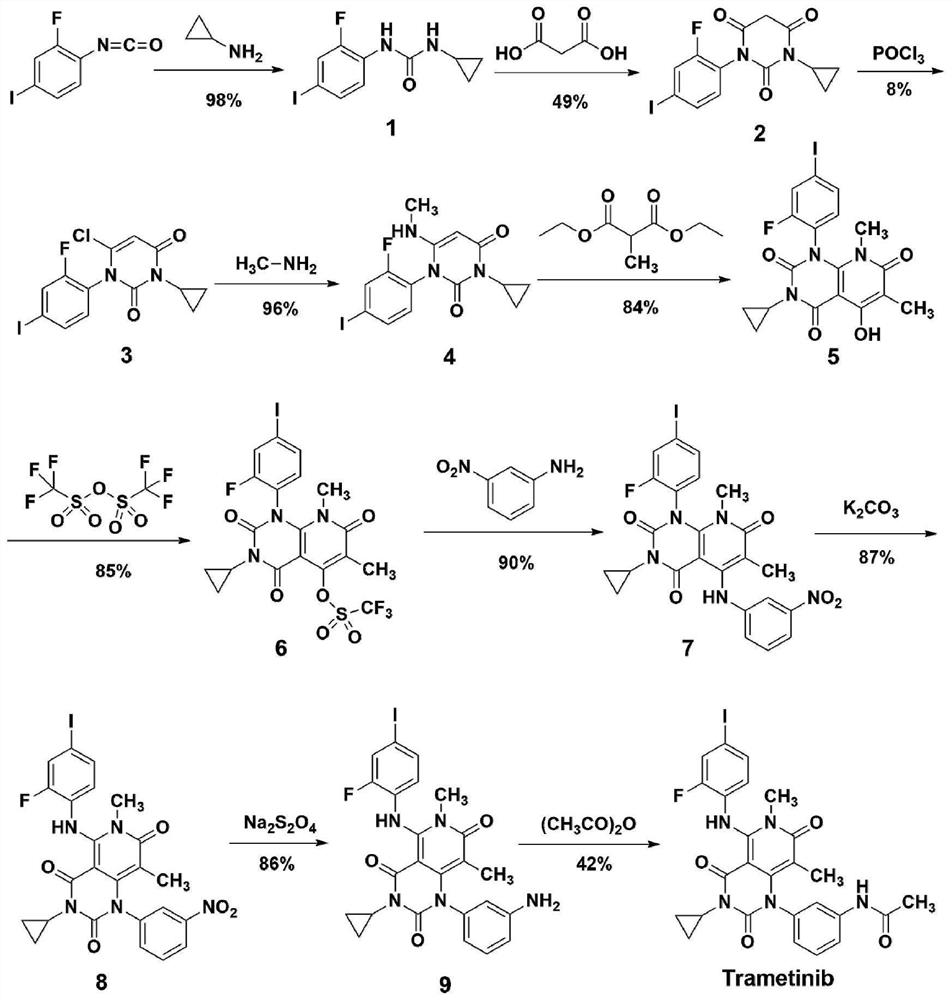
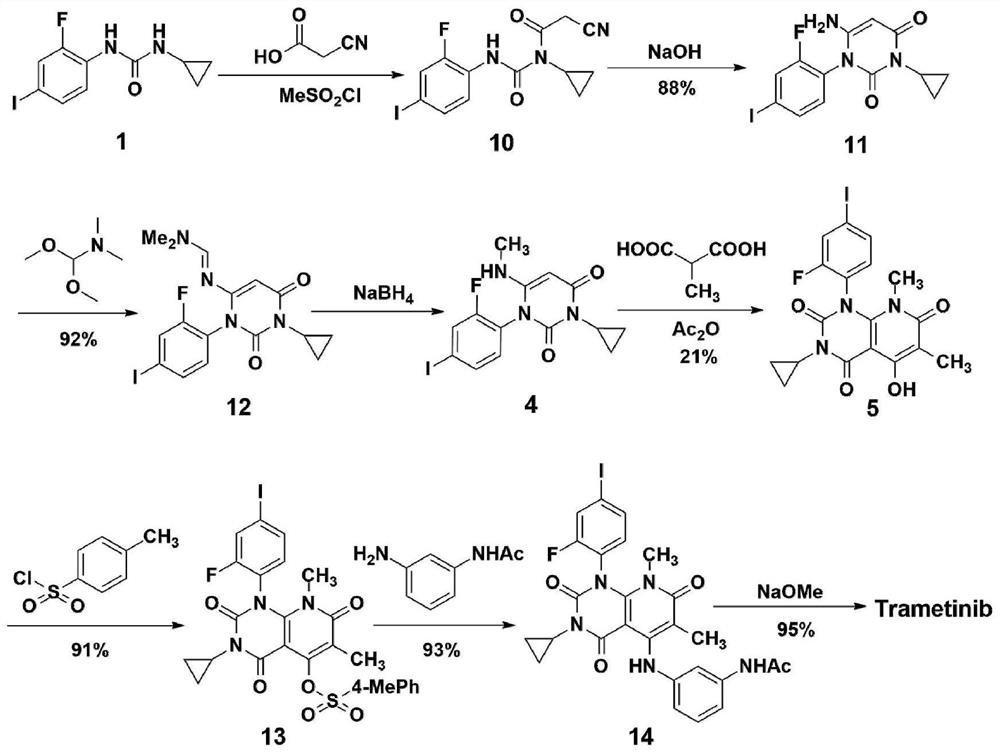
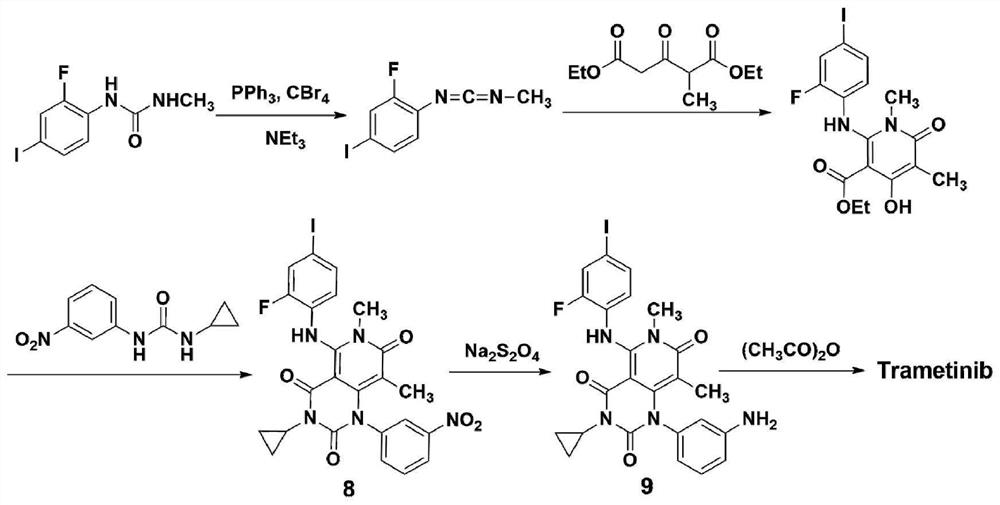
A kind of method of synthesizing trametinib
A synthesis method and technology of iodophenylamino group, applied in the field of pharmaceutical synthesis, can solve the problems of difficult control of intermediates, cumbersome routes, cumbersome operations and the like, and achieve the effects of low cost, easy operation and shortened process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] according to image 3 The shown route diagram prepares synthetic trametinib:
[0035] S1: In N-(2-fluoro-4-iodophenyl)-N'-methylurea (15g, 51.0mmol) and triethylamine (28.3mL, 204mmol) in dichloromethane (250mL), add CBr 4 (33.8g, 102mmol) and triphenylphosphine (26.8g, 102mmol), react at room temperature for 6h, and concentrate to obtain crude N-(2-fluoro-4-iodophenyl)-N'-methylcarbodiimide; To a solution of diethyl 2-methyl-3-oxo-glutaric acid (9.56g, 44.2mmol) in dry THF (100mL), add sodium ethoxide (50mmol) at 0°C, and slowly add the crude product in THF dropwise (150mL) solution, react at room temperature for 2h, and react at 50°C for 12h, add dilute hydrochloric acid (1mol / L, 80ml), ethyl acetate (50mL once, three times in total) to extract the aqueous phase, combine the organic layers, wash with water, and dry , filtered, concentrated, and separated on a silica gel column (200 mesh, mobile phase cyclohexane / ethyl acetate), to obtain oil 2-(2-fluoro-4-iodophenylam...
Embodiment 2
[0039] S1: In N-(2-fluoro-4-iodophenyl)-N'-methylurea (15g, 51.0mmol) and triethylamine (28.3mL, 204mmol) in dichloromethane (250mL), add CBr 4 (33.8g, 102mmol) and triphenylphosphine (26.8g, 102mmol), react at room temperature for 6h, and concentrate to obtain crude N-(2-fluoro-4-iodophenyl)-N'-methylcarbodiimide; To a solution of diethyl 2-methyl-3-oxo-glutaric acid (9.36g, 43.4mmol) in dry THF (100mL), add sodium ethoxide (45.9mmol) at 0°C, and slowly add the crude product THF (150mL) solution, reacted at room temperature for 2h, and reacted at 40°C for 14h, added dilute hydrochloric acid (1mol / L, 80ml), ethyl acetate (50mL once, three times in total) to extract the aqueous phase, combined the organic layers, washed with water, Dry, filter, concentrate, and separate on a silica gel column (200 mesh, mobile phase cyclohexane / ethyl acetate) to obtain the oil 2-(2-fluoro-4-iodophenylamino)-4-hydroxyl-1,5- Dimethyl-6-oxo-1,6-dihydropyridine-3-carboxylic acid ethyl ester (8.2g)...
Embodiment 3
[0043] S1: In N-(2-fluoro-4-iodophenyl)-N'-methylurea (15g, 51.0mmol) and triethylamine (28.3mL, 204mmol) in dichloromethane (250mL), add CBr 4 (33.8g, 102mmol) and triphenylphosphine (26.8g, 102mmol), react at room temperature for 6h, and concentrate to obtain crude N-(2-fluoro-4-iodophenyl)-N'-methylcarbodiimide; To a solution of diethyl 2-methyl-3-oxo-glutarate (11.03g, 51mmol) in dry THF (100mL), add sodium ethoxide (56.1mmol) at 0°C, and slowly add the crude product in THF dropwise (150mL) solution, react at room temperature for 2h, and react at 60°C for 10h, add dilute hydrochloric acid (1mol / L, 80ml), ethyl acetate (50mL once, three times in total) to extract the aqueous phase, combine the organic layers, wash with water, and dry , filtered, concentrated, and separated on a silica gel column (200 mesh, mobile phase cyclohexane / ethyl acetate), to obtain oil 2-(2-fluoro-4-iodophenylamino)-4-hydroxyl-1,5-di Methyl-6-oxo-1,6-dihydropyridine-3-carboxylic acid ethyl ester (8...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com