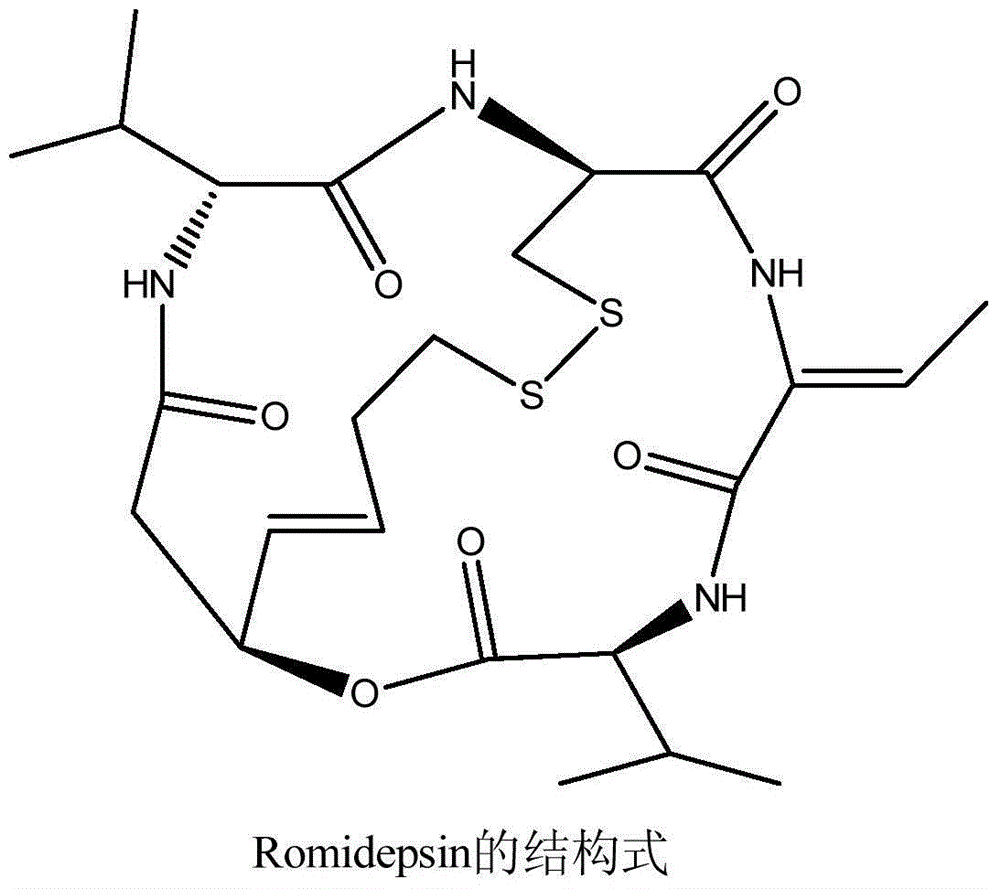
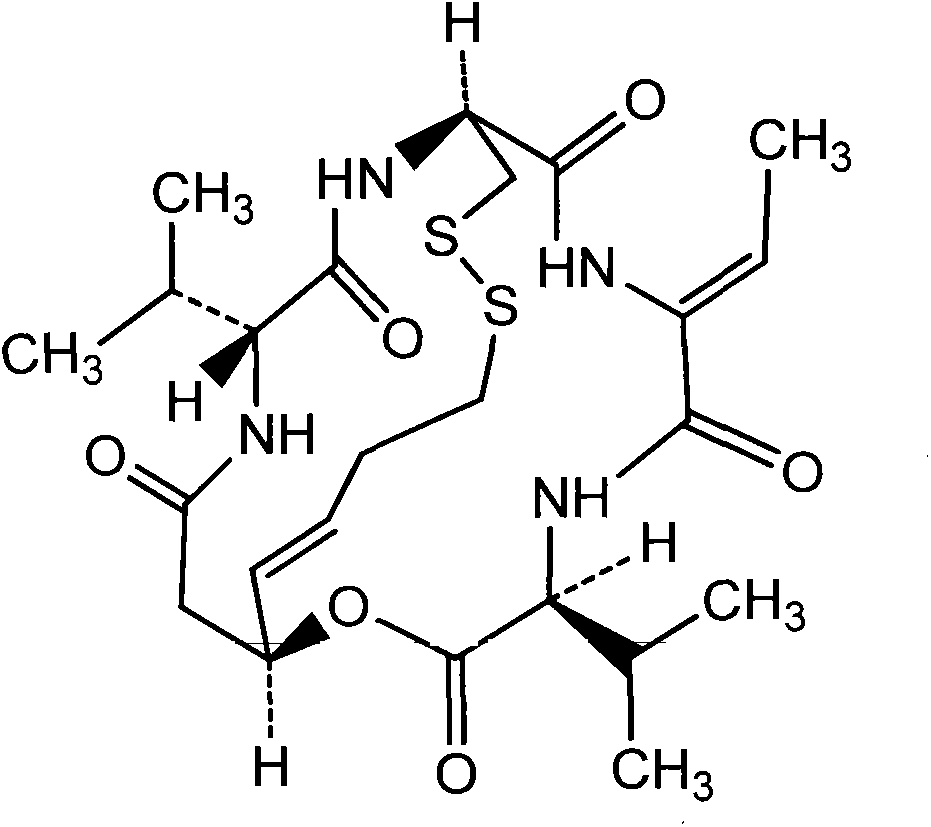
Patents
Literature
33 results about "Romidepsin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
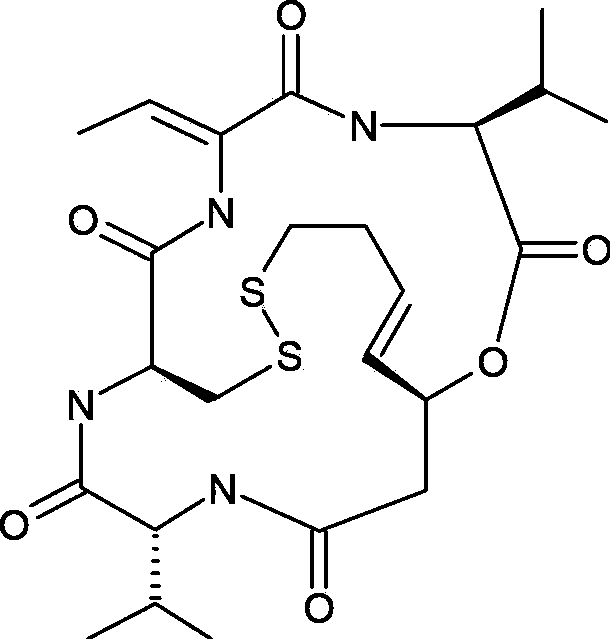
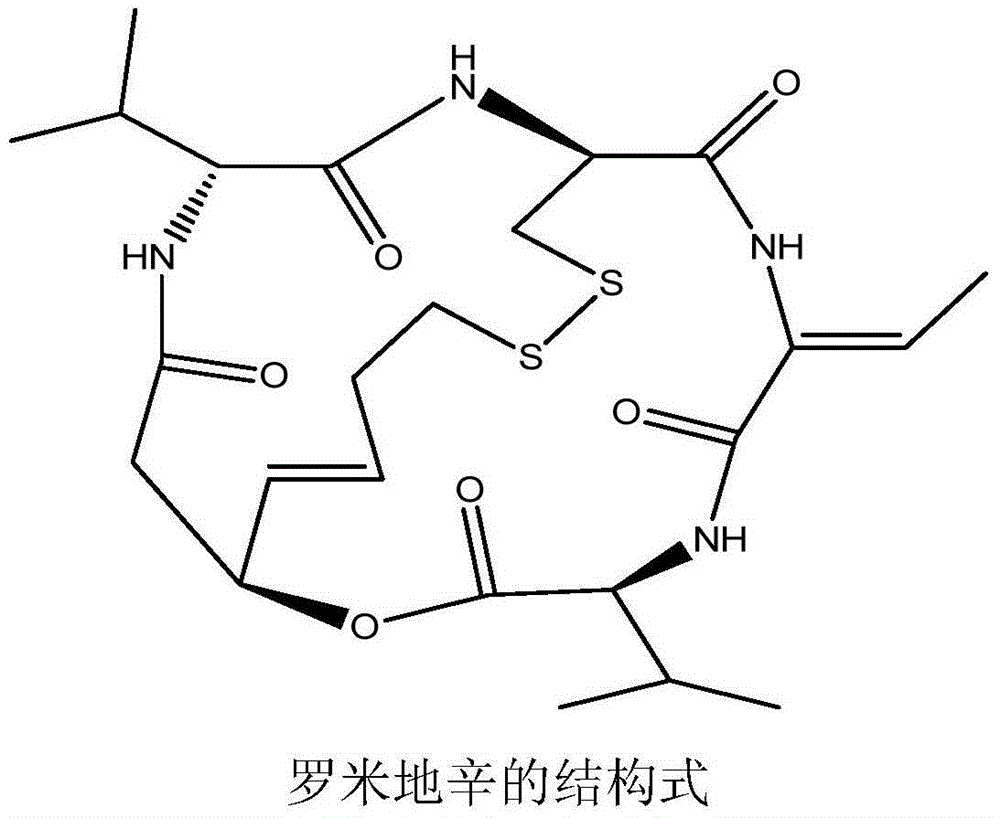
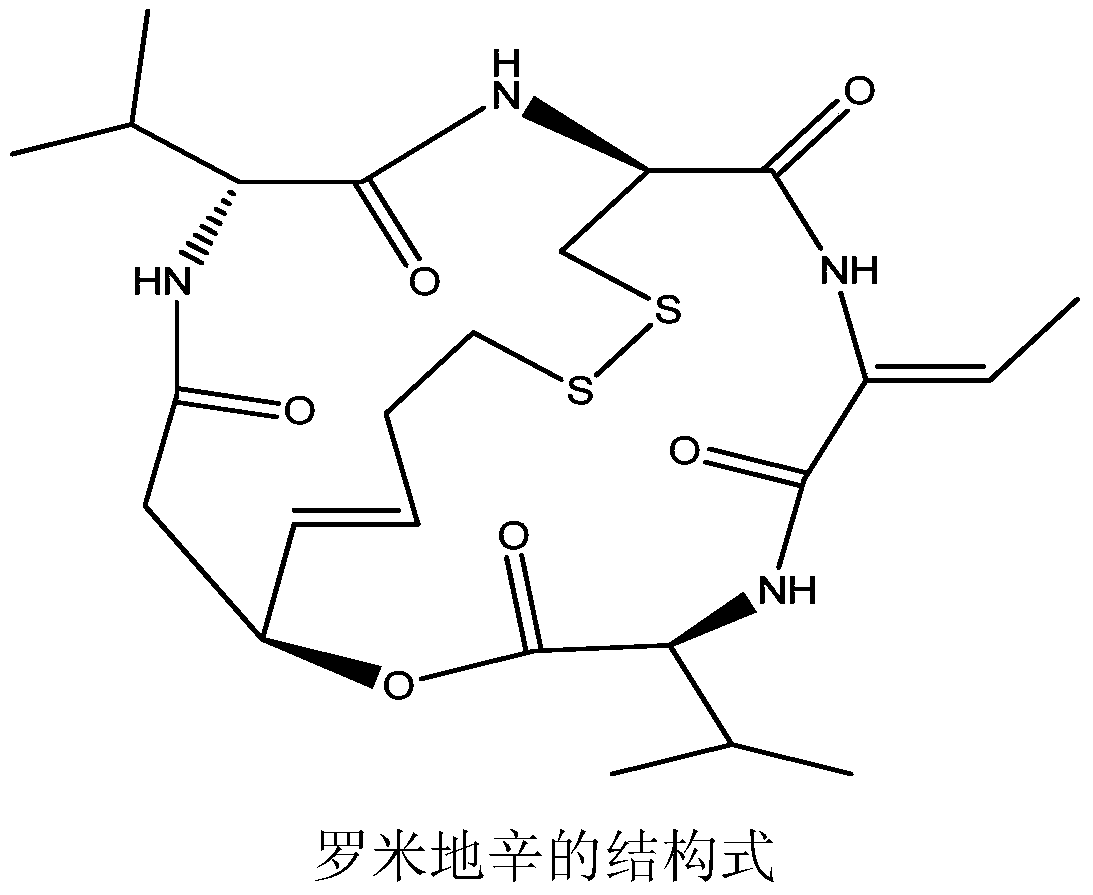
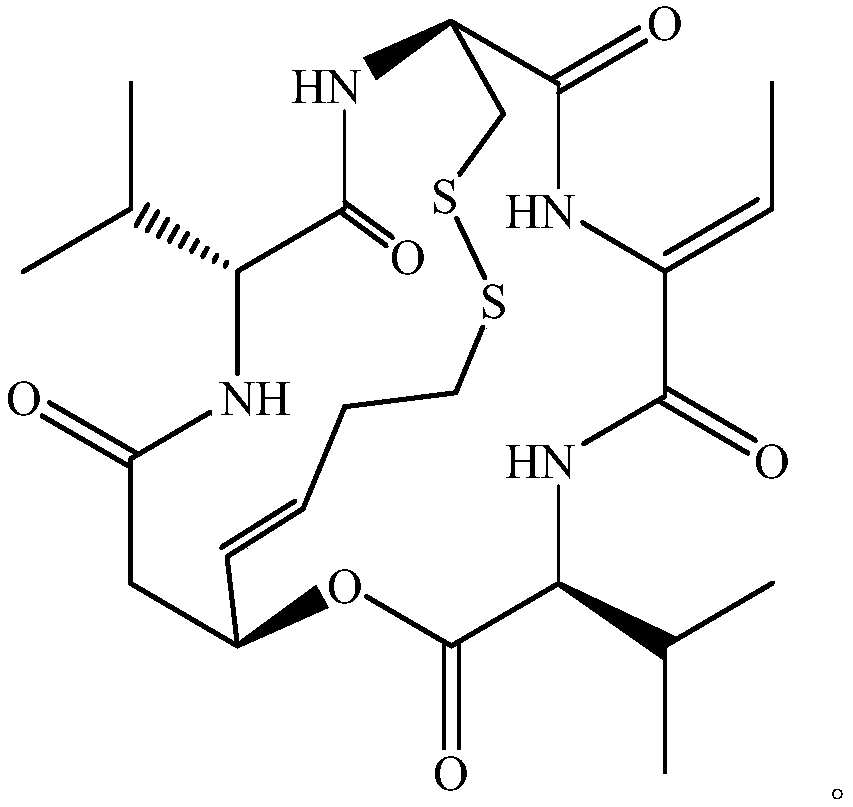
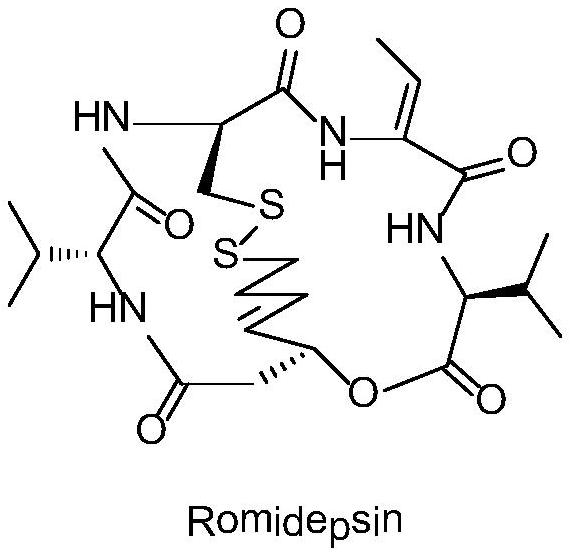
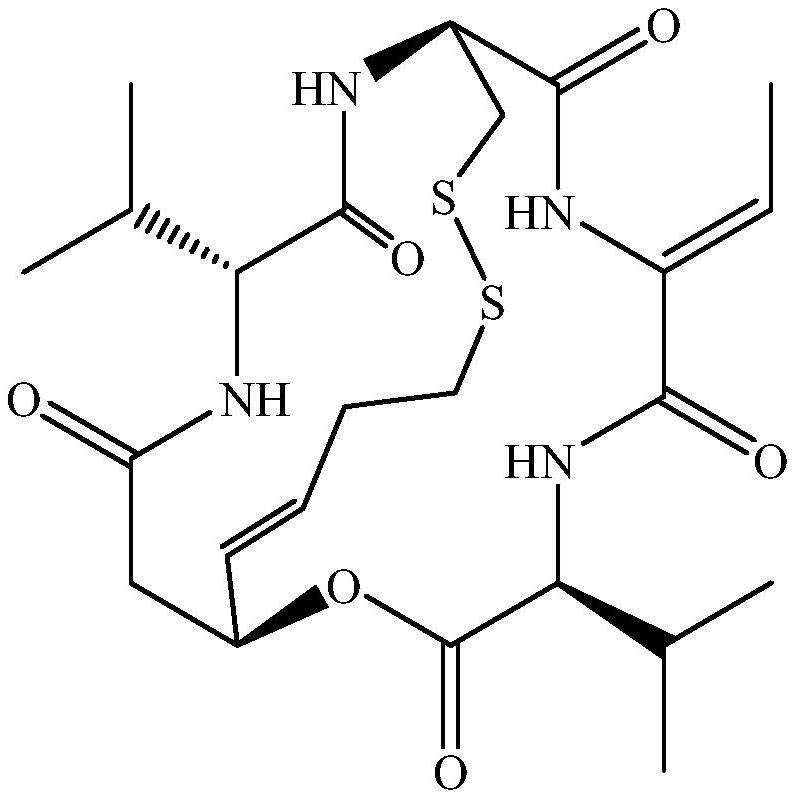
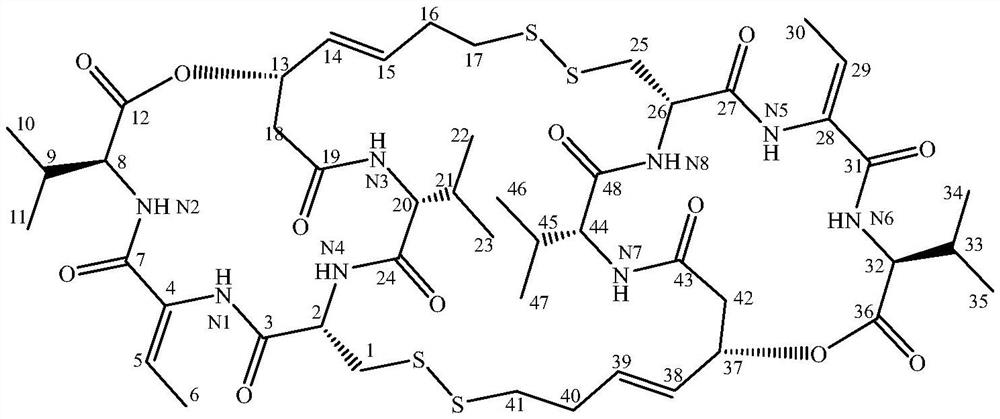
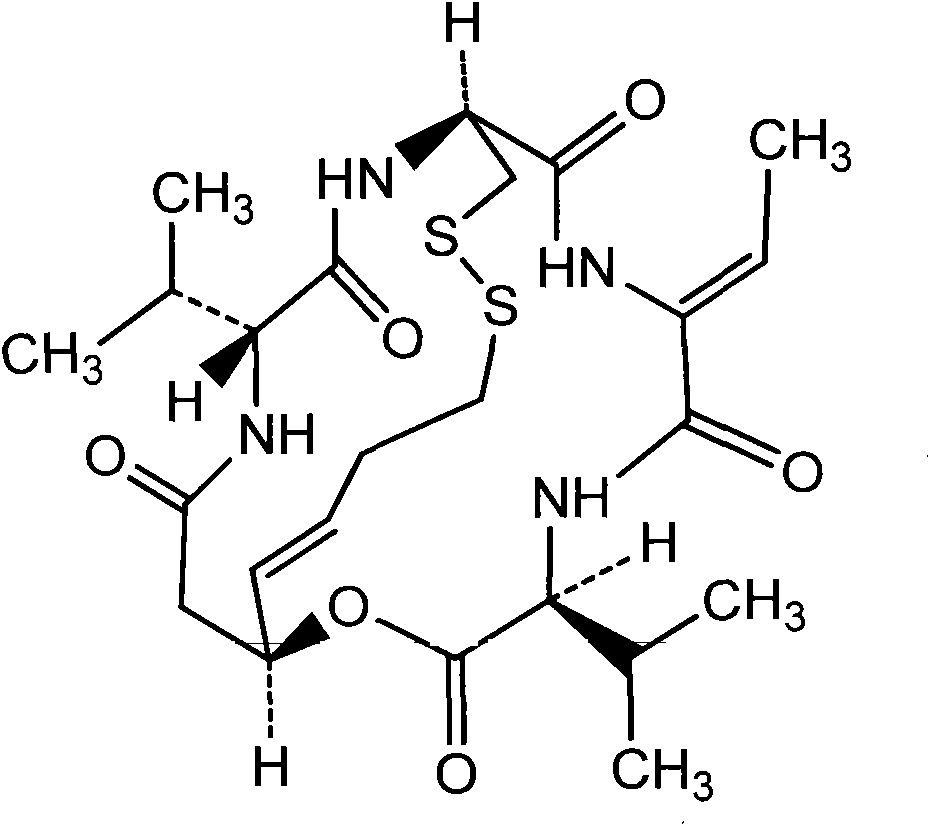
Romidepsin is used to treat certain types of cancer (cutaneous or peripheral T-cell lymphoma).
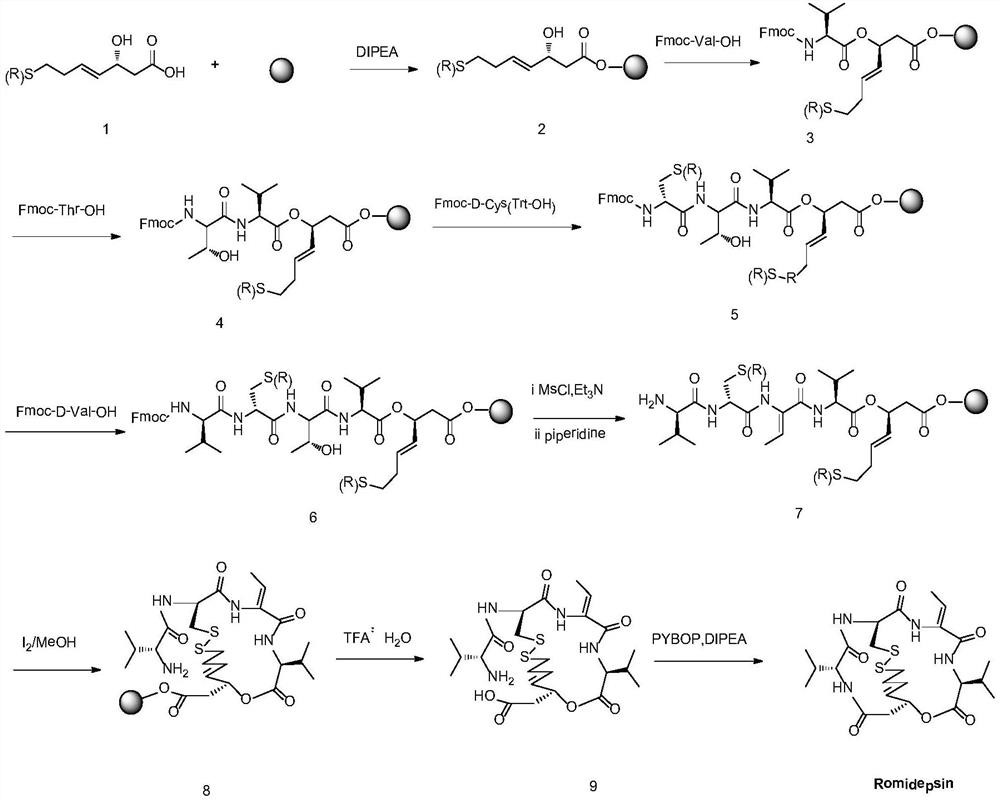
Preparation method for romidepsin
InactiveCN103897029AFew synthetic stepsHigh yieldPeptide preparation methodsCyclic peptidesCouplingMedicinal chemistry
Disclosed is a method for preparing Romidepsin. The present invention relates to the pharmaceutical synthesis. The present invention is based on a method of solid phase synthesis. First, coupling is performed between resin and carboxyl groups on 3-hydroxy-7-mercapto-4-heptenoic acid; then,coupling is performed in sequence between four pieces of amino acid on Romidepsin; then, hydroxyl groups are removed, and disulfide bonds and amido bonds are obtained by means of cyclization, so as to form Romidepsin. The purity the finally finished product is greater than 99%, the total yield is higher than 30%, and method features simple preparation, a short synthesis cycle and low cost, and helps to produce Romidepsin in a large scale.
Owner:HYBIO PHARMA
Treatment of Ras-Expressing Tumors
ActiveUS20090305956A1Effective treatmentOrganic active ingredientsCyclic peptide ingredientsWilms' tumorCancer research
The present invention provides compositions and methods for the treatment of / ?αs-expressing tumors using at least one DAC inhibitor (e.g., romidepsin).
Owner:CELGENE CORP
Pharmaceutical composition containing a hypomethylating agent and a histone deacetylase inhibitor
InactiveUS20110129521A1Good curative effectBiocideCarbohydrate active ingredientsTransdermal patchHistone methylation
A pharmaceutical composition for induction therapy which has a hypomethylating agent and a histone deacetylase inhibitor (“HDAC inhibitor”); wherein the hypomethylating agent is a DNA and histone methylation inhibitor such as cladribine and the HDAC inhibitor is, for example, entinostat, panobinostat, vorinostat, and / or romedepsin; further wherein the hypomethylating agent and the HDAC inhibitor are combined in formulations for various administrations including e.g., a continuous delivery system such as a transdermal patch of at least one reservoir or a plurality of reservoirs, oral, a fixed-dose oral combination, intravenous, and combinations thereof. This pharmaceutical composition for induction therapy is used with a monoclonal antibody in the treatment of various cancers, sarcomas, and other malignancies.
Owner:NIMBLE EPITECH
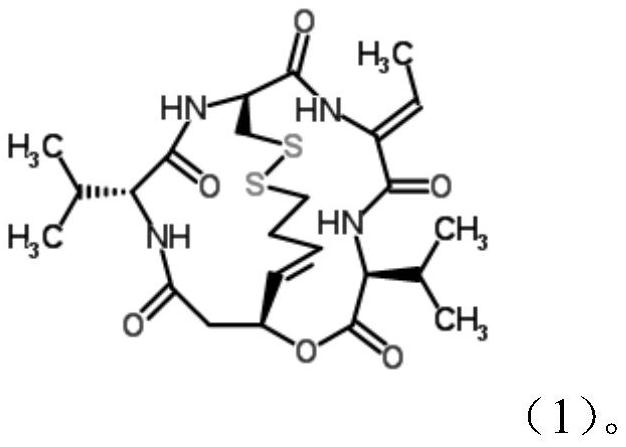
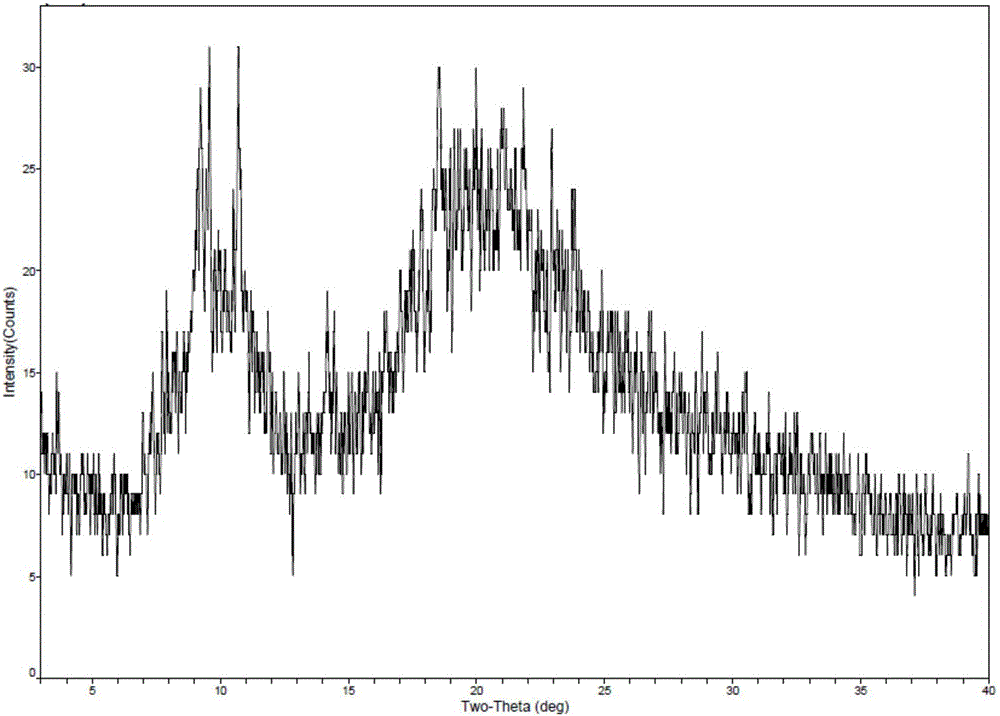
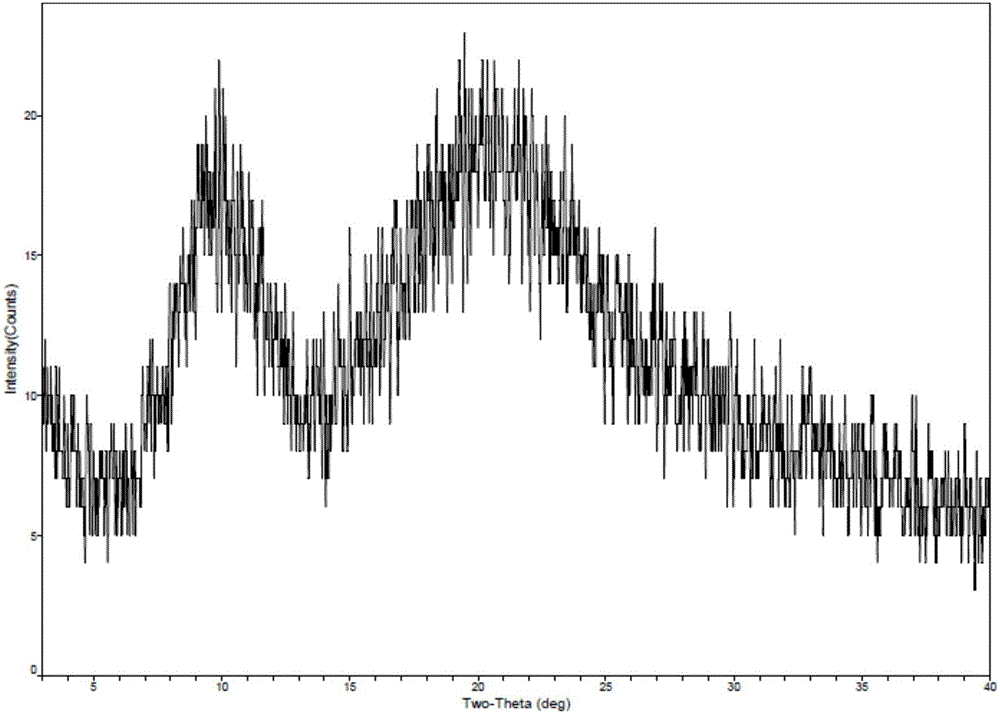
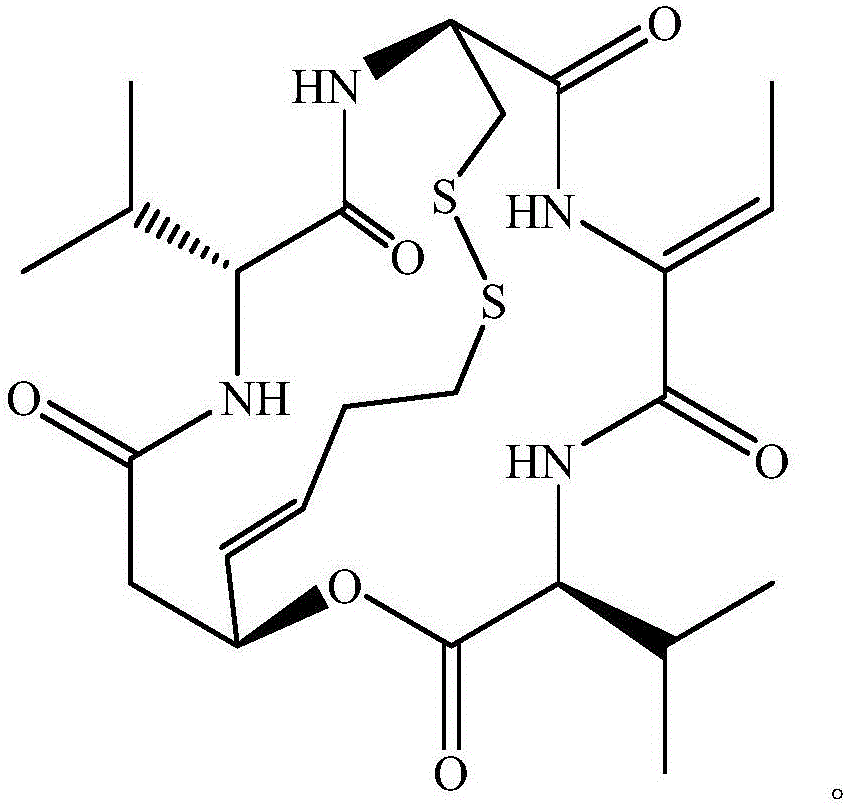
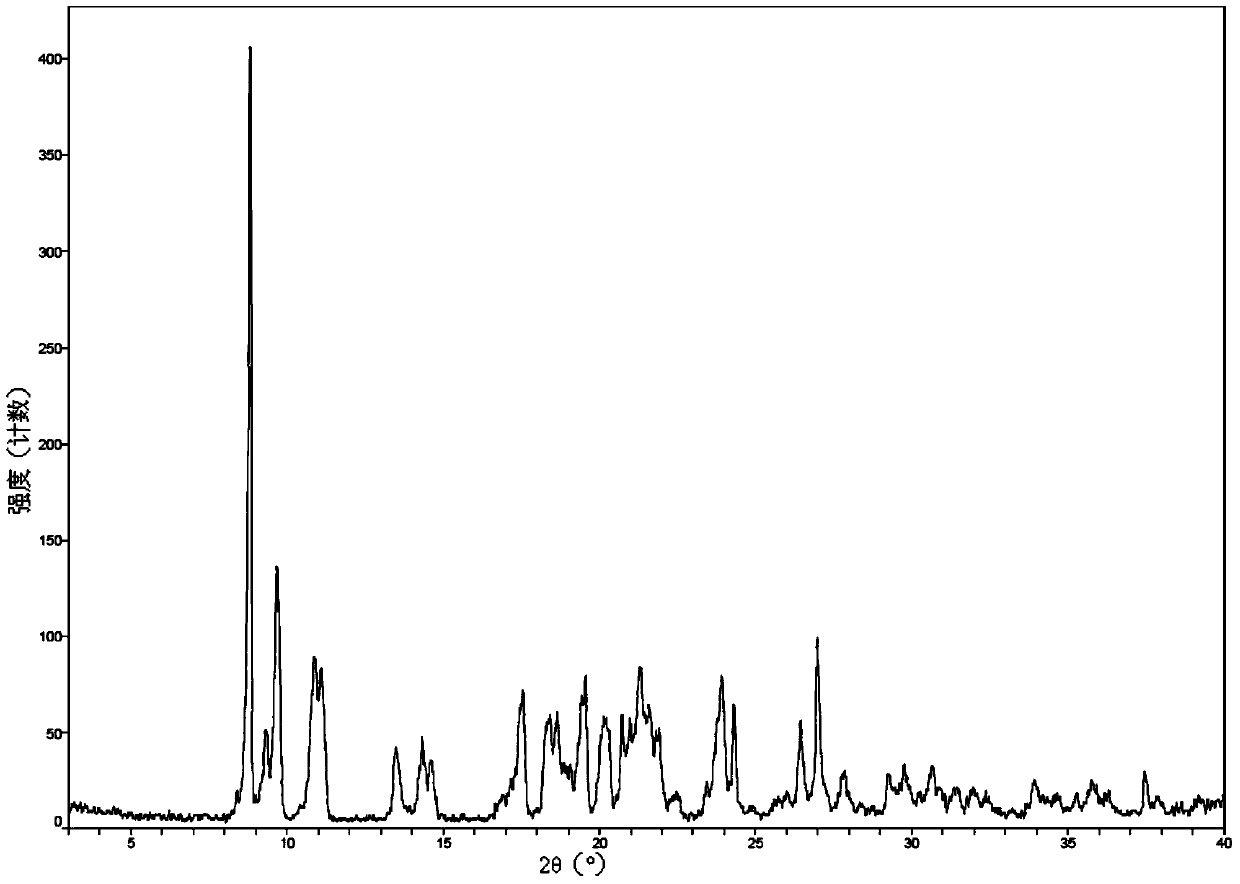
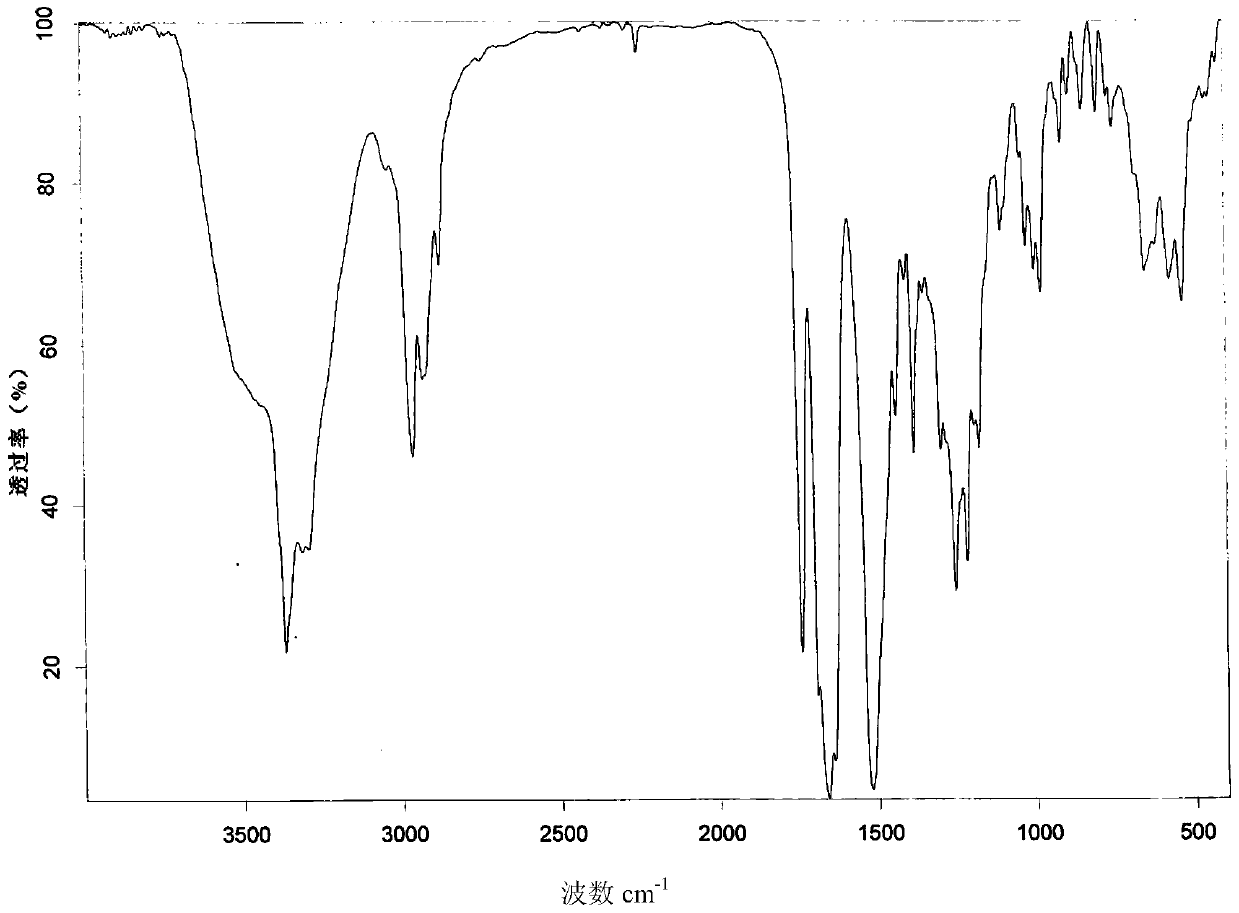
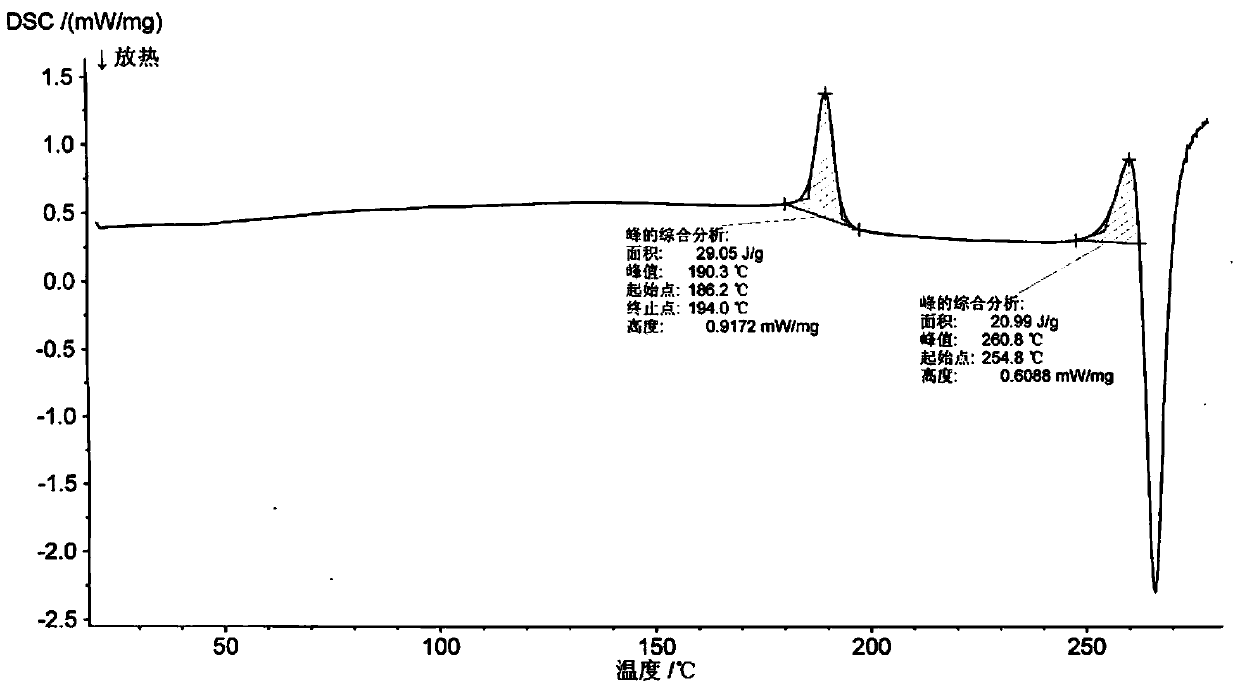
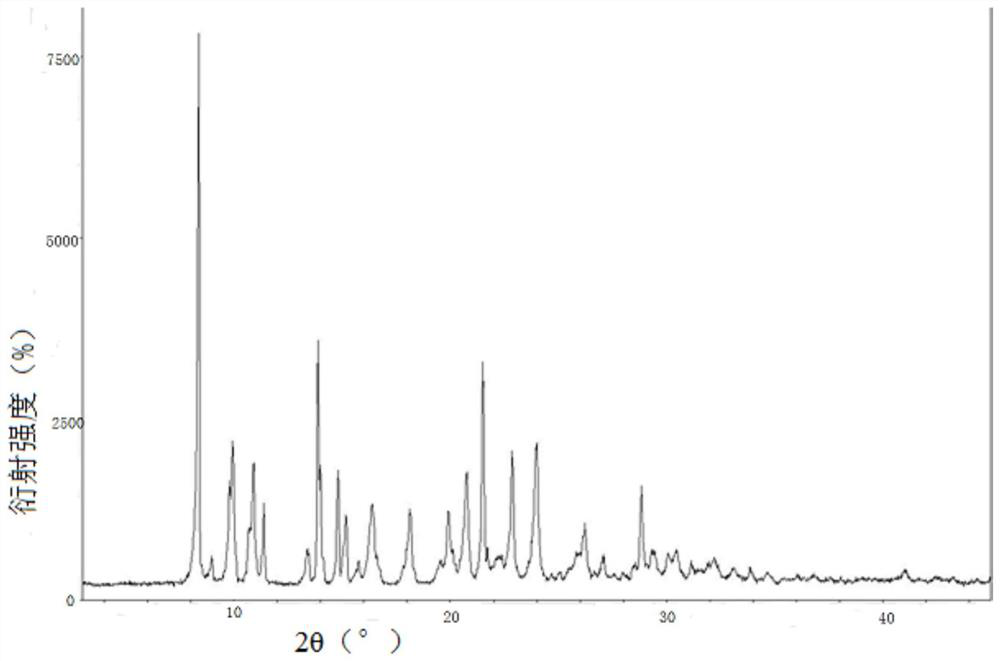
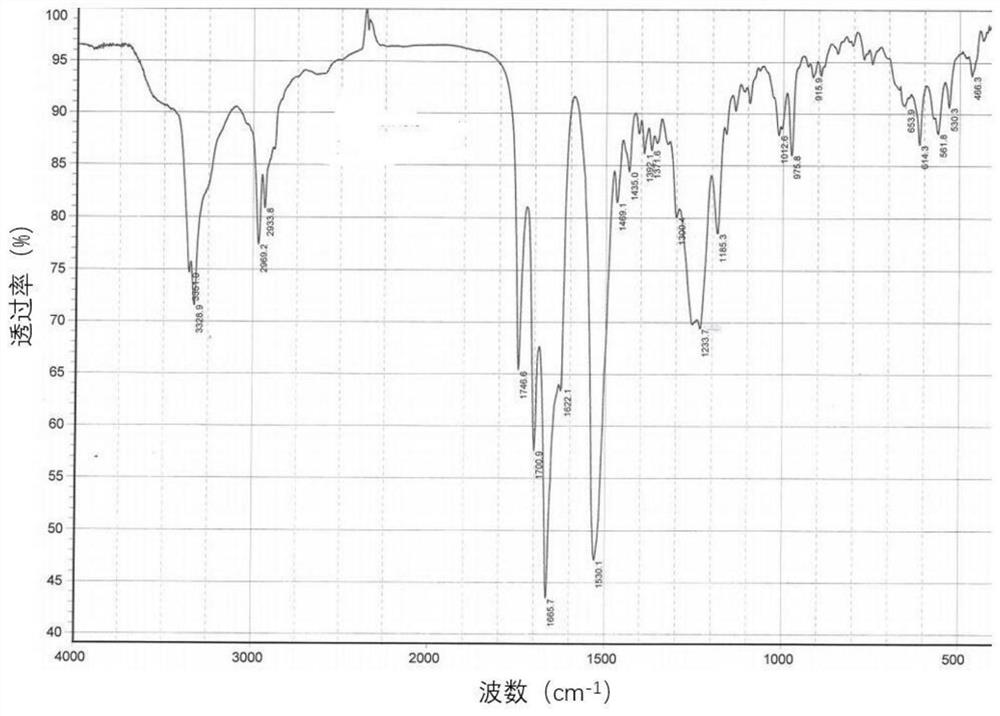
New crystal form of romidepsin, and preparation method and application thereof
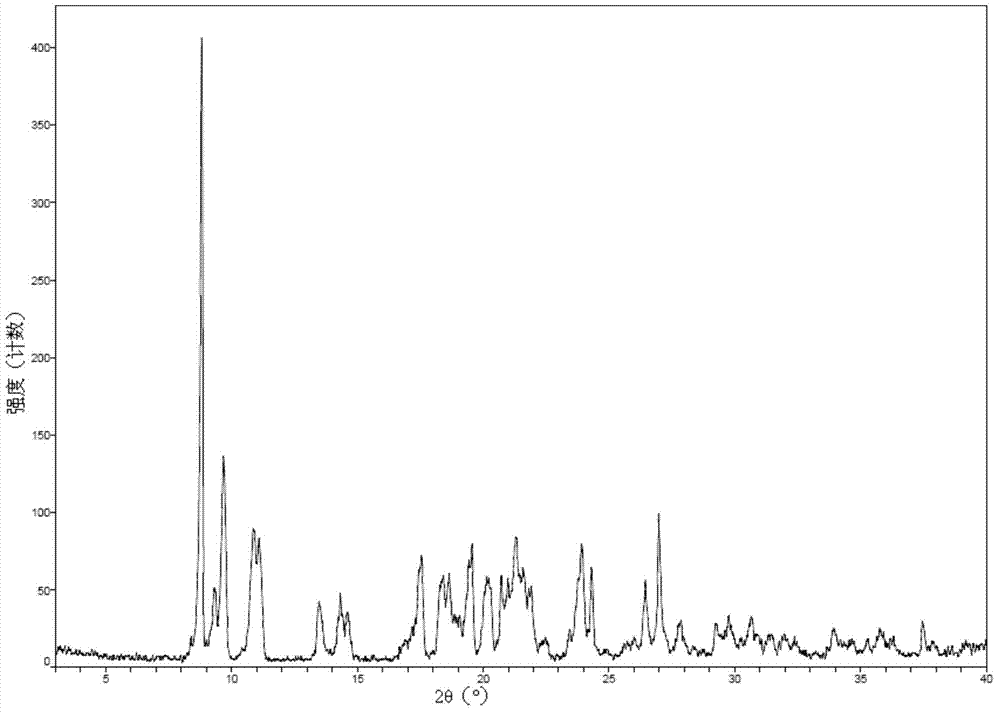
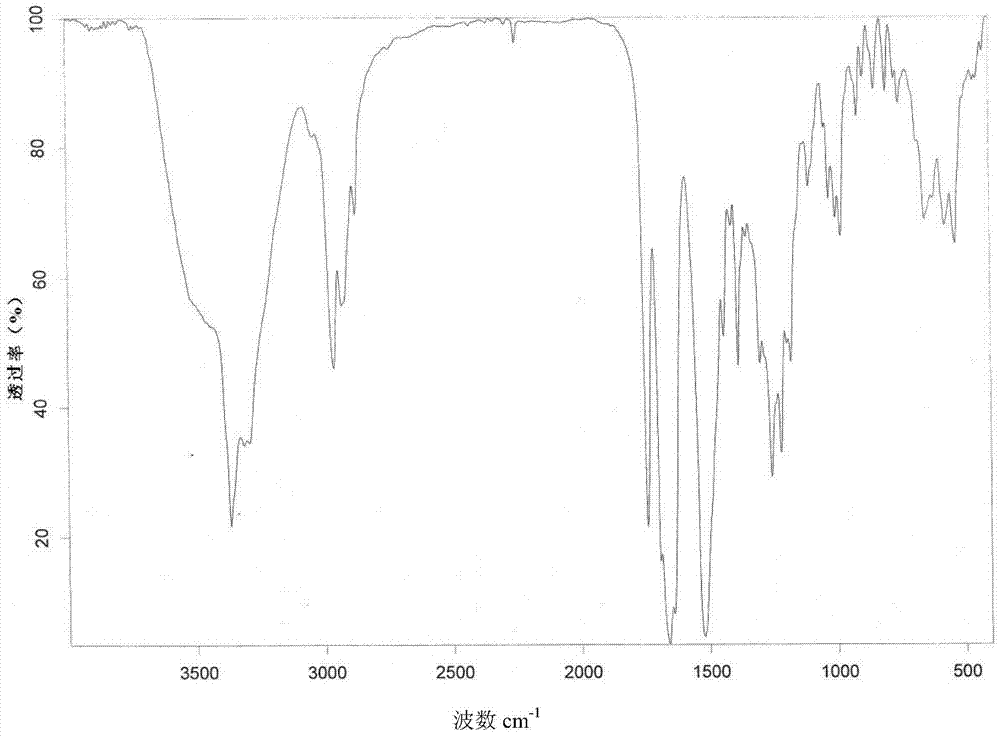
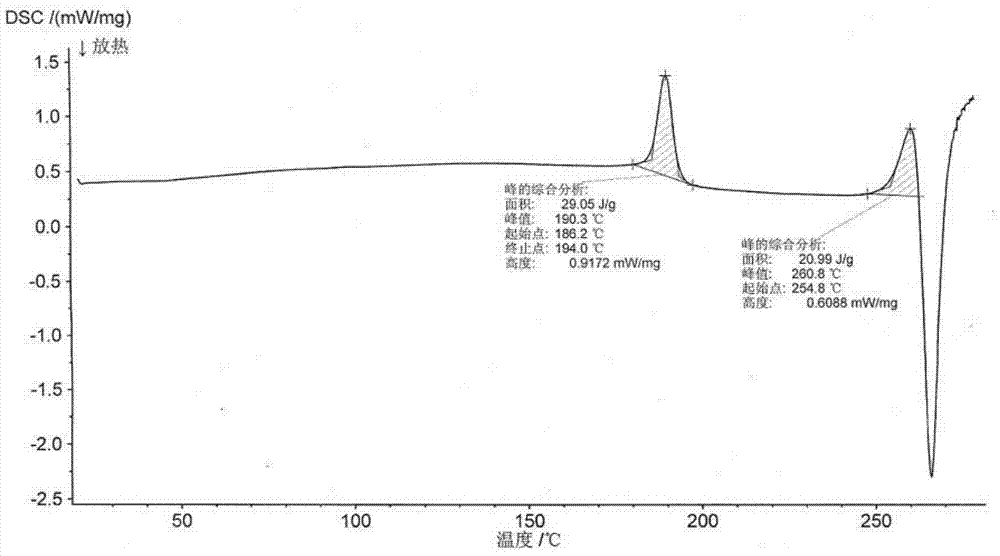
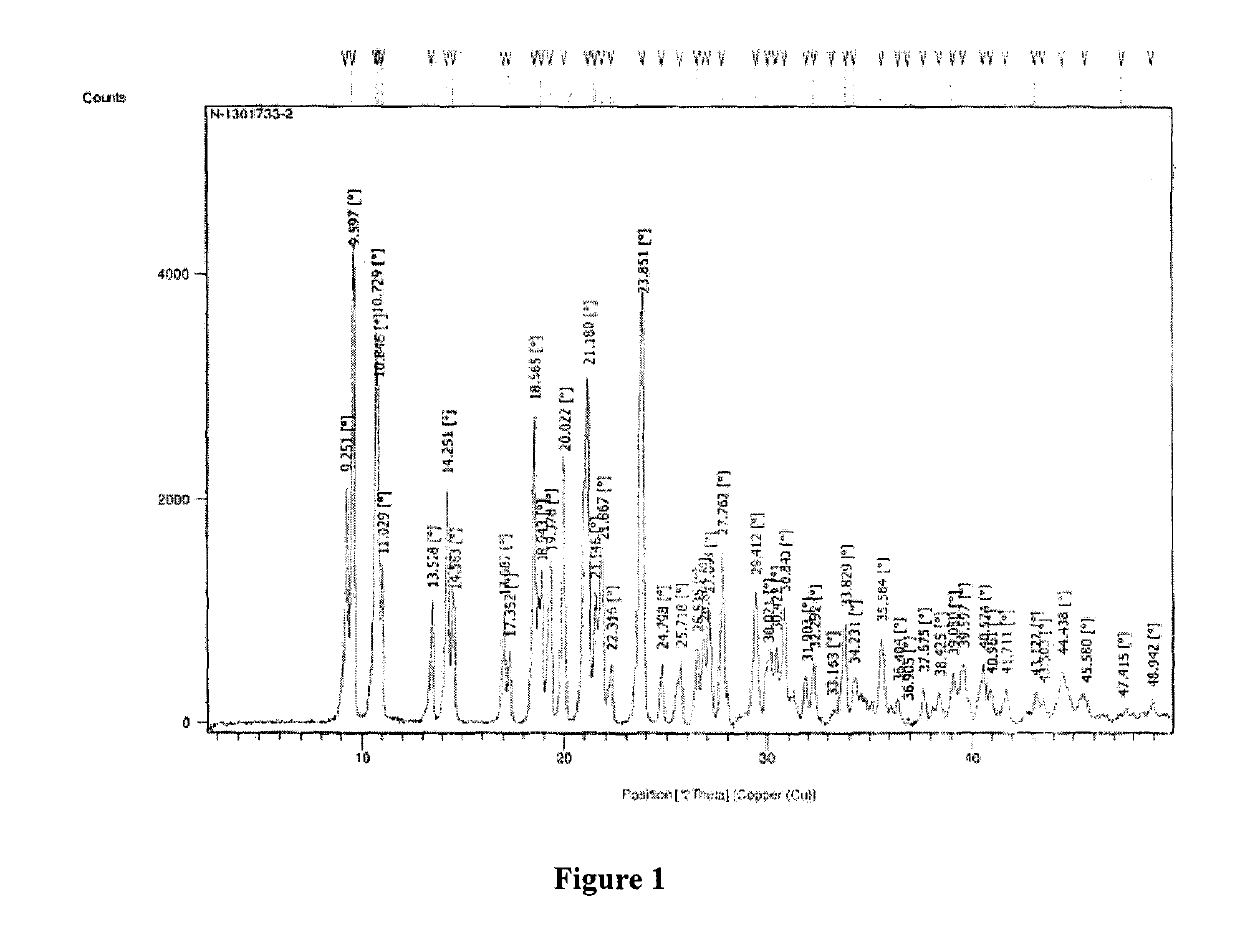
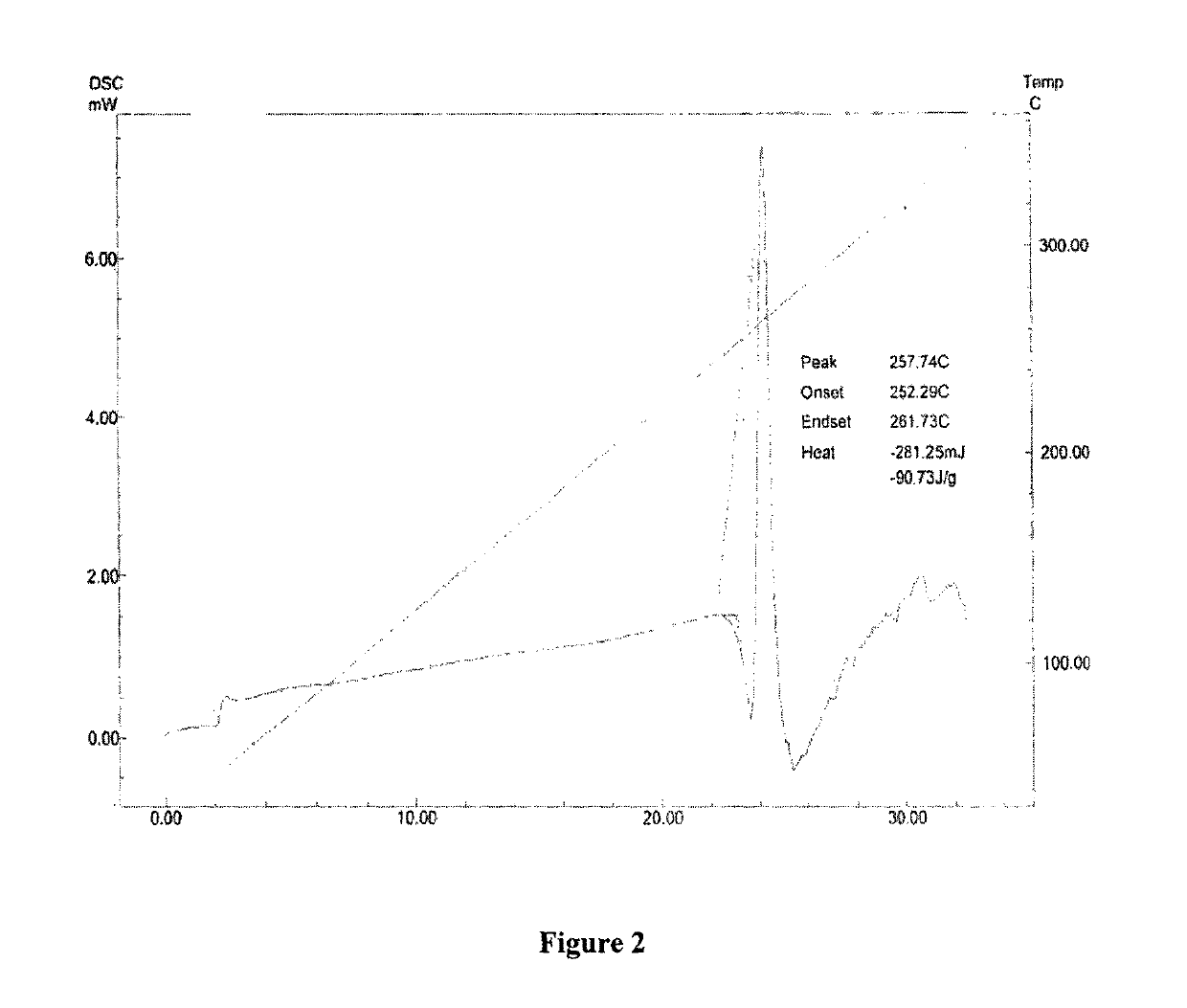
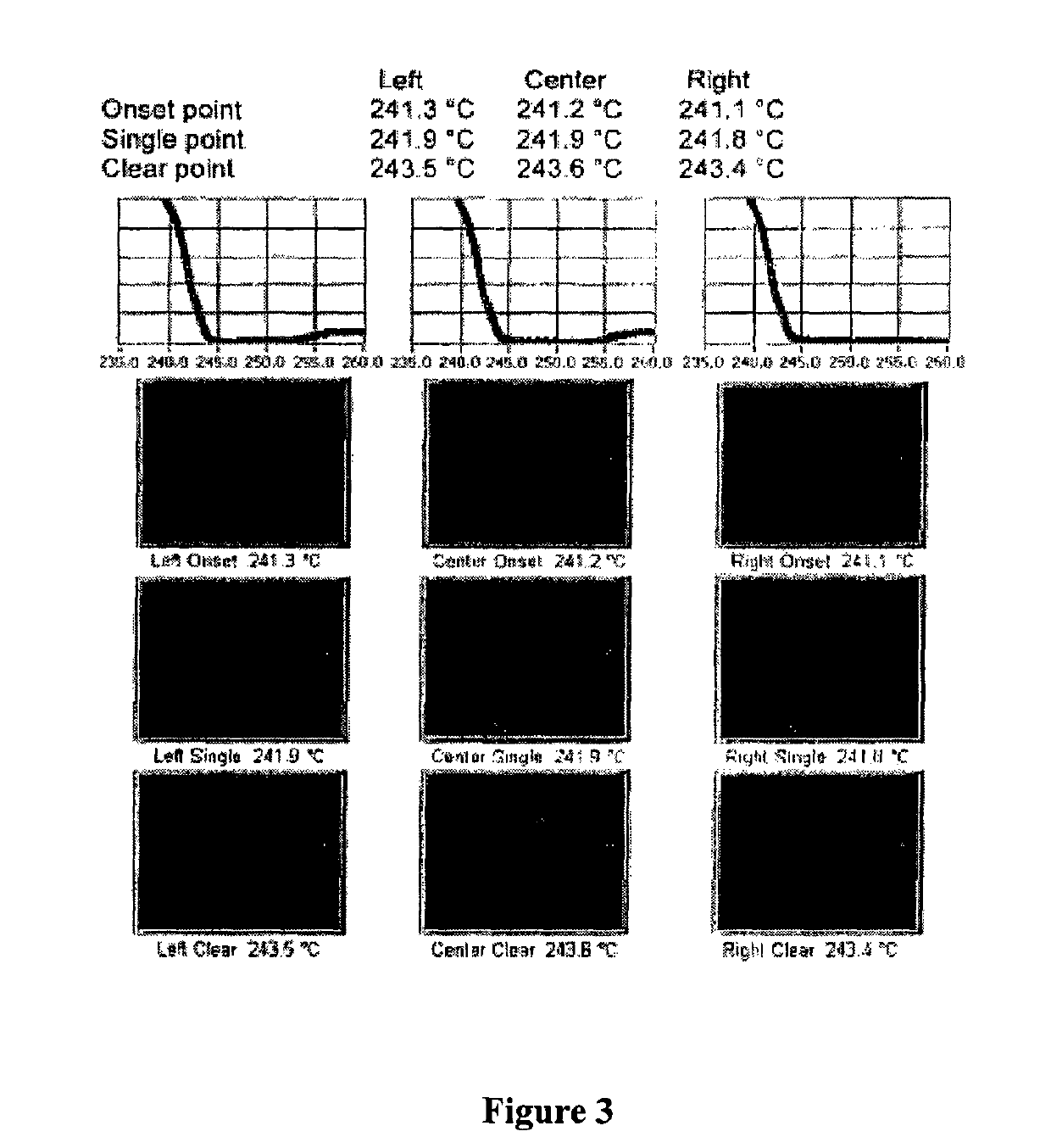
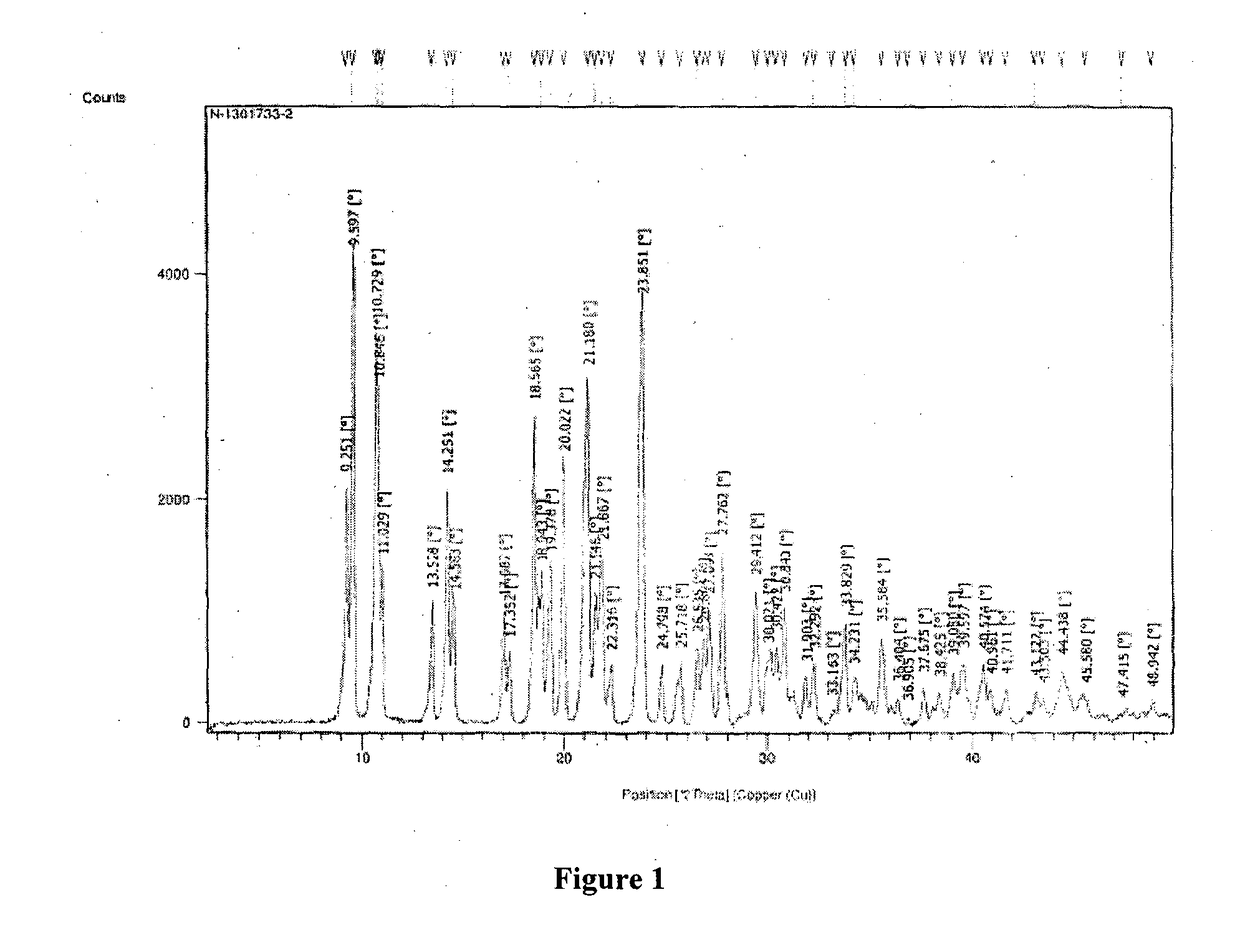
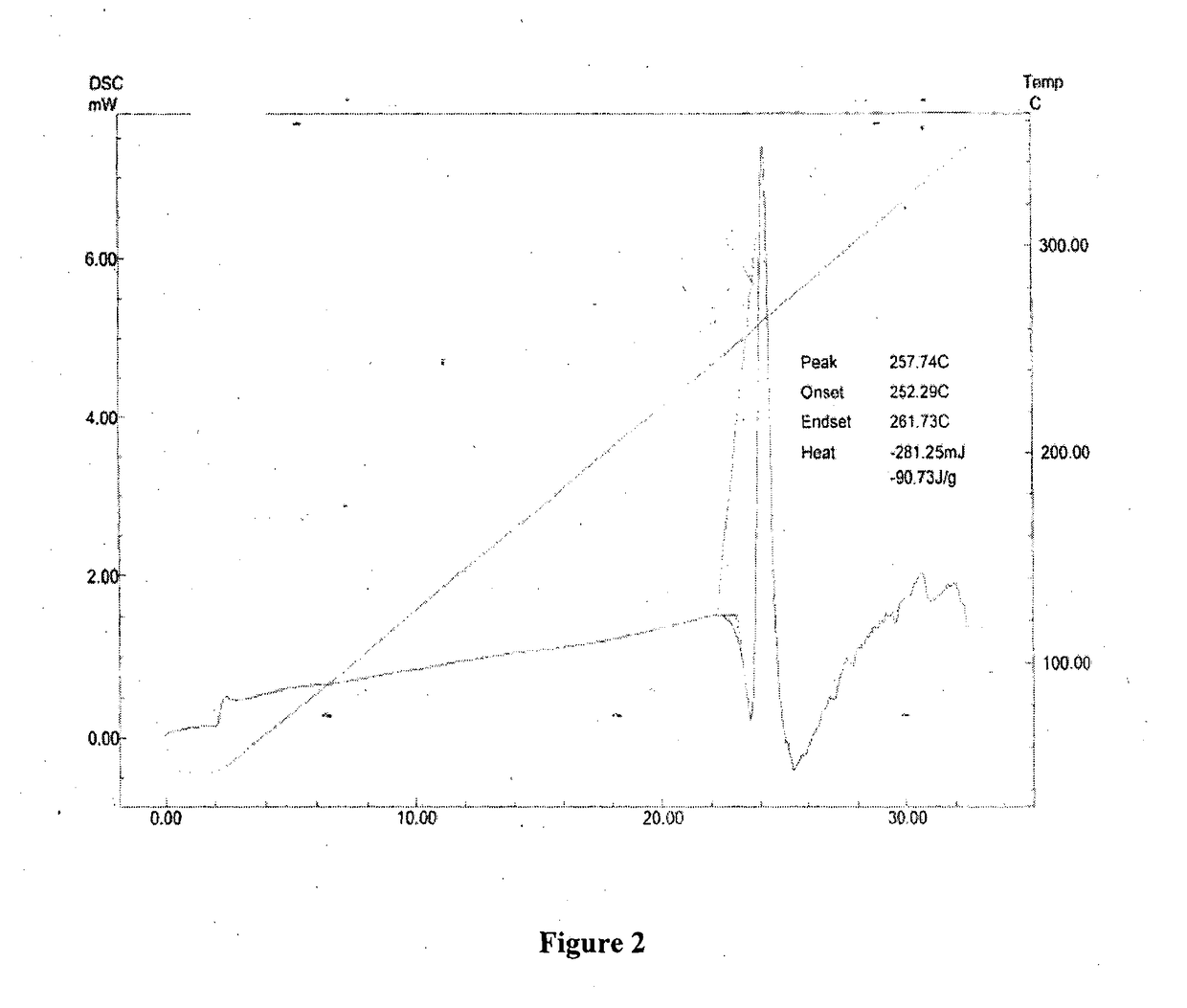
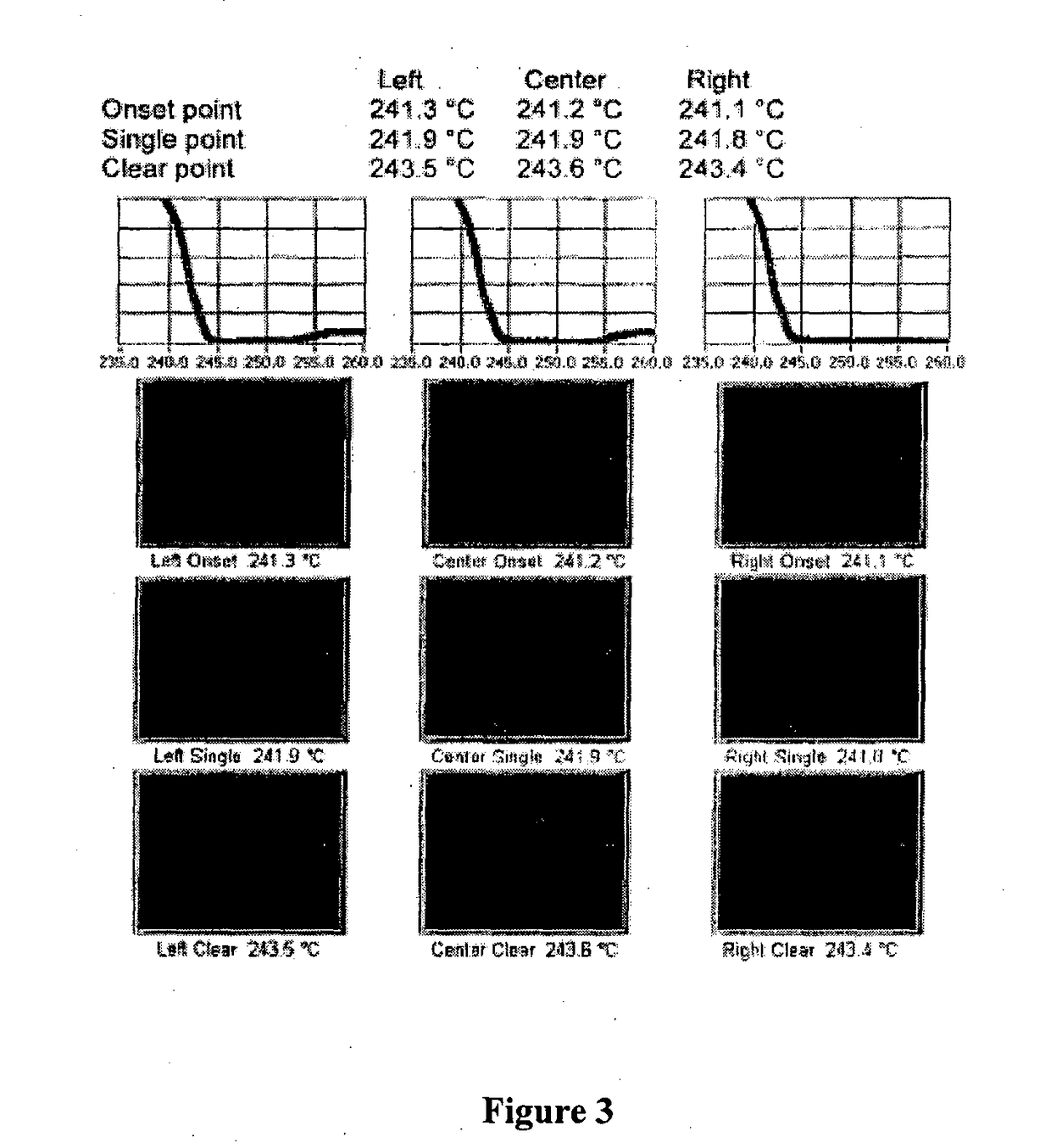
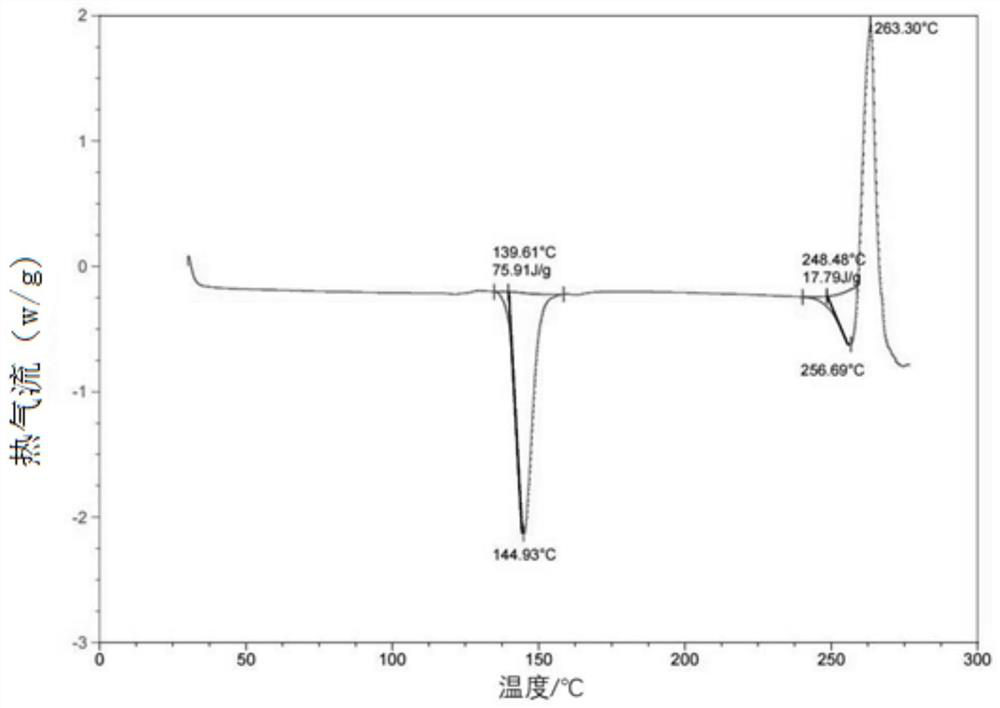
ActiveCN104262456ATetrapeptide ingredientsPeptide preparation methodsX-rayDifferential scanning calorimetry
The invention relates to a new crystal form O of romidepsin, which can be characterized by X-ray powder diffraction (XRD) spectrogram, differential scanning calorimetry (DSC) spectrogram, infrared absorption (UV) spectrogram and the like. Meanwhile, the invention also relates to a preparation method and application of the romidepsin crystal form O.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Romidepsin separation and purification method
InactiveCN104447950AHigh purityHigh yieldPeptide preparation methodsPurification methodsOrganic layer
The invention discloses a romidepsin separation and purification method. The method comprises the following steps: 1, mixing a romidepsin-containing Chromobacterium violaceum broth with macroporous adsorption resin, and stirring until complete adsorption; 2, separating out the macroporous adsorption resin adsorbing romidepsin from the broth, adding the macroporous adsorption resin adsorbing romidepsin to a column, cleaning, eluting to obtain an eluate 1, and concentrating to obtain a concentrate 1; 3, adding the concentrate 1 into macroporous adsorption resin, loading to the column, washing, eluting, detecting by using HPLC in the eluting process, collecting a romidepsin-containing elute 2, and concentrating to obtain a concentrate 2; 4, extracting the concentrate 2 to obtain an organic layer, and concentrating to obtain a crude product; and 5, purifying the crude product to obtain romidepsin. The separation and purification method has the advantages of high extraction purity, high yield, simple purification process and low cost.
Owner:SHANGHAI INST OF PHARMA IND +1
Accelerated therapy
InactiveUS20100152100A1Without increasing rateIncrease rate increaseOrganic active ingredientsBiocideMedicineRomidepsin
The present invention encompasses the surprising finding that romidepsin can safely be administered to humans on an accelerated dosing schedule.
Owner:CELGENE CORP +1
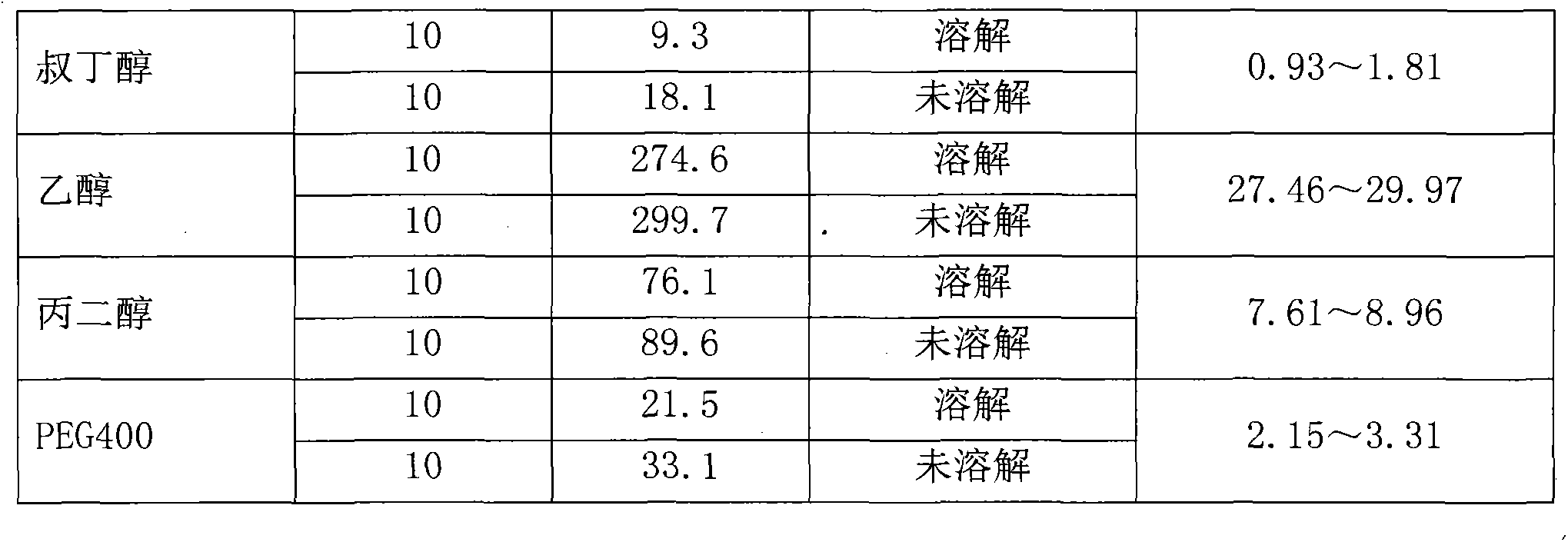
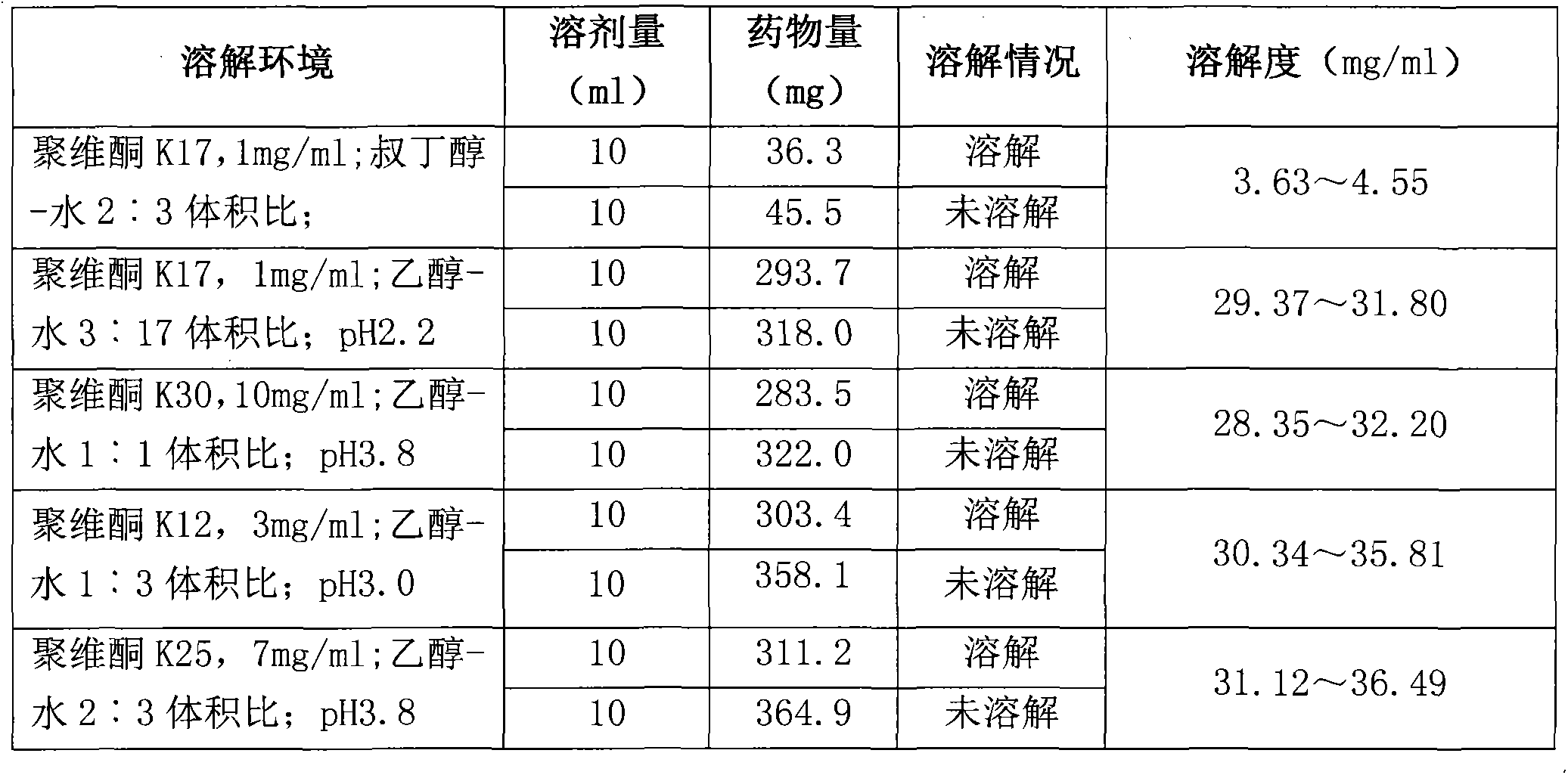
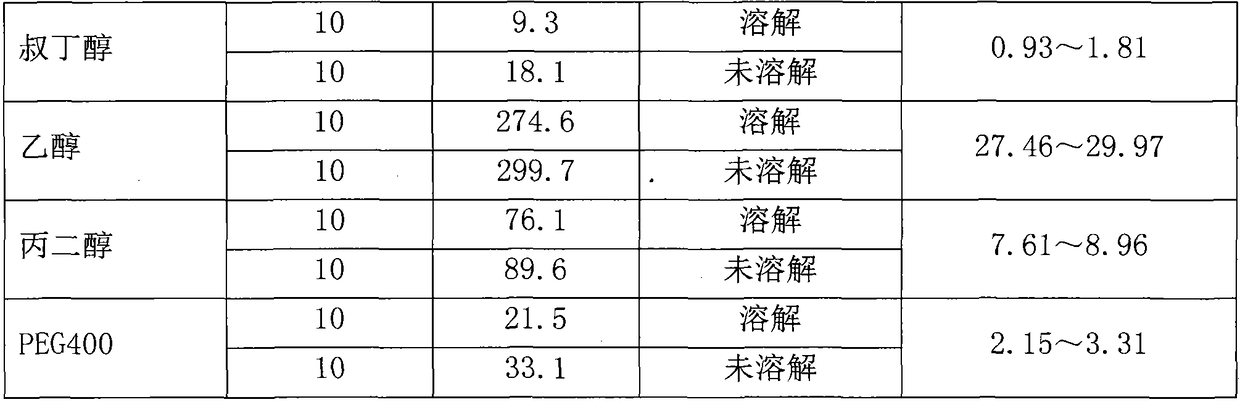
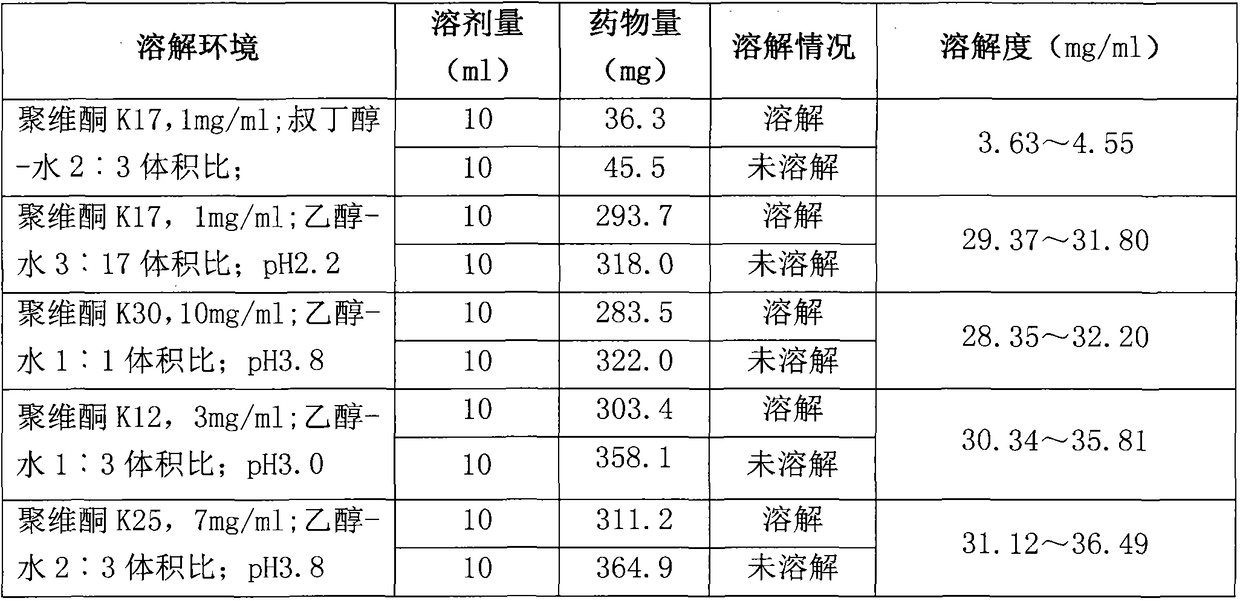
Preparation method of Romidepsin solution
ActiveCN103877010AImprove solubilityEasy to getPowder deliveryPharmaceutical non-active ingredientsFreeze-dryingDissolution
The invention relates to a preparation method of a Romidepsin solution. Specifically, the method mainly includes the following three steps of: 1. preparing a povidone-containing ethanol water solution; 2. adjusting the pH of the solution obtained in step 1; and 3. controlling the solution temperature and maintaining the pH of the solution, adding Romidepsin until complete dissolution, thus obtaining the Romidepsin solution with a concentration of 0.5-6mg / ml. The invention also discloses application of the Romidepsin solution prepared by the method in preparation of freeze-dried powder injections.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
Method of blocking transmission of malarial parasite
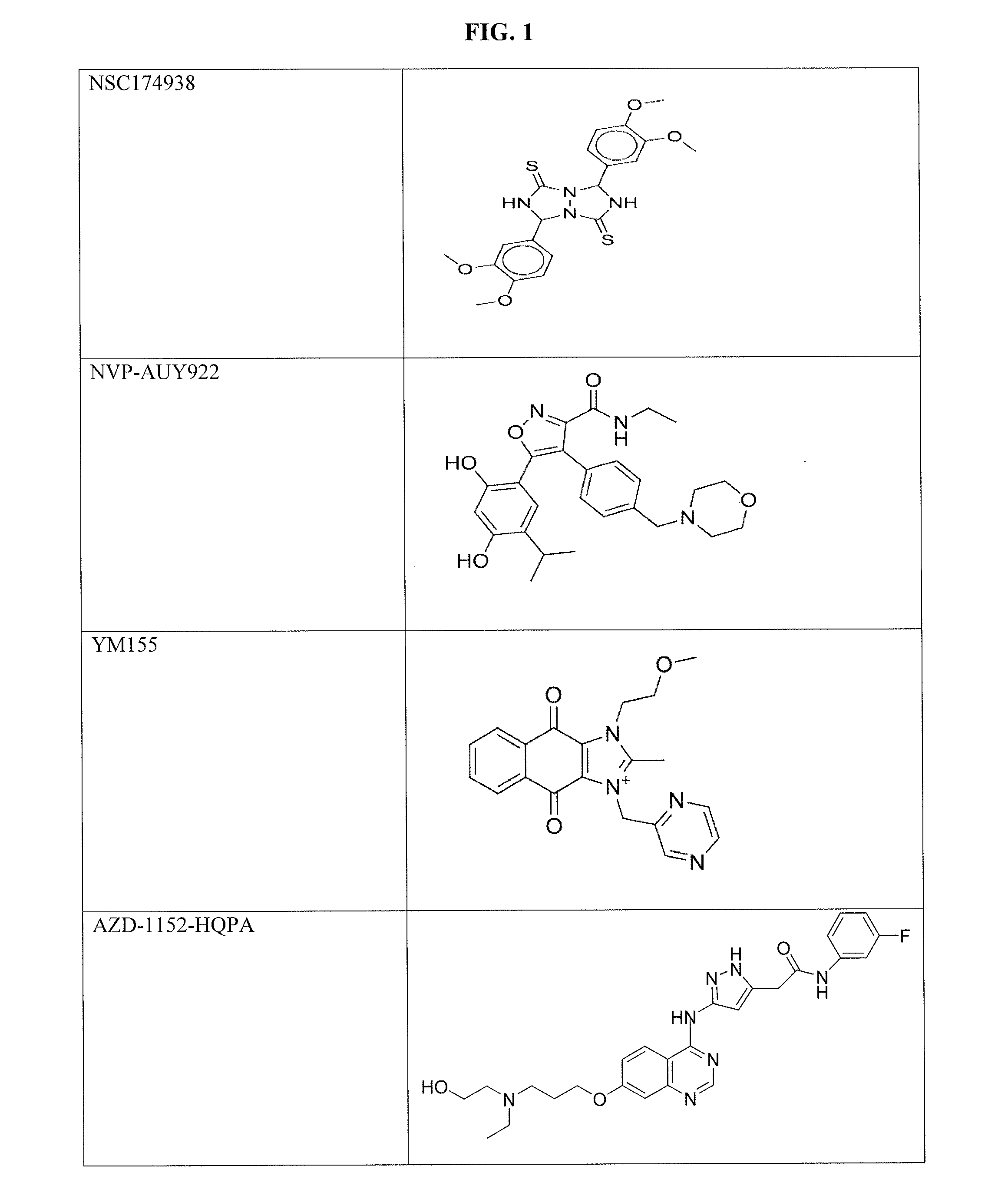
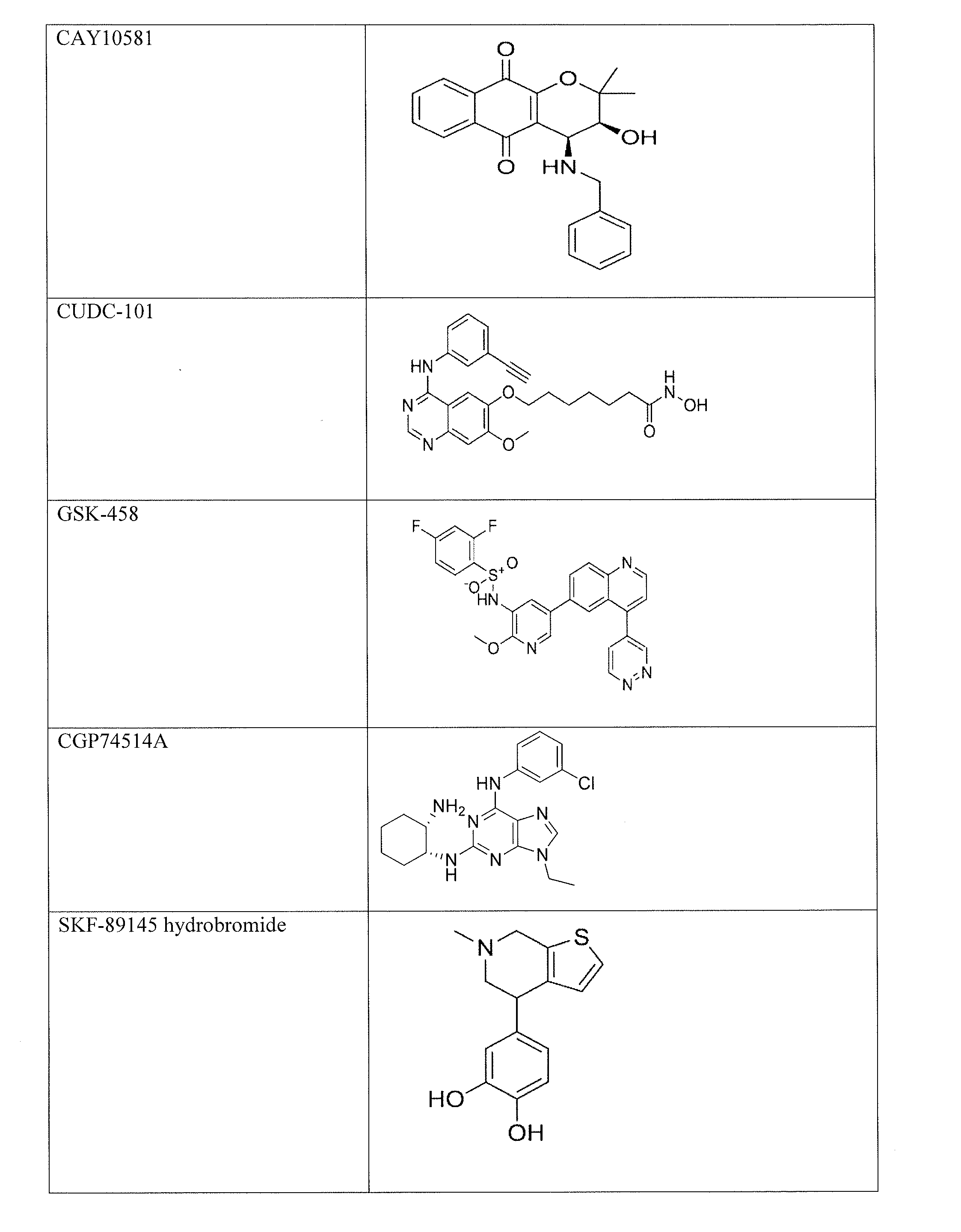
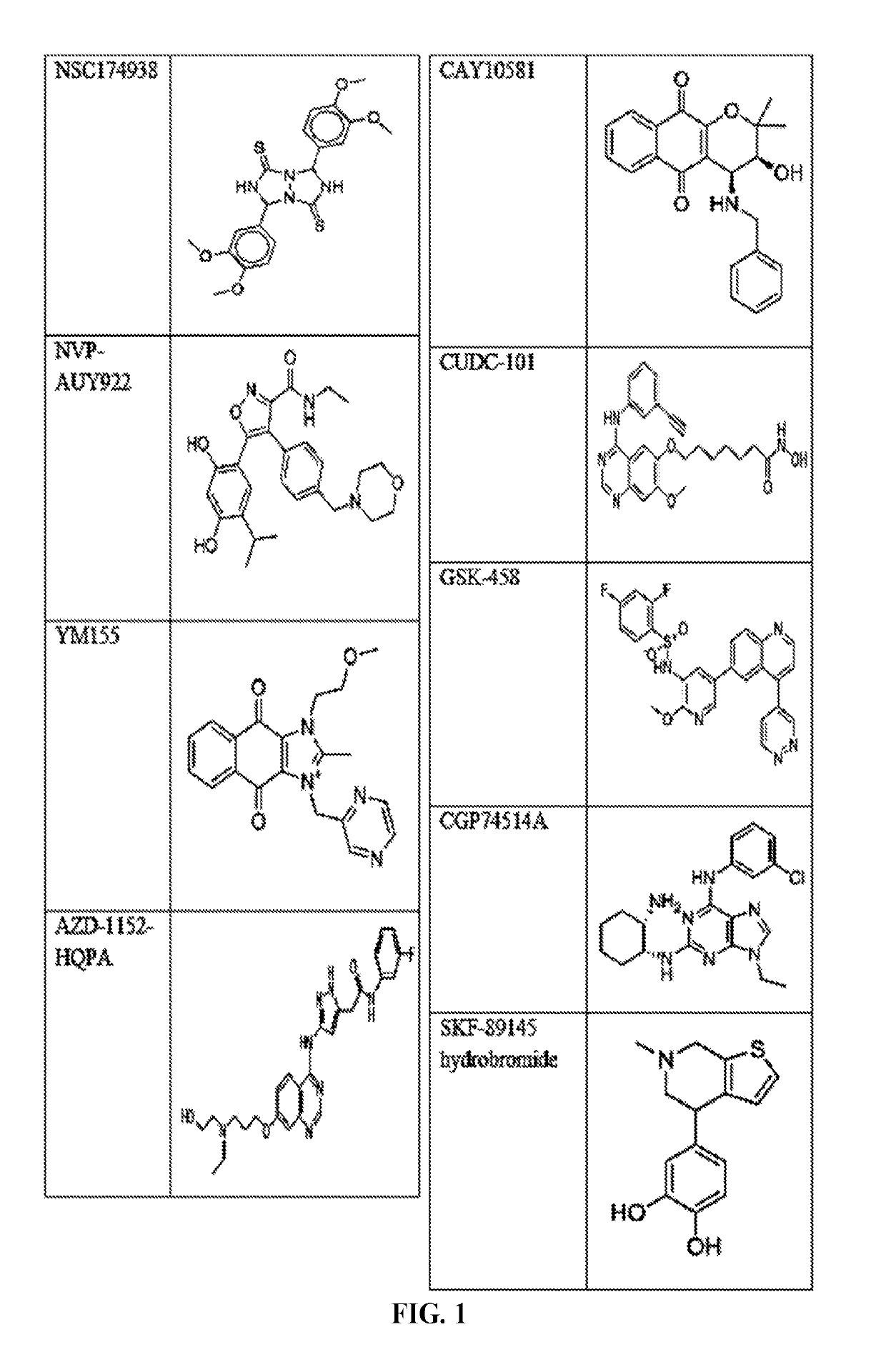
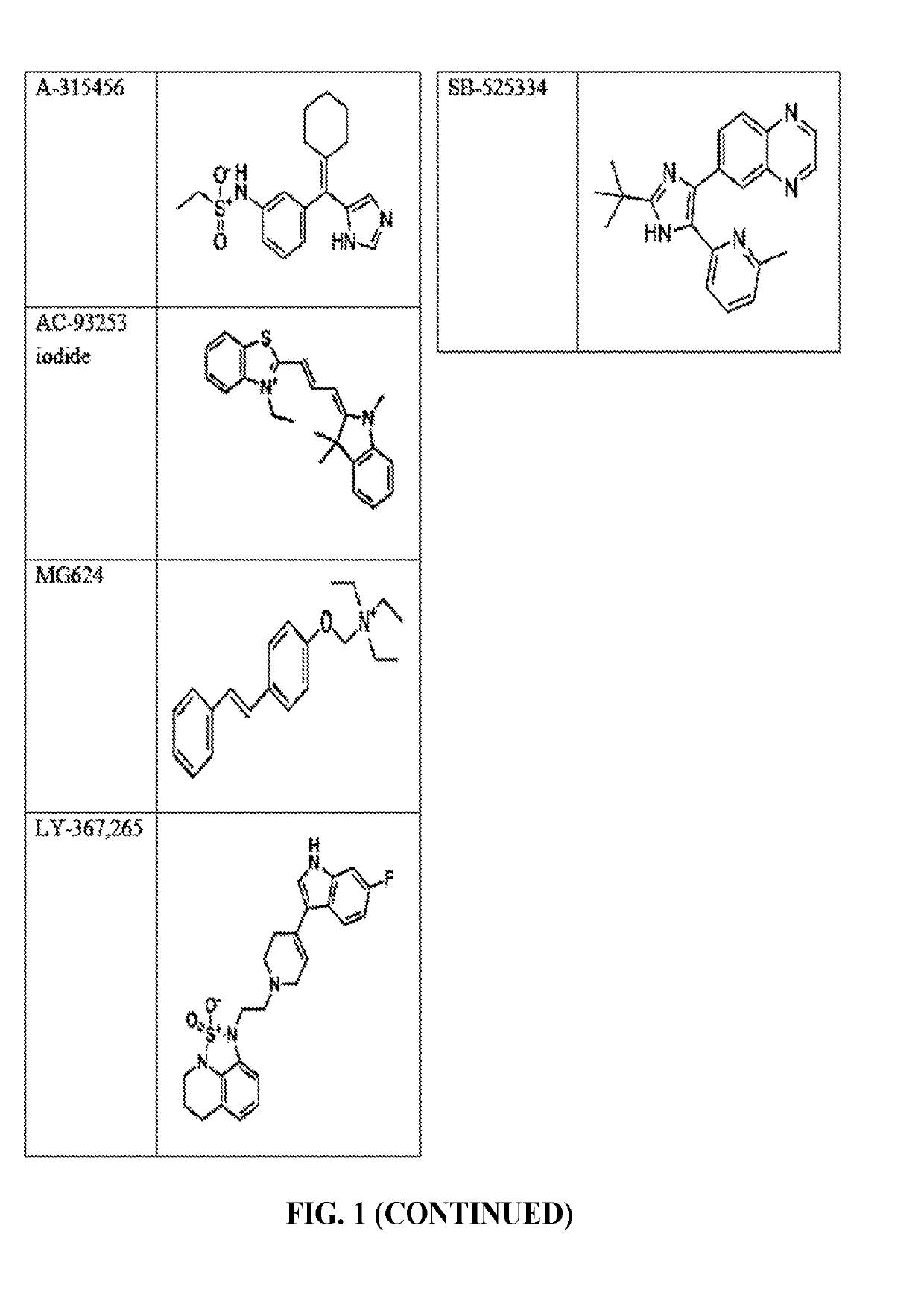
The invention provides a method of blocking transmission of a Plasmodium parasite and a method of treating or preventing malaria comprising administering to an animal an effective amount of a first compound of formula I: wherein A, B, R1, R2, R10, and R11 are described herein, either alone or in combination with a second compound selected from elesclomol, NSC 174938, NVP-AUY922, Maduramicin, Narasin, Alvespimycin, Omacetaxine, Thiram, Zinc pyrithione, Phanquinone, Bortezomib, Salinomycin sodium, Monensin sodium, Dipyrithione, Dicyclopentamethylene-thiuram disulfide, YM155, Withaferin A, Adriamycin, Romidepsin, AZD-1 152-HQPA, CAY10581, Plicamycin, CUDC-101, Auranofin, Trametinib, GSK-458, Afatinib, and Panobinostat.
Owner:UNITED STATES OF AMERICA +1
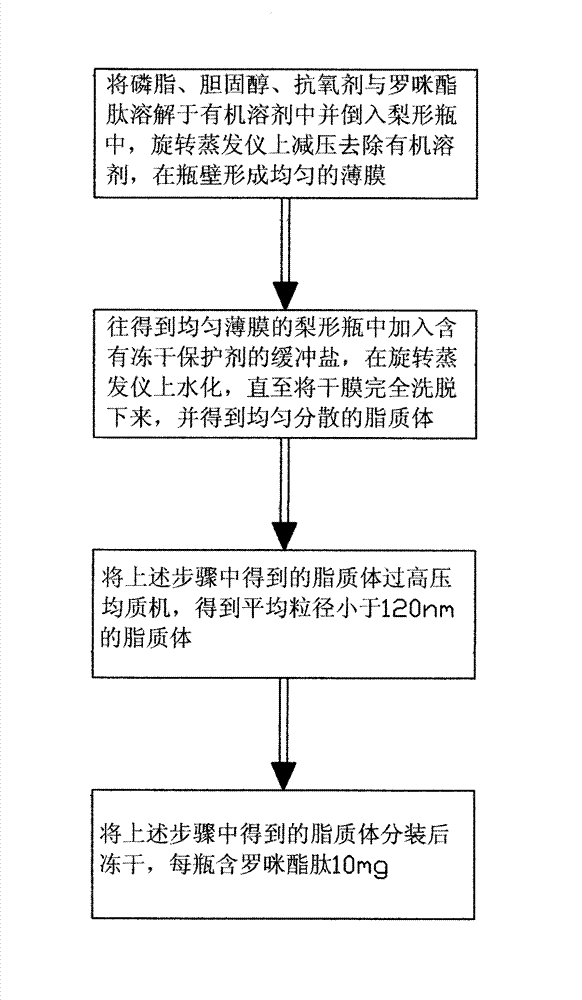
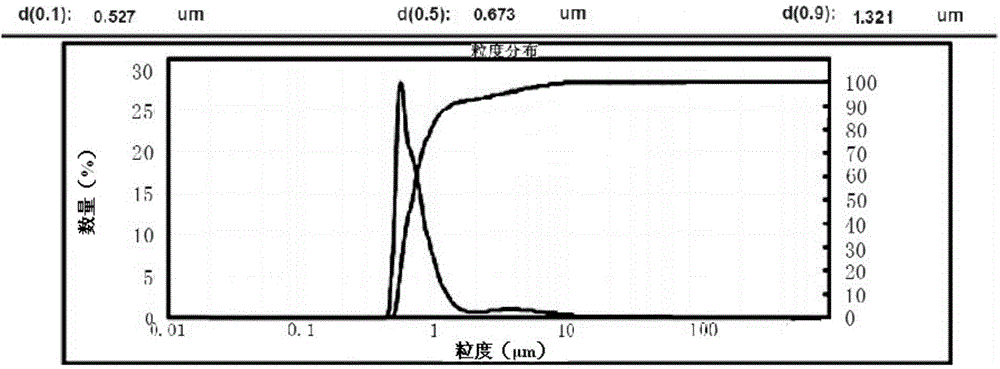
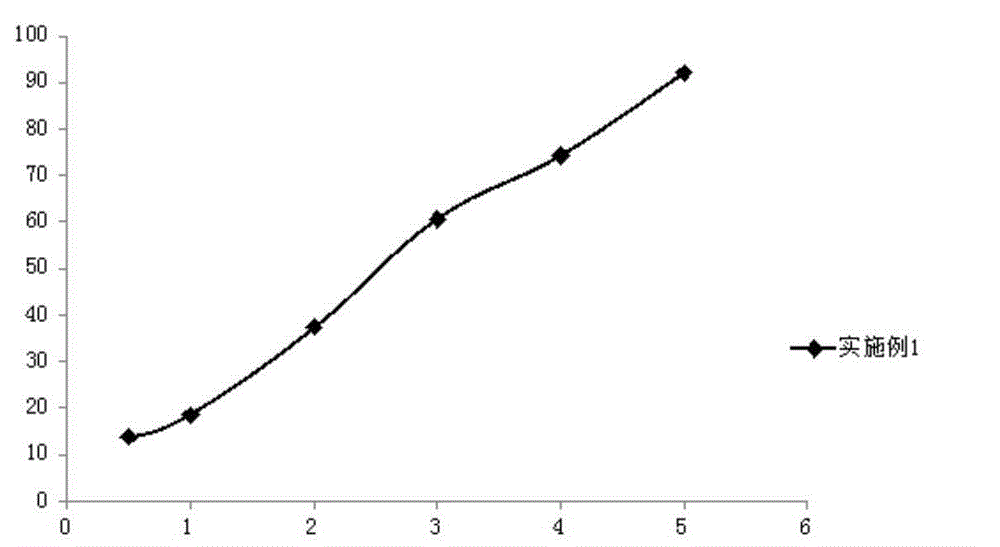
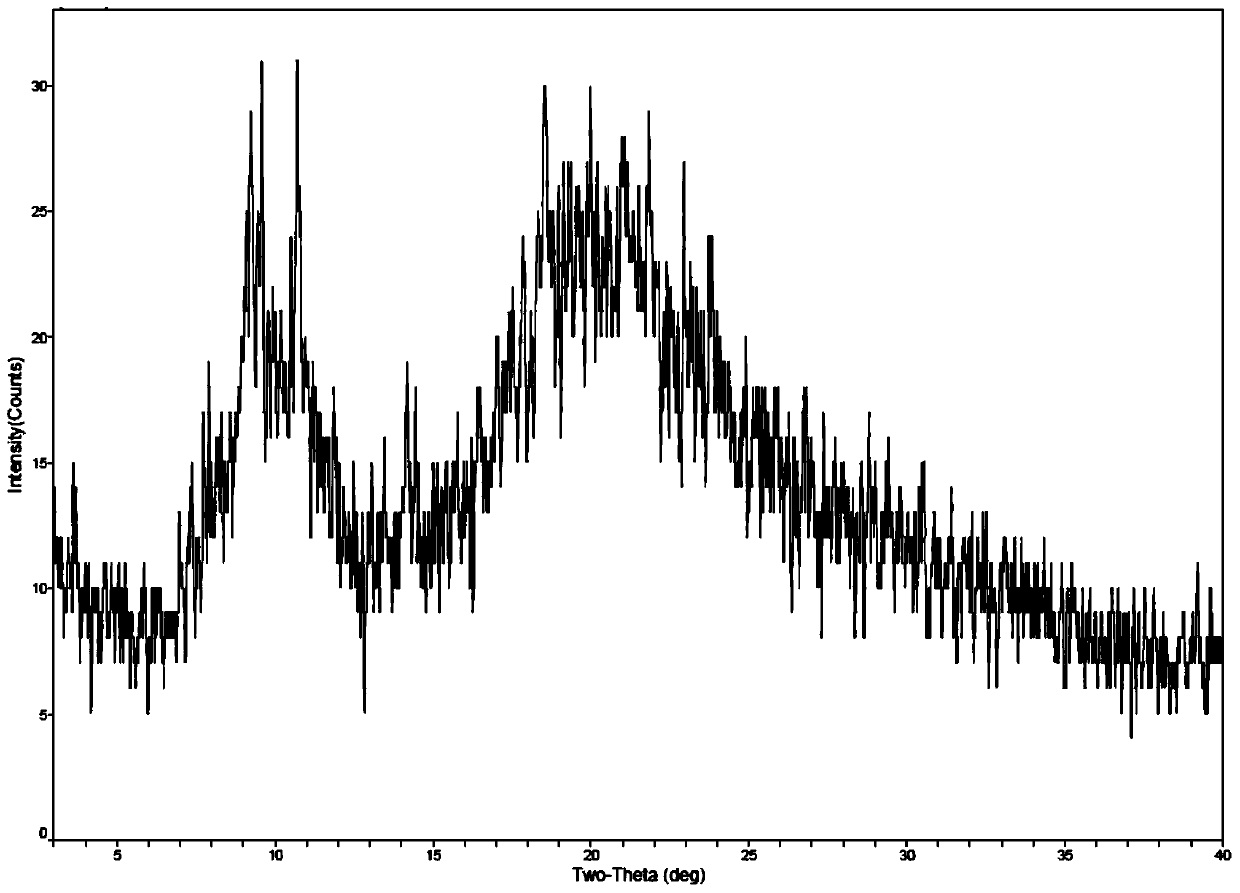
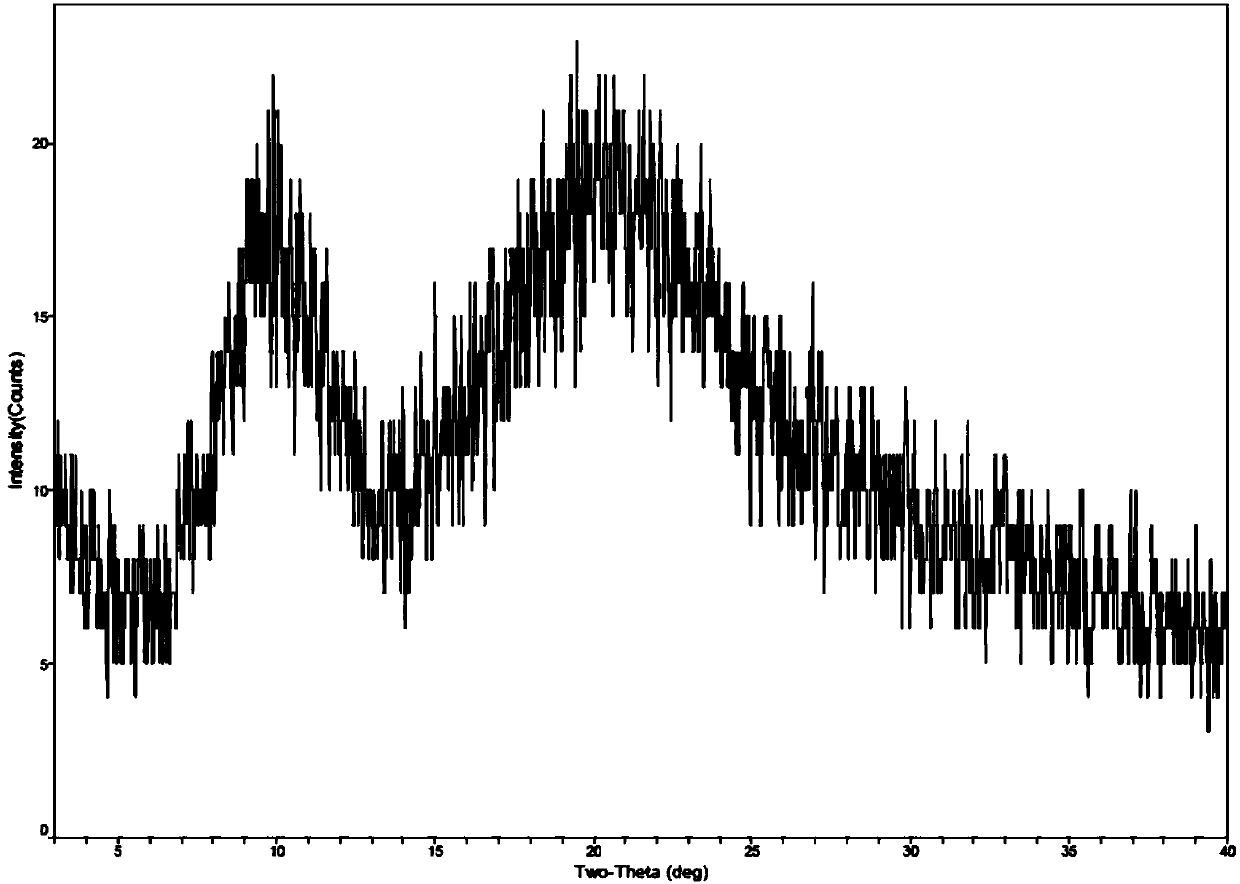
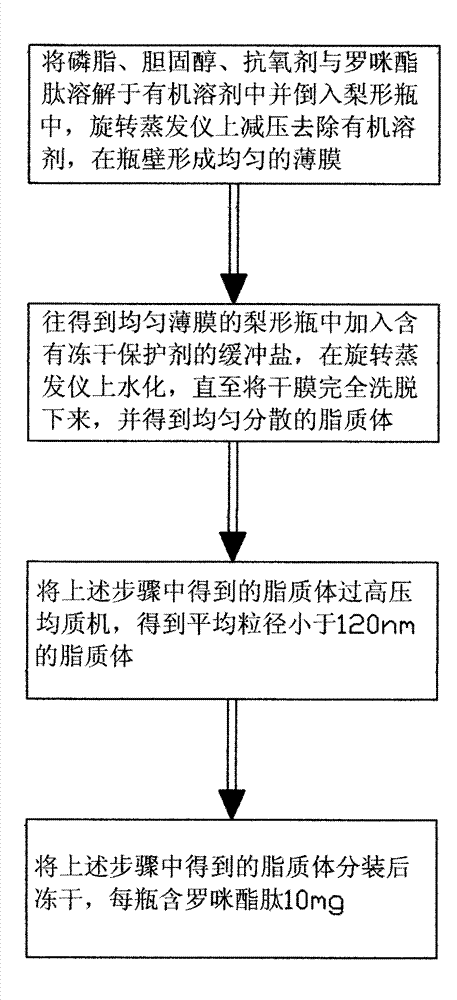
Method for preparing romidepsin lipidosome
ActiveCN103110931AReduce degradationImprove targetingCyclic peptide ingredientsAntineoplastic agentsSide effectAdditive ingredient
The invention discloses a method for preparing romidepsin lipidosome. The romidepsin lipidosome is prepared by using a film dispersion method through regarding cholesterol as a stabilizer, cane sugar as a cryoprotectant and vitamin E as an antioxidant, wherein the particle size of the lipidosome is made to be smaller than 120nm and the encapsulation efficiency is made to be greater than 85% by a high pressure homogenization method at the same time. The romidepsin lipidosome prepared by the method is capable of reducing the degradation of romidepsin, prolonging the administration time, improving the adaptability of patients, improving the targeting of romidepsin, increasing the anti-tumor effect, and reducing the toxic and side effects of drugs as all ingredients in the formula are physically compatible materials with high safety. Moreover, the method for preparing romidepsin lipidosome disclosed by the invention has the advantages of simple process and low cost, and is suitable for industrial production.
Owner:广州迈凯安生物医药研究院有限公司
Romidepsin fat microsphere preparation and preparing method thereof
ActiveCN106137980AImprove targetingLittle side effectsPowder deliveryTetrapeptide ingredientsSide effectMicrosphere
The invention relates to a romidepsin fat microsphere preparation and a preparing method thereof. The romidepsin fat microsphere preparation is prepared from romidepsin, oil for injection, an emulsifying agent, a stabilizing agent, an isoosmotic adjusting agent, a pH modifier and water for injection. The romidepsin fat microsphere preparation has high stability and a good slow-release feature, the targeting ability of romidepsin is improved, and the toxic and side effects of romidepsin are reduced.
Owner:HYBIO PHARMA
Compounds and method for blocking transmission of malarial parasite
Owner:UNITED STATES OF AMERICA +1
Romidepsin production method and fermentation medium thereof
ActiveCN104450837AIncrease productionMicroorganism based processesFermentationInorganic phosphateMANNITOL/SORBITOL
The invention discloses a romidepsin production method and a fermentation medium thereof. The fermentation medium for romidepsin production includes a carbon source, an organic nitrogen source and an inorganic phosphate; the carbon source comprises glucose and mannitol, and the organic nitrogen source comprises corn steep liquor, casein and soybean powder; the carbon source accounts for 4-10% of the weight of the fermentation medium; the organic nitrogen source accounts for 2-5% of the weight of the fermentation medium; and the inorganic phosphate accounts for 0.1-2.1% of the weight of the fermentation medium. The invention also discloses the romidepsin production method. When the fermentation medium is used in the romidepsin production, the output is increased to 500-600mg / L and is above 150-200% higher than the output realized by using fermentation media in the prior art.
Owner:SHANGHAI INST OF PHARMA IND +1
Application of romidepsin in preparation of medicine for preventing and treating novel coronavirus of COVID-19
PendingCN113813366AImprove therapeutic indexLow half effective concentrationAntiviralsDepsipeptide ingredientsDiseasePharmaceutical drug
The invention relates to application of romidepsin in preparation of a medicine for preventing and treating novel coronavirus of COVID-19, and particularly discloses application of romidepsin or pharmaceutically acceptable salts, isotopes, stereoisomers, mixtures of stereoisomers, tautomers, esters, amides or prodrugs of romidepsin in preparation of medicine for preventing and / or treating diseases caused by coronavirus. The coronavirus is a novel coronavirus SARS-Cov-2, SARS-CoV, HCoV 229E, NL63, OC43, HKU1 and MERS-CoV. The half effective concentration of the romidepsin to the novel coronavirus of COVID-19 is 200nM, and the half effective concentration is low, so that the antiviral effect is good.
Owner:SHENZHEN INST OF ADVANCED TECH CHINESE ACAD OF SCI
Method for preparing amorphous-form Romidepsin
ActiveCN105801667AIncrease concentrationImprove developmentTetrapeptide ingredientsPeptide preparation methodsAlcoholFreeze-drying
The invention relates to a method for preparing amorphous-form Romidepsin. The method comprises the steps that Romidepsin is dissolved in an alcoholic solution, water is added, the mixture is stirred to be uniform before being put in a pre-cooled freeze-drying bin, and lastly pre-freezing and drying are conducted to obtain amorphous-form Romidepsin. According to the method, technological operation is simple, cost is low, and prepared amorphous-form Romidepsin is good in stability and quite suitable for industrial production.
Owner:SHANGHAI ARYL PHARMTECH CO LTD +1
A new crystal form of romidepsin and its preparation method and application
ActiveCN104262456BTetrapeptide ingredientsPeptide preparation methodsX-rayDifferential scanning calorimetry
The present invention relates to a novel polymorph form O of romidepsin. Said polymorph can be expressed by an X-ray powder diffraction (XRPD) spectrogram, a differential scanning calorimetry (DSC) spectrogram, and an infrared (IR) absorption spectrogram. At the same time, the present invention also relates to a pharmaceutical composition of the remidepsin polymorph form O, a preparation method for same, and uses thereof.
Owner:ZHEJIANG HISUN PHARMA CO LTD
Process for isolation of romidepsin from fermentation broth and preparation of crystals of romidepsin
The present invention describes a process for isolation of romidepsin from fermentation broth and preparation of crystals of romidepsin. The process of the invention includes fewer purification steps and provides romidepsin having purity of greater than 99.5% area by HPLC. The process of the invention involves simple purification steps and hence, does not require multiple chromatographic purification steps to achieve desired quality of romidepsin. The process is advantageous over reported processes in terms of 99.5% pure yield, fast process, less expensive and less cumbersome as multiple chromatographic purification is not necessary to achieve desired quality. The process for the preparation of crystals of romidepsin provides advantages like simple steps and involves use of single solvent. The process is advantageous in terms of time, cost, and simplicity.
Owner:CONCORD BIOTECH
A kind of method and fermentation medium for producing romidepsin
ActiveCN104450837BIncrease productionMicroorganism based processesFermentationInorganic phosphateMANNITOL/SORBITOL
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
A kind of method for preparing amorphous romidepsin
ActiveCN105801667BWill not precipitateIncrease concentrationTetrapeptide ingredientsPeptide preparation methodsAlcoholFreeze-drying
The invention relates to a method for preparing amorphous-form Romidepsin. The method comprises the steps that Romidepsin is dissolved in an alcoholic solution, water is added, the mixture is stirred to be uniform before being put in a pre-cooled freeze-drying bin, and lastly pre-freezing and drying are conducted to obtain amorphous-form Romidepsin. According to the method, technological operation is simple, cost is low, and prepared amorphous-form Romidepsin is good in stability and quite suitable for industrial production.
Owner:SHANGHAI ARYL PHARMTECH CO LTD +1
The method for preparing romidolipid liposome
ActiveCN103110931BReduce degradationImprove targetingCyclic peptide ingredientsAntineoplastic agentsSucroseSide effect
The invention discloses a method for preparing romidepsin lipidosome. The romidepsin lipidosome is prepared by using a film dispersion method through regarding cholesterol as a stabilizer, cane sugar as a cryoprotectant and vitamin E as an antioxidant, wherein the particle size of the lipidosome is made to be smaller than 120nm and the encapsulation efficiency is made to be greater than 85% by a high pressure homogenization method at the same time. The romidepsin lipidosome prepared by the method is capable of reducing the degradation of romidepsin, prolonging the administration time, improving the adaptability of patients, improving the targeting of romidepsin, increasing the anti-tumor effect, and reducing the toxic and side effects of drugs as all ingredients in the formula are physically compatible materials with high safety. Moreover, the method for preparing romidepsin lipidosome disclosed by the invention has the advantages of simple process and low cost, and is suitable for industrial production.
Owner:广州迈凯安生物医药研究院有限公司
A process for isolation pf romidepsin from fermentation broth and preparation of crystals of romidepsin
The present invention describes a process for isolation of romidepsin from fermentation broth and preparation of crystals of romidepsin. The process of the invention includes fewer purification steps and provides romidepsin having purity of greater than 99.5% area by HPLC. The process of the invention involves simple purification steps and hence, does not require multiple chromatographic purification steps to achieve desired quality of romidepsin. The process is advantageous over reported processes in terms of 99.5% pure yield, fast process, less expensive and less cumbersome as multiple chromatographic purification is not necessary to achieve desired quality. The process for the preparation of crystals of romidepsin provides advantages like simple steps and involves use of single solvent. The process is advantageous in terms of time, cost, and simplicity.
Owner:CONCORD BIOTECH
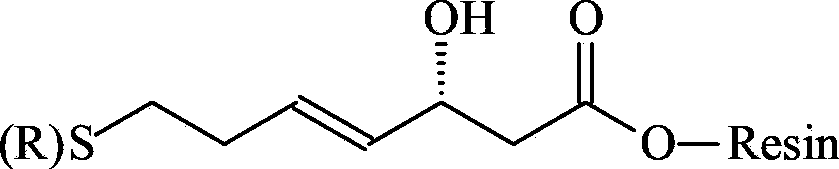
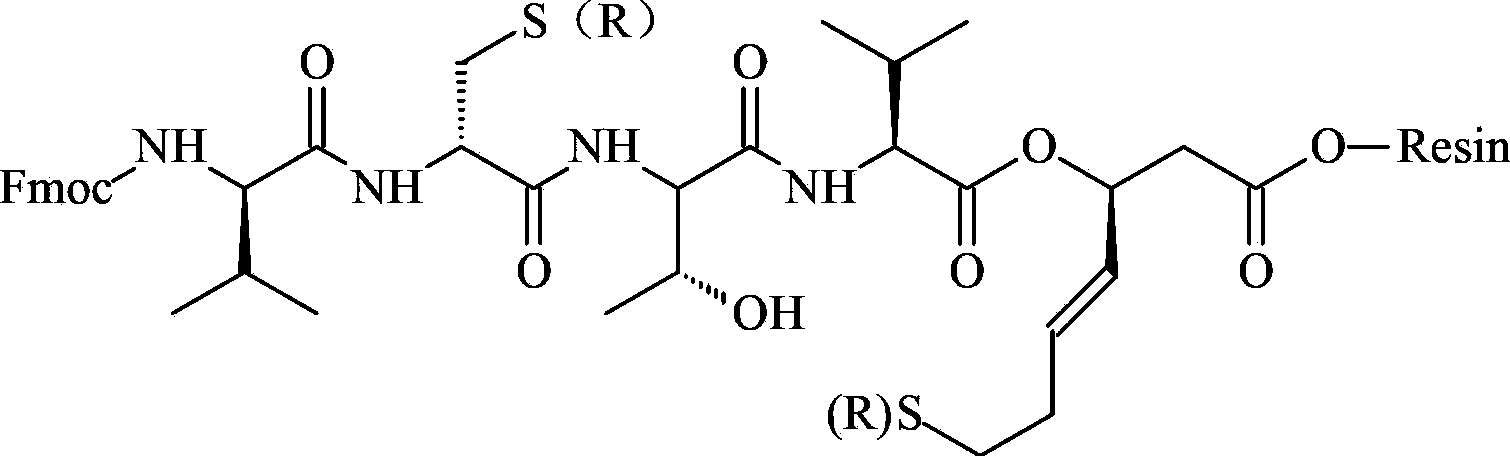
A kind of synthetic method of romidepsin
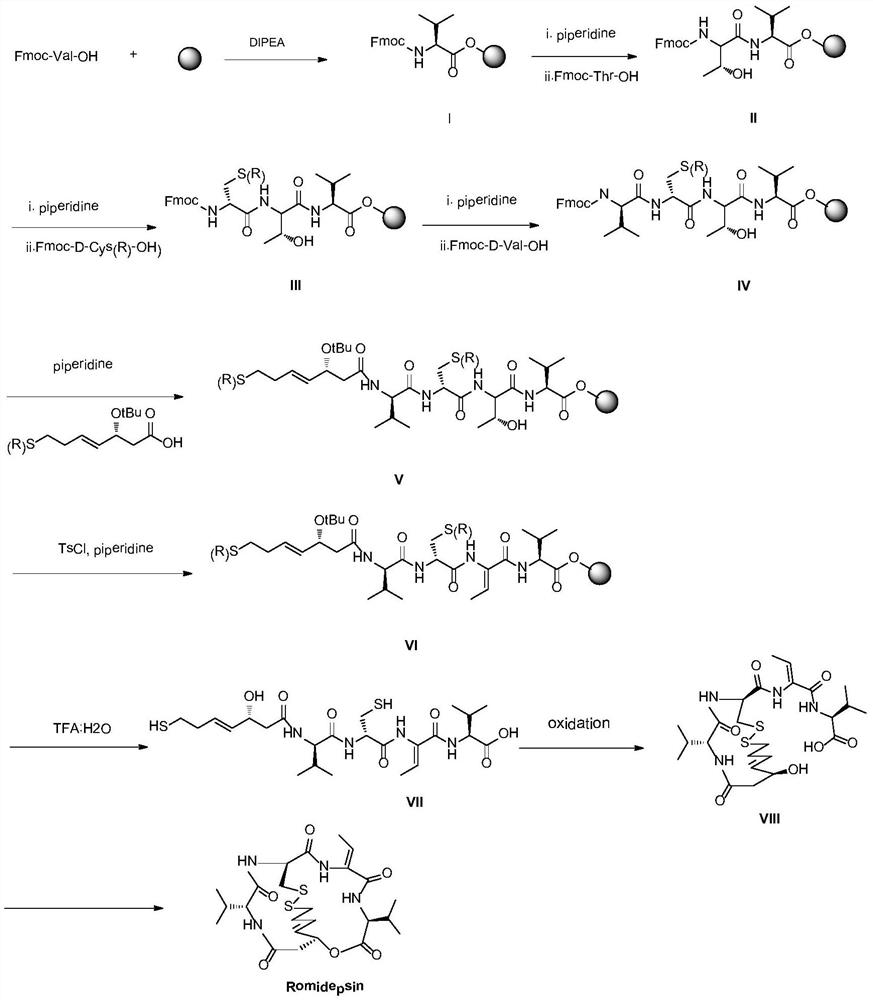
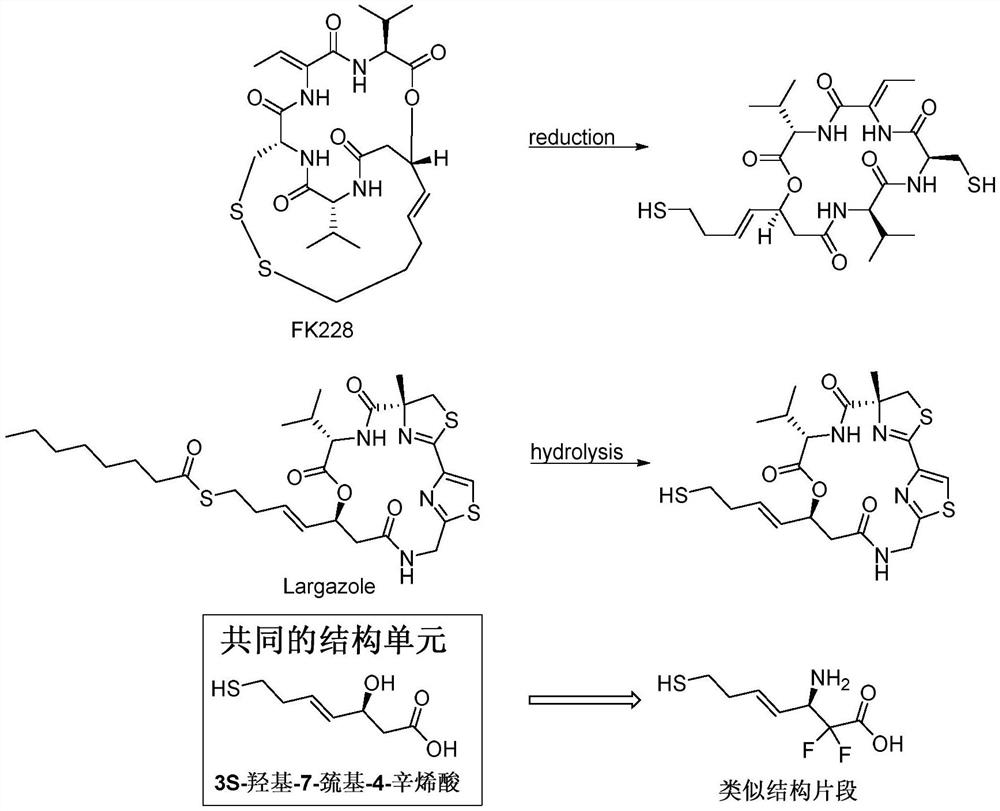
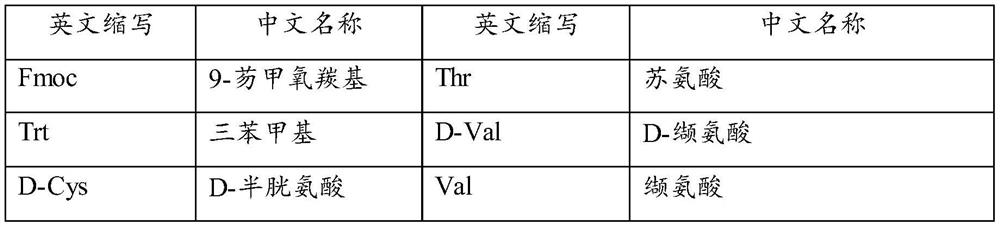
ActiveCN111333697BHigh yieldThe method steps are simplePeptide preparation methodsBulk chemical productionDisulfide bondingSide chain
The invention provides a synthetic method of romidepsin, which comprises the following steps: 1) under the action of an activator, solid-phase synthetic resin is coupled with Fmoc-L-Val-OH to obtain intermediate I; 2) According to the Fmoc solid-phase synthesis strategy, Fmoc‑L‑Thr‑OH, Fmoc‑D‑Cys(R)‑OH, Fmoc‑D‑Val‑OH, (R)‑3‑tert-butoxy‑7‑ ‑Mercapto‑4‑heptenoic acid is subjected to polypeptide chain extension coupling to obtain intermediate V; 3) intermediate V removes the hydroxyl group on the side chain of the L‑Thr residue to form a double bond to obtain intermediate VI; 4) intermediate Cleavage of VI to remove resin and side chain protecting groups to obtain intermediate VII; 5) intermediate VII is oxidized to form an intramolecular disulfide bond to obtain intermediate VIII; 6) intermediate VIII is obtained through intramolecular esterification pungent.
Owner:GANSU CHANGEE BIO PHARMA
A kind of romidepsin acetate crystal form and preparation method thereof
ActiveCN109796521BImprove solubilityImprove stabilityPeptide preparation methodsTumor therapyOrganic chemistry
The invention discloses a crystal form of romidepsin acetate and a preparation method thereof. The romidepsin acetate crystal form of the present invention improves the solubility and stability of romidepsin, can expand the application of romidepsin in the field of tumor treatment and HIV treatment, and can also provide more romidepsin The development of octyl acetate dosage forms provides a research basis.
Owner:SHANGHAI INST OF PHARMA IND CO LTD +1
Preparation method for synthesizing romidepsin dipolymer romipeptide A
The invention relates to a preparation method for synthesizing a romidepsin dipolymer romipeptide A. According to the preparation method, romidepsin is used as a precursor, the romipeptide A is synthesized in one step, the process is simple, and a large amount of romipeptide A can be prepared.
Owner:TAIZHOU VOCATIONAL COLLEGE OF SCI & TECH
A kind of romidepsin lipid microsphere preparation and preparation method thereof
ActiveCN106137980BImprove targetingLittle side effectsPowder deliveryTetrapeptide ingredientsMicrosphereBiology
The application relates to a romidepsin lipid microsphere preparation and a preparation method thereof. It may contain romidepsin, oil for injection, emulsifier, stabilizer, isotonicity regulator, pH regulator and water for injection. The romidepsin lipid microsphere preparation of the invention has good stability and slow-release characteristics, improves the targeting of romidepsin, and reduces its toxic and side effects.
Owner:HYBIO PHARMA
A kind of preparation method of romidepsin solution
ActiveCN103877010BImprove solubilityEasy to getPowder deliveryPharmaceutical non-active ingredientsPresent methodFreeze-drying
The present invention relates to a preparation method of romidepsin solution, which mainly includes the following three steps: 1. preparing an aqueous ethanol solution containing povidone; 2. adjusting the pH of the solution obtained in step 1; 3. controlling the temperature of the solution and maintaining the pH of the solution, add romidepsin until completely dissolved to obtain the solution, the concentration of romidepsin is 0.5-6mg / ml. And the application of the romidepsin solution prepared by the method in the preparation of freeze-dried powder injection.
Owner:CHIA TAI TIANQING PHARMA GRP CO LTD
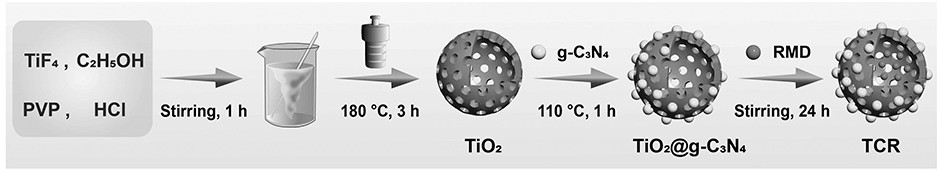
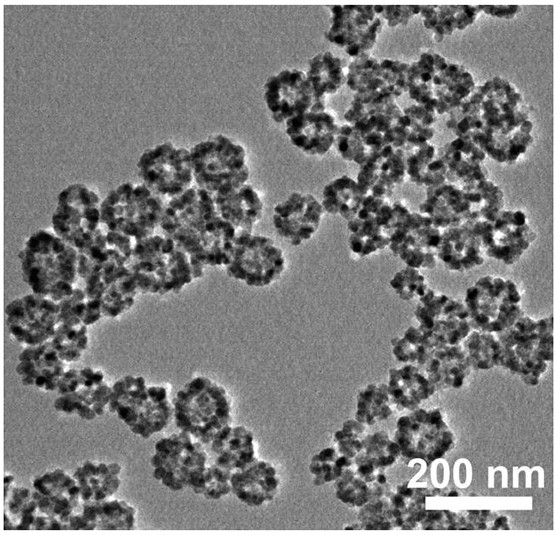
Preparation of romidepsin-loaded heterojunction nanoparticles with sonodynamic effect
PendingCN114652686AGood dispersionGood biocompatibilitySonopheresisPowder deliveryTumor therapyBiocompatibility
The invention discloses preparation of a romidepsin-loaded heterojunction nanoparticle with a sonodynamic effect, which is a novel nanoparticle material taking pharmaceutical chemistry as a synthesis basis and is used for tumor treatment by utilizing the specific excellent properties of the romidepsin-loaded heterojunction nanoparticle. The preparation method specifically comprises the following steps: (1) preparing mesoporous titanium dioxide; (2) preparation of carbon nitride quantum dots (g-C3N4 QD) and composite preparation of the carbon nitride quantum dots and a titanium dioxide material; (3) loading an endogenous response small molecule drug romidepsin, so as to prepare a romidepsin-loaded heterojunction nano particle with a sonodynamic effect; and (4) verifying the successful synthesis of the nanoparticles and the synergistic treatment effect of sonodynamic and chemotherapy through experiments. The finally obtained nanoparticle complex has good dispersibility, biocompatibility and good tumor treatment effect, and has great application potential in the field of biomedicine and other fields of social economy.
Owner:SOUTHWEST UNIVERSITY
A kind of preparation method of romidepsin
InactiveCN103897029BEasy to operateShort synthesis cyclePeptide preparation methodsTetrapeptidesCouplingMedicinal chemistry
Disclosed is a method for preparing Romidepsin. The present invention relates to the pharmaceutical synthesis. The present invention is based on a method of solid phase synthesis. First, coupling is performed between resin and carboxyl groups on 3-hydroxy-7-mercapto-4-heptenoic acid; then,coupling is performed in sequence between four pieces of amino acid on Romidepsin; then, hydroxyl groups are removed, and disulfide bonds and amido bonds are obtained by means of cyclization, so as to form Romidepsin. The purity the finally finished product is greater than 99%, the total yield is higher than 30%, and method features simple preparation, a short synthesis cycle and low cost, and helps to produce Romidepsin in a large scale.
Owner:HYBIO PHARMA
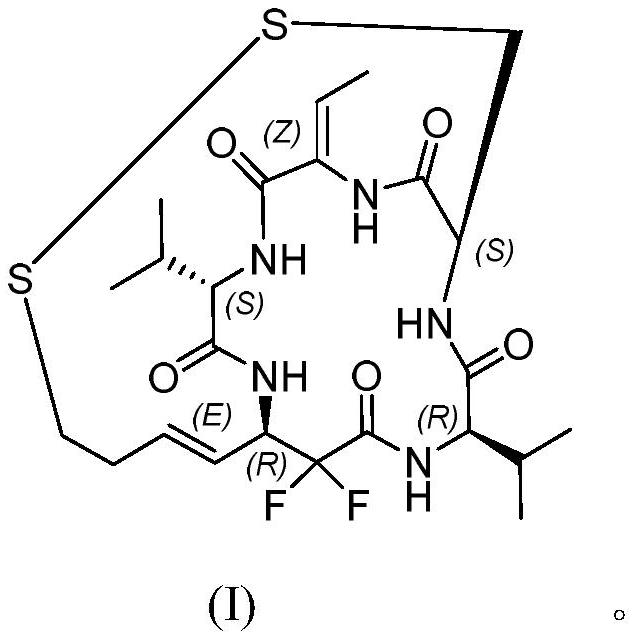
Difluoro-substituted romidepsin analogue as well as preparation method and application thereof
PendingCN114380888APrevent proliferationTetrapeptide ingredientsPeptide preparation methodsDisulfide bondingCarboxyl radical
The invention belongs to the technical field of chemical pharmacy, and relates to a difluoro-substituted romidepsin analogue, a preparation method thereof and application of the analogue as an antitumor agent.According to the difluoro-substituted romidepsin analogue, a compound 1 reacts with optically pure tert-butyl sulfinamide to obtain a compound 2, and an organic zinc reagent reacts with the compound 2 chemically to generate a compound 3; carrying out ester hydrolysis to obtain a compound 4; reacting the compound 4 with an intermediate to obtain a compound 5; performing deprotection reaction on the compound 5 to obtain a compound 6 containing free amino groups; carrying out ester hydrolysis on the compound 6 to obtain a compound 7 containing free carboxyl; carrying out intramolecular condensation reaction on the compound 7 and an intermediate to prepare a compound 8; the compound 8 is subjected to a protection group removal reaction and intramolecular disulfide bond generation, and a compound 9 is prepared. The compound disclosed by the invention has a strong effect of inhibiting various tumor cells, and particularly can inhibit cell proliferation of gastric cancer, bladder cancer, colon cancer, liver cancer, lymph cancer or breast cancer, so that the compound has potential anti-tumor application.
Owner:FUDAN UNIV +1
A method for synthesizing romidepsin
ActiveCN107778350BHigh purityHigh yieldPeptide preparation methodsDisulfide bondingCombinatorial chemistry
Owner:CHENGDU SHENGNUO BIOPHARM
Compounds and method for blocking transmission of malarial parasite
ActiveUS20190315740A1Avoid spreadingOrganic chemistryAntiparasitic agentsSodium PyrithioneMalarial parasite
Disclosed are compounds of formula (I) and formula (II): (I) (II) wherein R1, R2, A, and B are as defined herein. Also disclosed is a method of blocking transmission of a Plasmodium parasite and a method of treating or preventing malaria comprising administering to an animal an effective amount of a first compound of formula (I) or (II) either alone or in combination with a second compound selected from elesclomol, NSC174938, NVP-AUY922, Maduramicin, Narasin, Alvespimycin, Omacetaxine, Thiram, Zinc pyrithione, Phanquinone, Bortezomib, Salinomycin sodium, Monensin sodium, Dipyrithione, Dicyclopentamethylene-thiuram disulfide, YM155, Withaferin A, Adriamycin, Romidepsin, AZD-1152-HQPA, CAY10581, Plicamycin, CUDC-101, Auranofin, Trametinib, GSK-458, Afatinib, and Panobinostat.
Owner:US DEPT OF HEALTH & HUMAN SERVICES +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com