A new crystal form of romidepsin and its preparation method and application
A technology of romidepsin and crystal form, applied in the field of pharmacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
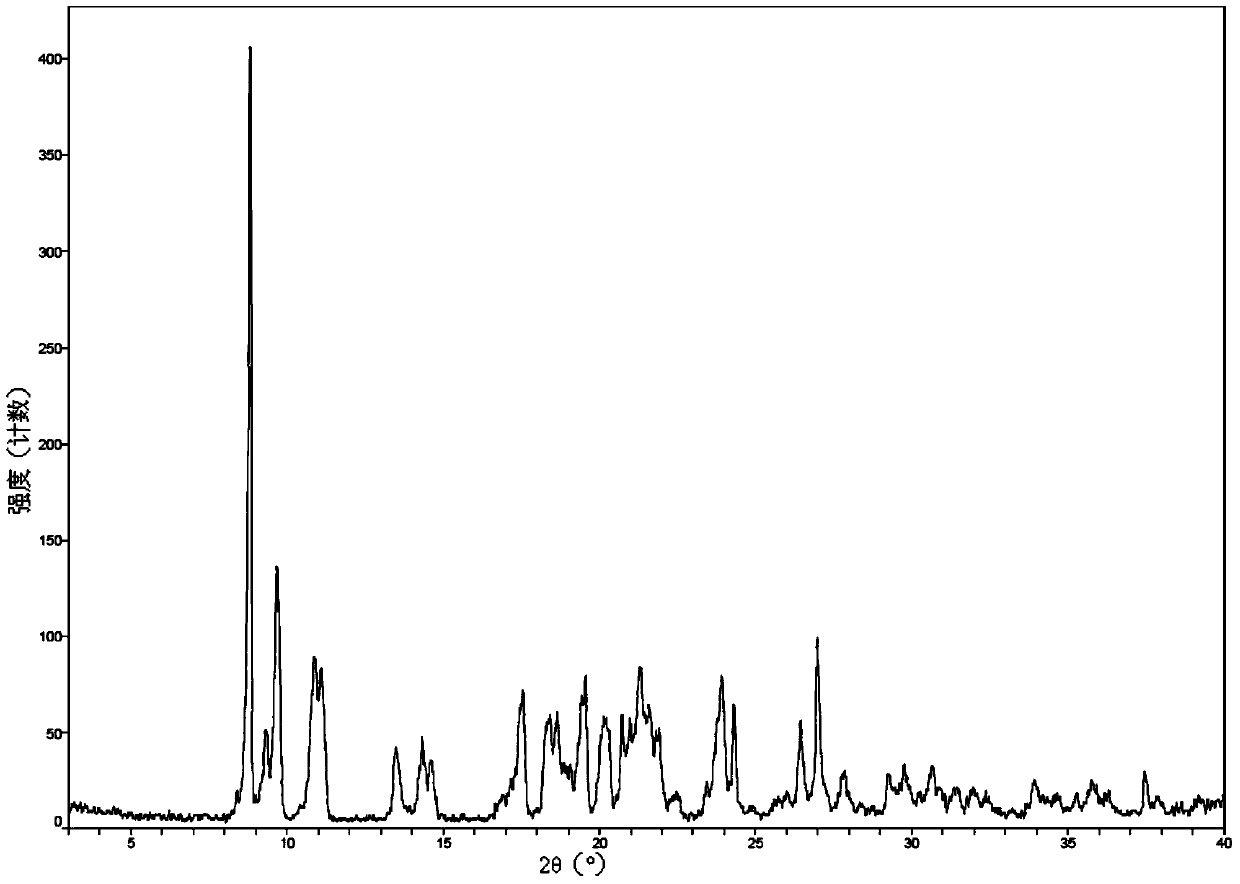
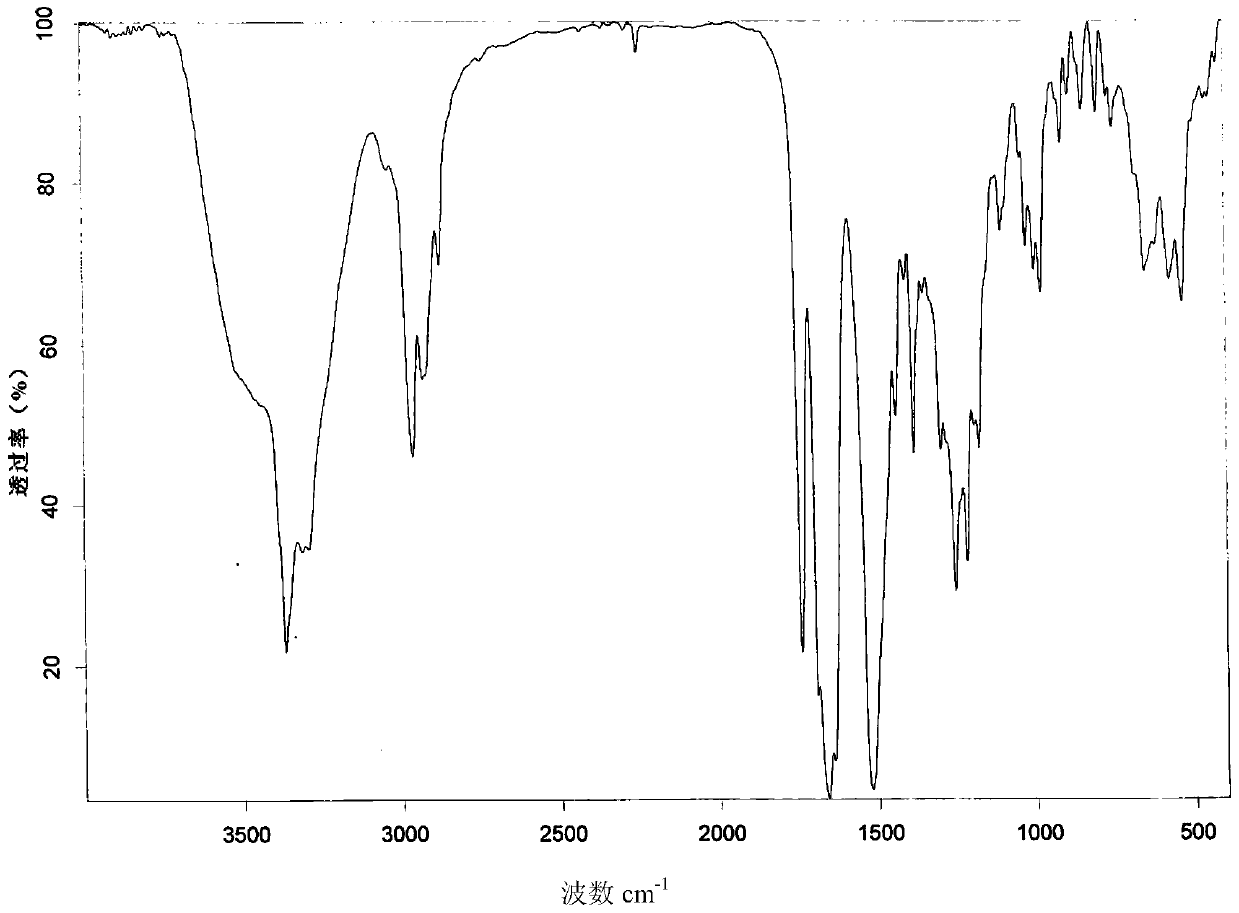
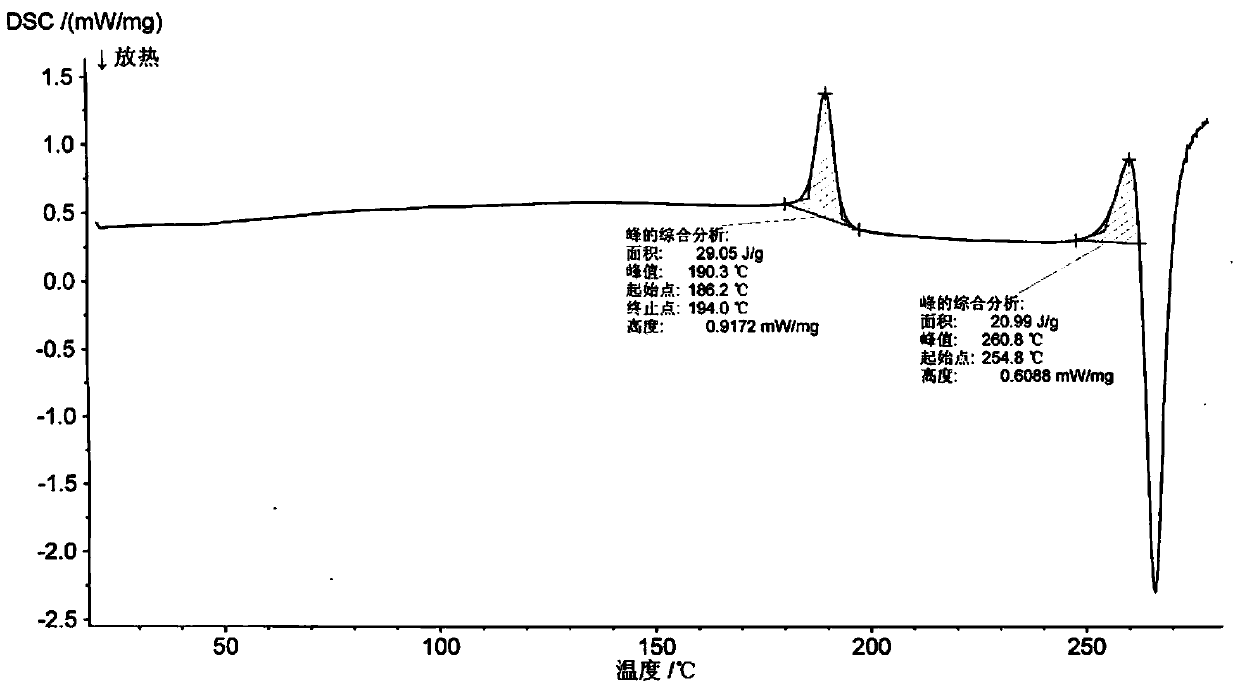
[0045] 2.0 g romidepsin (HPLC purity greater than 99%) was dissolved in 5 ml of chloroform:methanol=9 / 1 (volume ratio) mixed solvent to form a saturated solution (some crystals were not completely dissolved), and the filtrate was obtained by filtration. Take 3ml of the filtrate, then add 27ml of acetonitrile to the filtrate, mix well and place in a refrigerator at 4°C in the dark, after 72h, filter to obtain crystals, and dry the crystals in vacuum at 45°C for 48h to obtain romidepsin crystal form O. Get sample detection, its X-ray powder diffraction pattern is as follows figure 1 As shown, the infrared absorption spectrum is shown as figure 2 As shown, the DSC spectrum is as image 3 As shown, the TGA spectrum is as Figure 4 shown.
Embodiment 2
[0047] 10.0 g of romidepsin (HPLC purity greater than 99%) was dissolved in 25 ml of chloroform:methanol=9 / 1 (volume ratio) mixed solvent to form a saturated solution (with crystals not completely dissolved), and filtered to obtain the filtrate. Take 11 parts of 1ml filtrate, then add 1ml, 2ml, 3ml, 4ml, 5ml, 6ml, 7ml, 8ml, 9ml of acetonitrile to the filtrate respectively, mix well and place in a refrigerator at 4°C in the dark, and filter to obtain crystals after 48 hours , the crystals were vacuum-dried at 45°C for 48 hours. After testing, the crystal forms obtained in each solvent system are shown in the table below.
[0048] Acetonitrile (m1)
Embodiment 3
[0050] 1.0g romidepsin (HPLC purity greater than 99%) was dissolved in 2.5ml methylene chloride: in the mixed solvent of ethanol=9 / 1 (volume ratio), then added 35ml acetonitrile, stirred overnight at room temperature, filtered, 45 ℃ vacuum After drying for 48 hours, 0.92 g of solid was obtained with a yield of 92%. After detection, it was confirmed that romidepsin crystal form O was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com