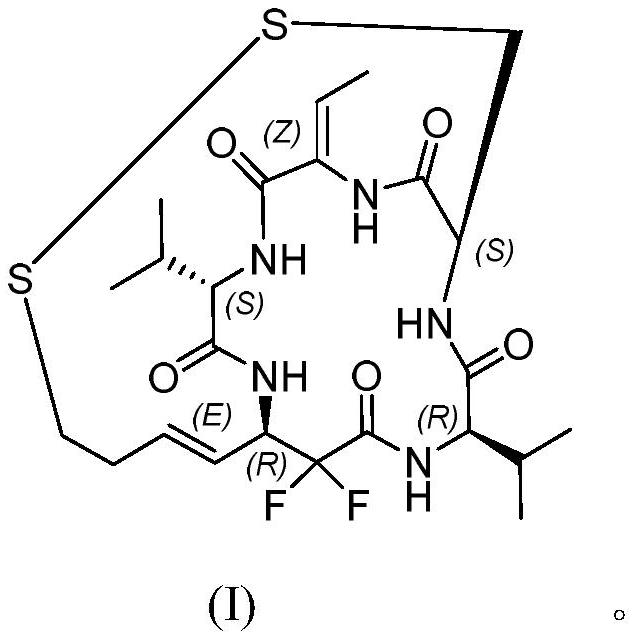
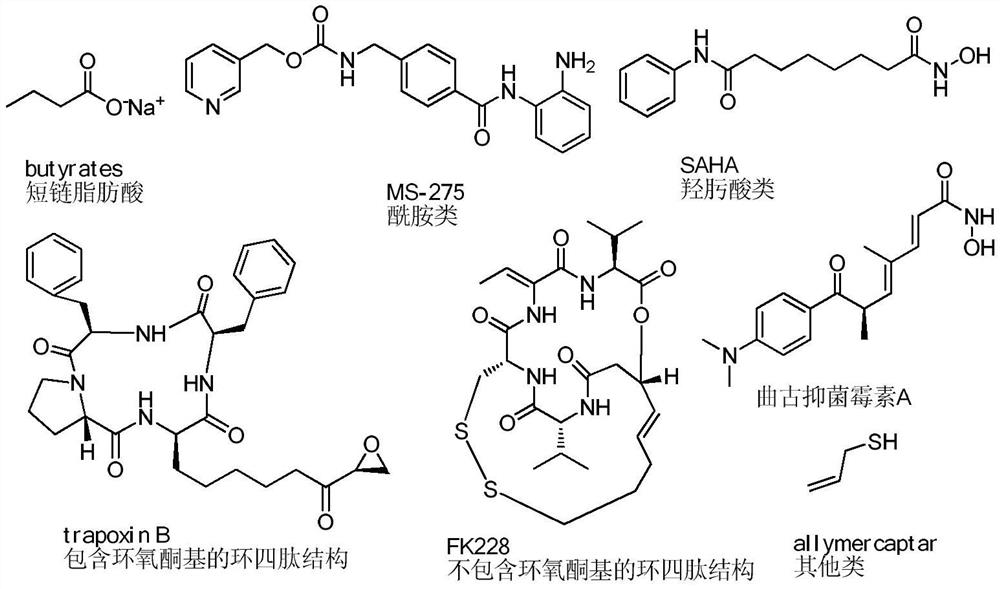
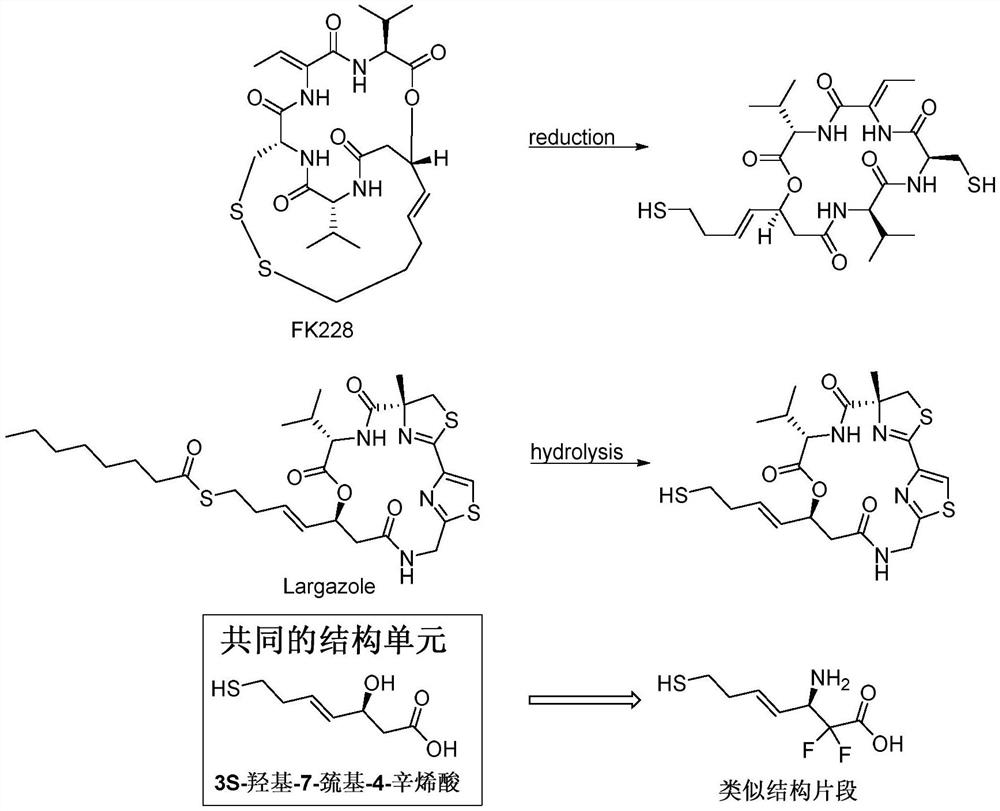
Difluoro-substituted romidepsin analogue as well as preparation method and application thereof
A technology of romidepsin and analogues, applied in the field of chemical pharmaceuticals, can solve problems such as lack of high selectivity or specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] Synthesis of Romidepsin Difluoro Substituted Analog 10
[0079] Step a:
[0080]
[0081] Take a clean and dry 100mL eggplant-shaped reaction bottle, protect it with argon, add raw materials aldehyde 1 (2.59g, 7.23mmol), R-sulfinamide (1.23g, 10.1mmol) and KHSO 4 (1.18g, 8.7mmol), dissolved in 40mL of anhydrous toluene, reacted at 45°C for 1h, then stopped the reaction, filtered off KHSO 4, concentrated, and purified by silica gel column chromatography (elution condition: PE / EA=10:1) to obtain 2.82 g of a colorless viscous solid with a yield of 85%.R f =0.2(PE / EA=10:1) -159.9 (c=1.9CHCl3). 1 H NMR (400MHz, CDCl 3 )δ8.11–8.09(m,1H),7.48–7.09(m,15H),6.47–6.15(m,2H),2.35–2.20(m,4H),1.19(s,9H). 13 C NMR (151MHz, CDCl 3 )δ163.81,148.63,144.65,129.53,127.92,126.71,66.90,61.17,57.24,48.53,32.05,30.40,28.12,24.72,22.43.ESI-MS(m / z):484.2[M+Na] + .HRMS-ESI(m / z):[M+Na] + Calcd. For C 28 h 31 NOS 2 Na:484.1739,found:484.1744;
[0082] Step b:
[0083]
[0084] T...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com