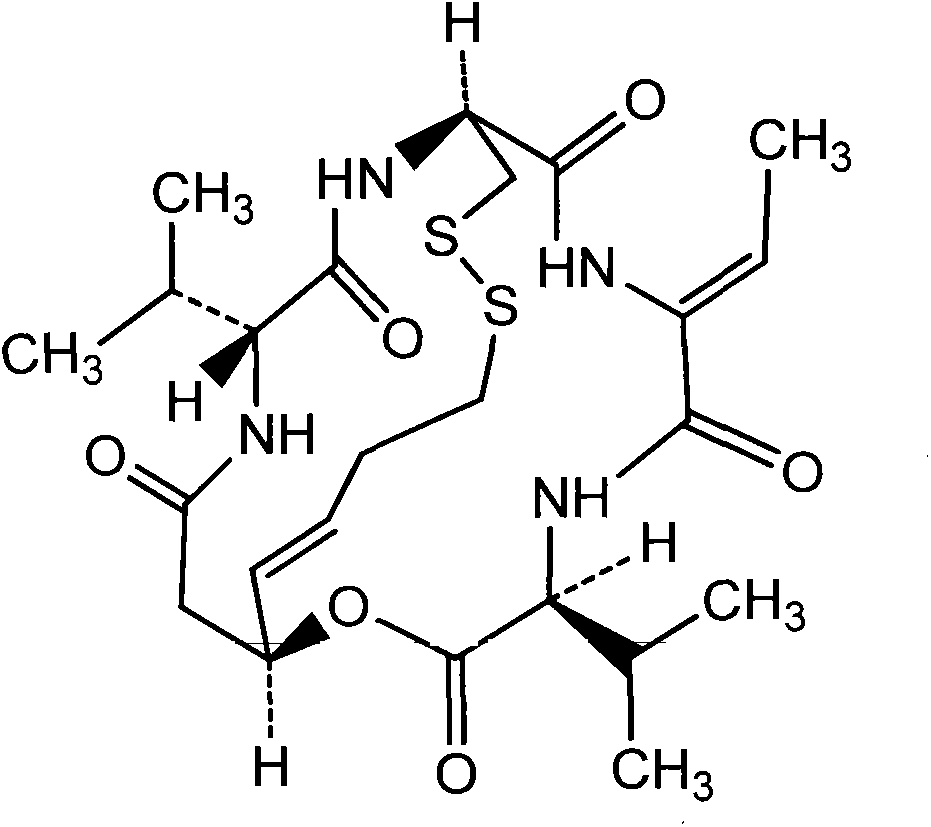
Preparation method of Romidepsin solution
A technology of romidepsin and preparation method, which is applied in the field of medicine, can solve problems such as limitations, and achieve the effects of stable and reliable quality, stable and reliable solution quality, and low irritation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1 Solubility investigation
[0029] Weigh about 10 mg of romidepsin, accurately weighed, add 10 ml of solvent, mix, airtight, ultrasonic for 30 minutes, observe under the light inspection box, if it is completely dissolved, continue to add about 10 mg of romidepsin (accurately weighed), mix, Airtight, ultrasonic (the temperature of the system gradually rises to about 45-55°C), and observe under the light inspection box. Repeat the operation until it cannot be dissolved completely. The test results are shown in Table 1 and Table 2.
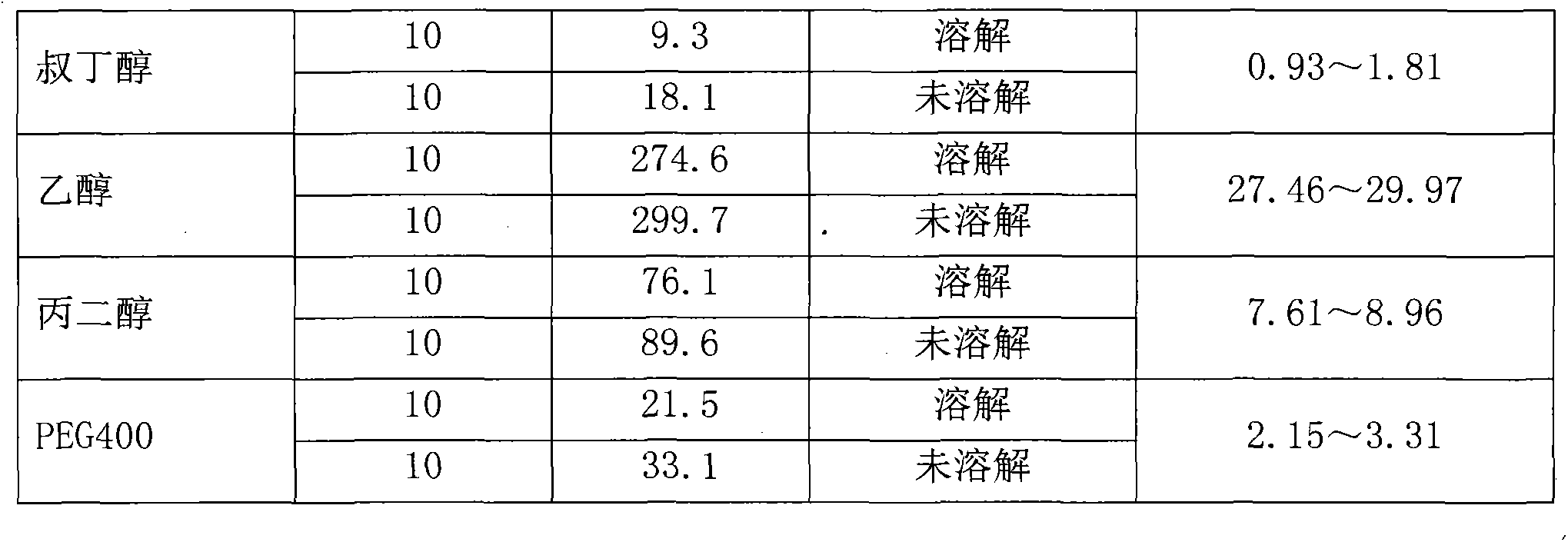
[0030] The solubility of table 1 romidepsin in a single solvent
[0031]
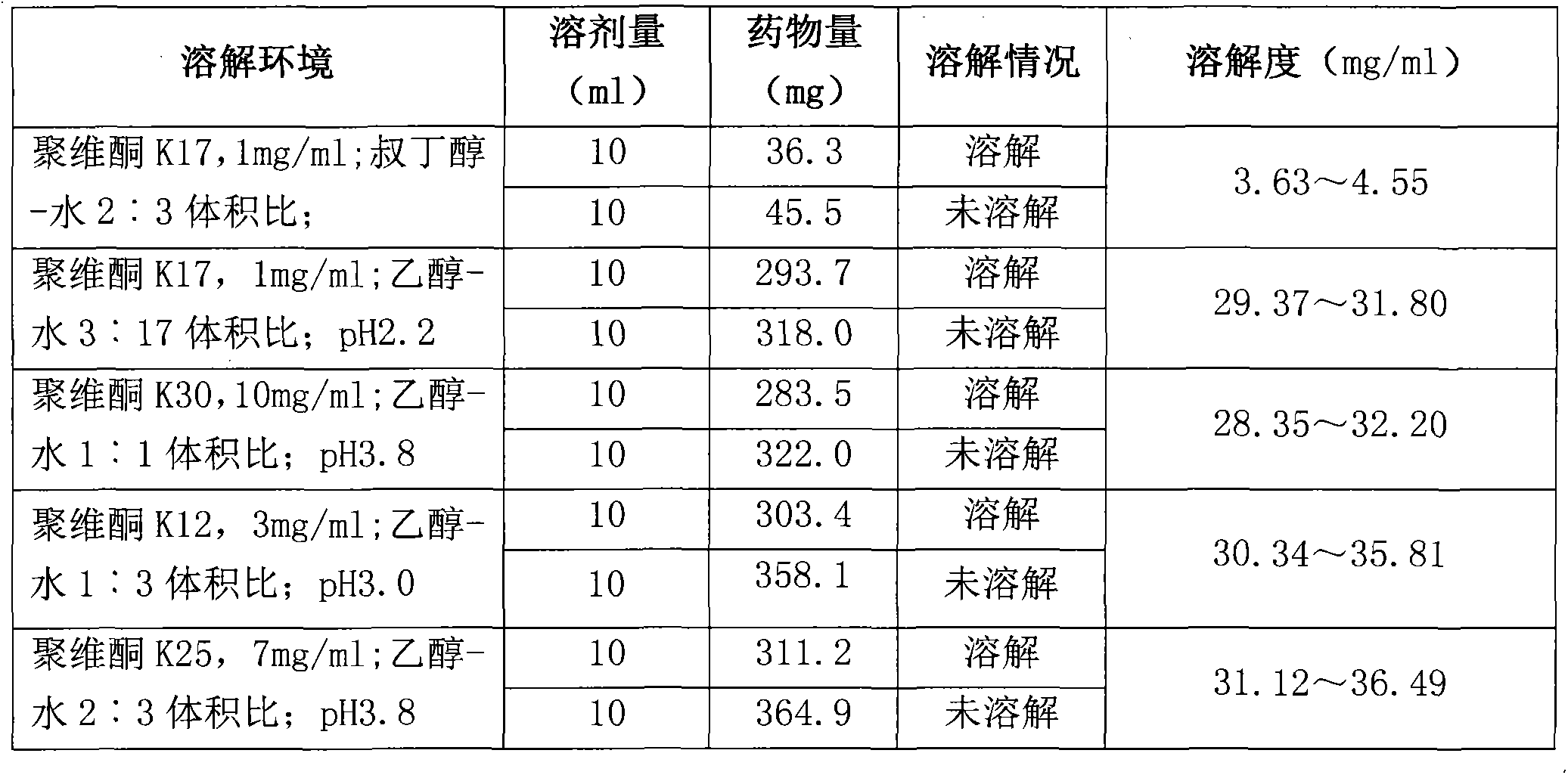
[0032] The solubility of table 2 romidepsin in mixed solvents
[0033]
[0034] Test results: In a single solvent, romidepsin has poor solubility in PEG400 and tert-butanol, better solubility in propylene glycol and ethanol, and the best solubility in ethanol; It has the best solubility in ketone-ethanol water mixed solution. Moreover, tert-butan...
Embodiment 2
[0035] Embodiment 2 prepares different concentration romidepsin solutions
[0036] Solution I
[0037] 1. Prepare an aqueous ethanol solution containing povidone K17, wherein the ethanol concentration is 15% (volume ratio), and the concentration of povidone is 1 mg / ml;
[0038] 2. Adjust the pH of the solution obtained in step 1 to 2.2;
[0039] 3. Control the solution temperature to 45°C, maintain the pH of the solution to 2.2, add romidepsin until it is completely dissolved to obtain a clear and transparent solution, and the concentration of romidepsin is 0.5 mg / ml.
[0040] Solution II
[0041] 1. Prepare an aqueous ethanol solution containing povidone K30, wherein the concentration of ethanol is 50% (volume ratio), and the concentration of povidone is 10 mg / ml;
[0042] 2. Adjust the pH of the solution obtained in step 1 to 3.8;
[0043] 3. Control the temperature of the solution at 60°C, maintain the pH of the solution to 3.8, add romidepsin until it is completely dis...
Embodiment 3
[0052] Embodiment 3 long-term stability investigation
[0053] Take the romidepsin solutions of various concentrations prepared in Example 2, fine filter and terminal filter with a 0.2um microporous membrane, fill, half-tamp, and freeze-dry. The long-term stability of each freeze-dried powder was investigated. Packaging: 10ml neutral borosilicate glass control injection bottle; inspection conditions: 25±2°C, RH 60%±10%. The content detection of romidepsin was carried out according to the method reported in the reference literature. Xiaohong Chen, Erin R. Gardner, and William D. Figg, Determination of the cyclic depsipeptide FK228 in human and mouse plasma byliquid chromatography with mass-spectrometric detection. J Chromatogr B AnalytTechnol Biomed Life Sci.
[0054] Table 3 Long-term stability of solution I freeze-dried powder
[0055]
[0056] Table 4 Solution II freeze-dried powder long-term stability
[0057]
[0058] Table 5 Solution III freeze-dried powder long...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com