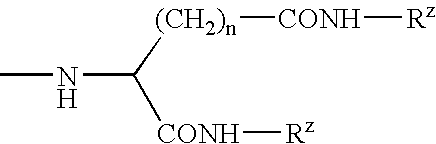
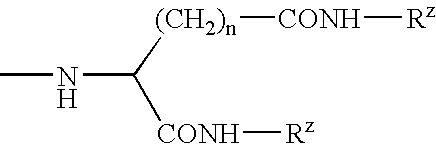
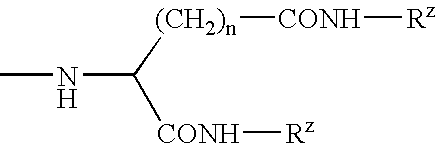
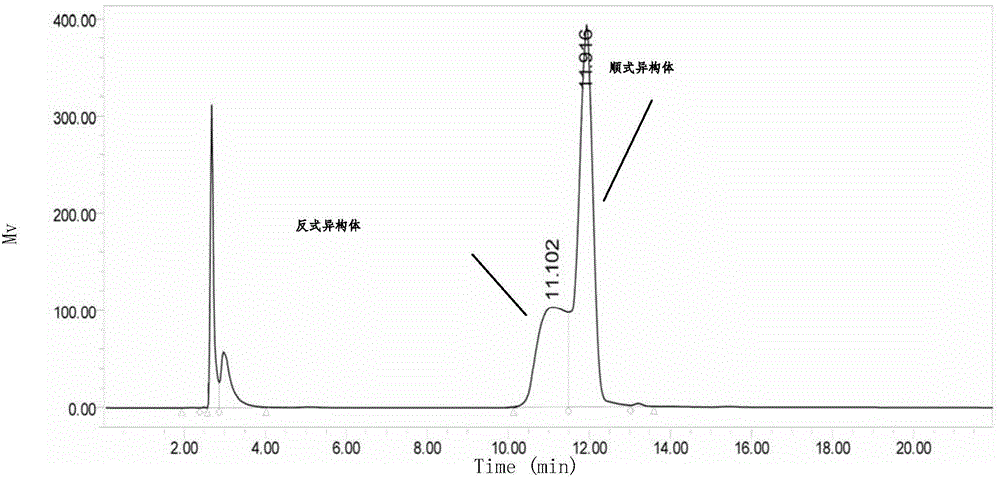
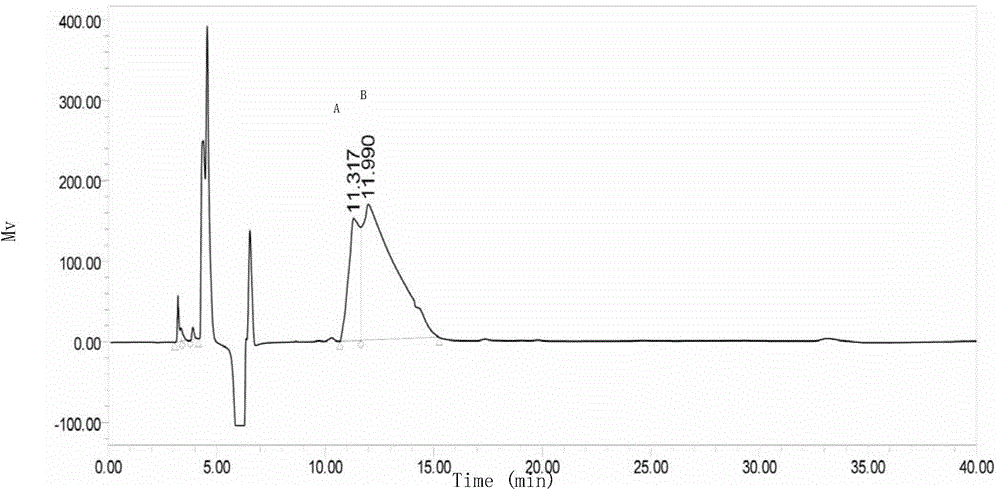
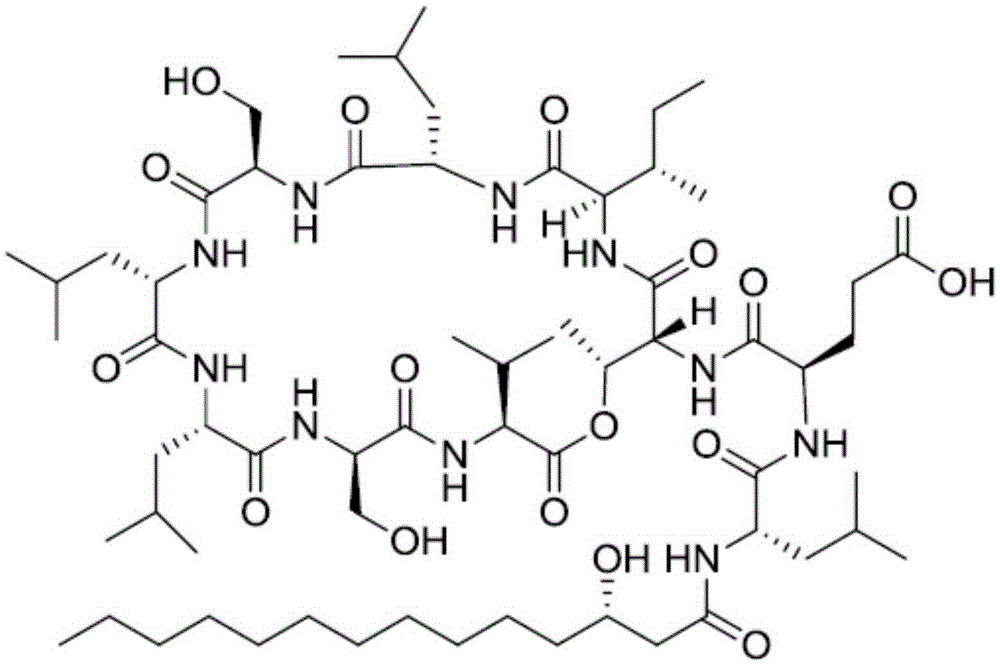
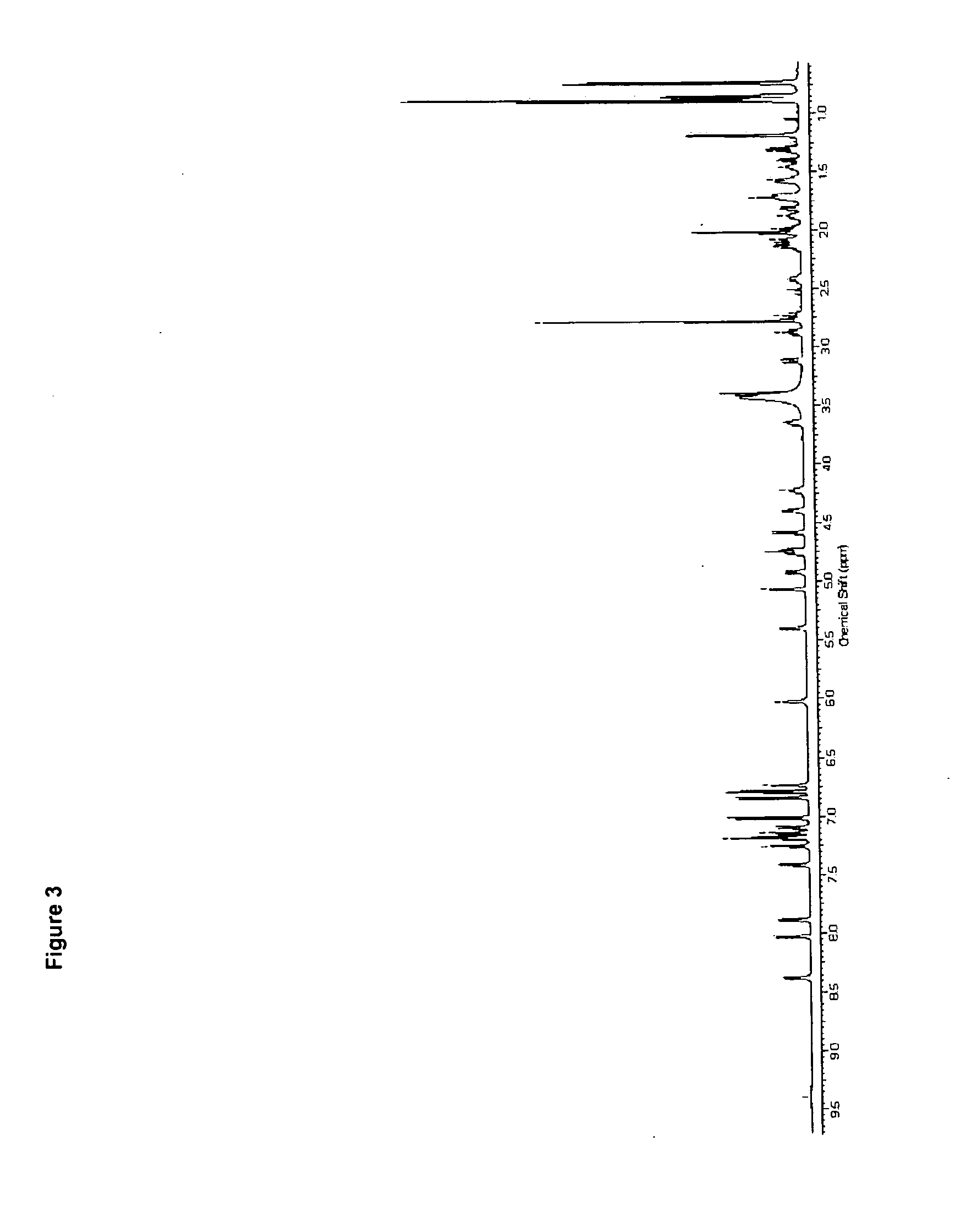
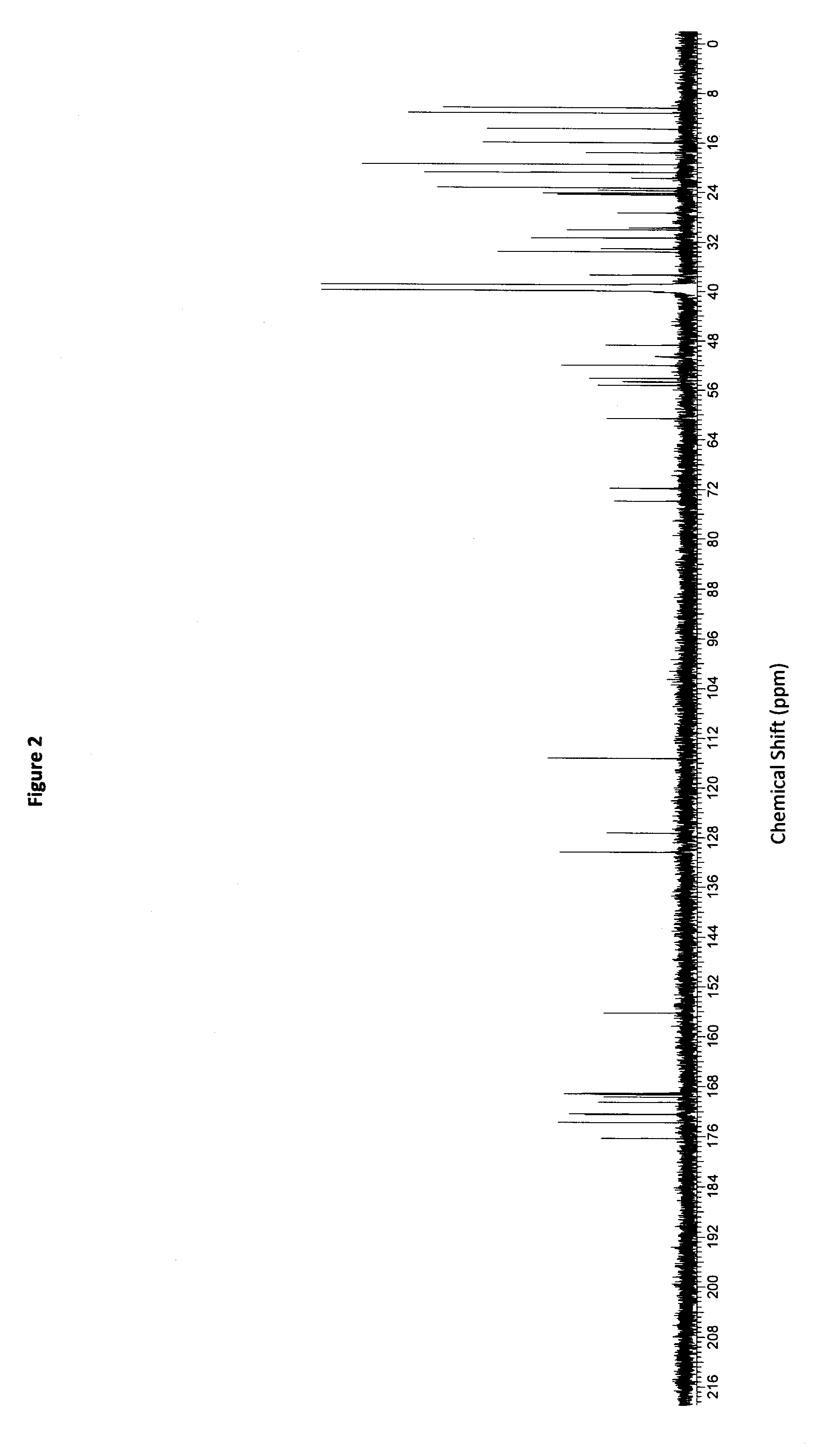
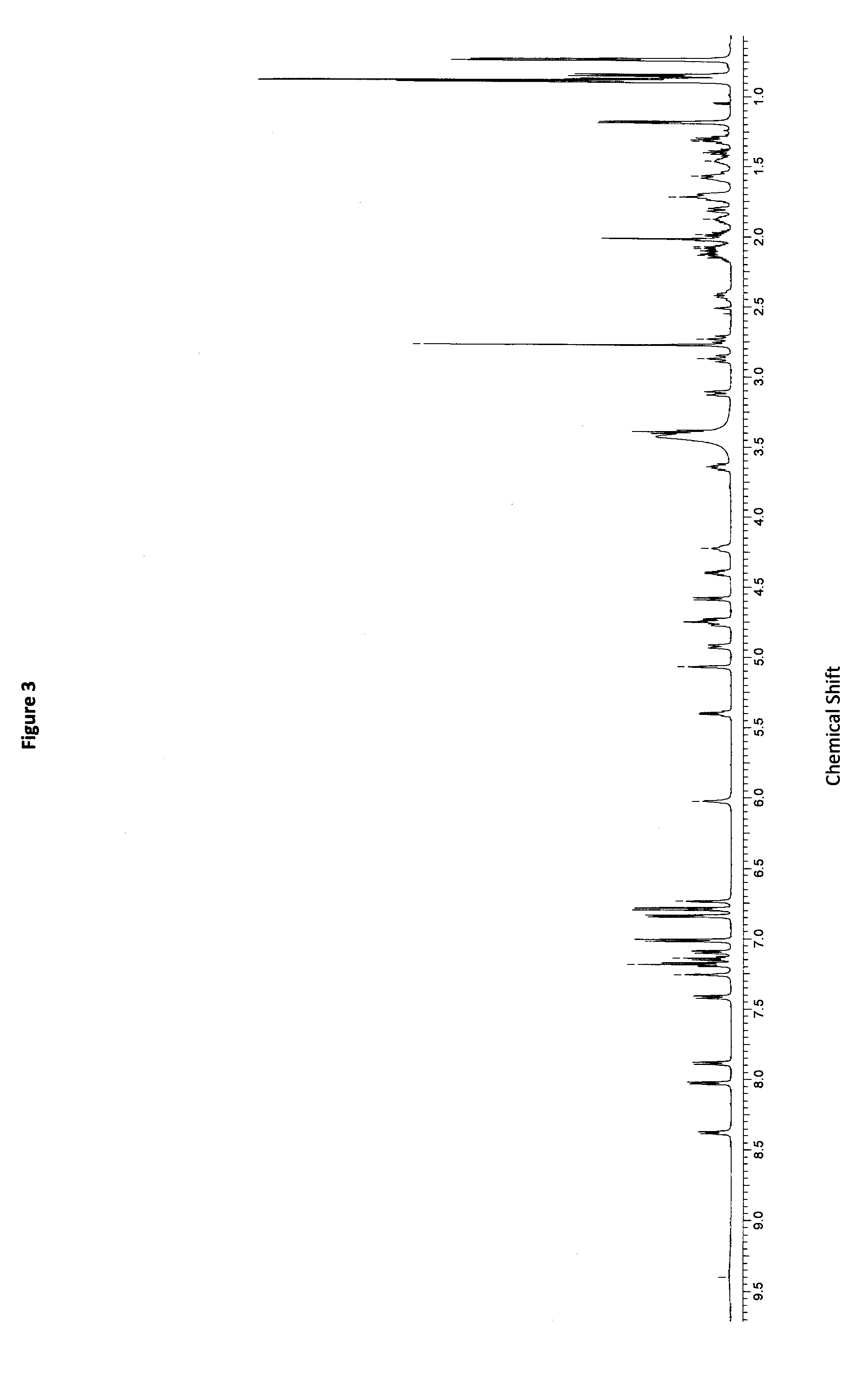
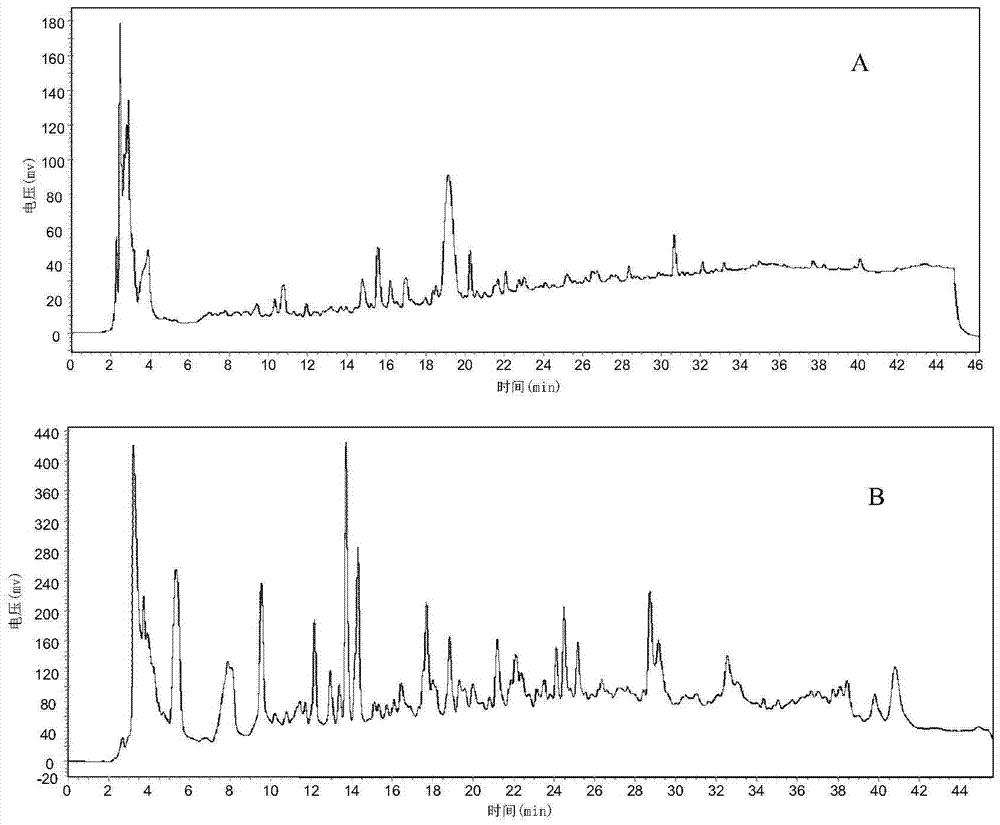
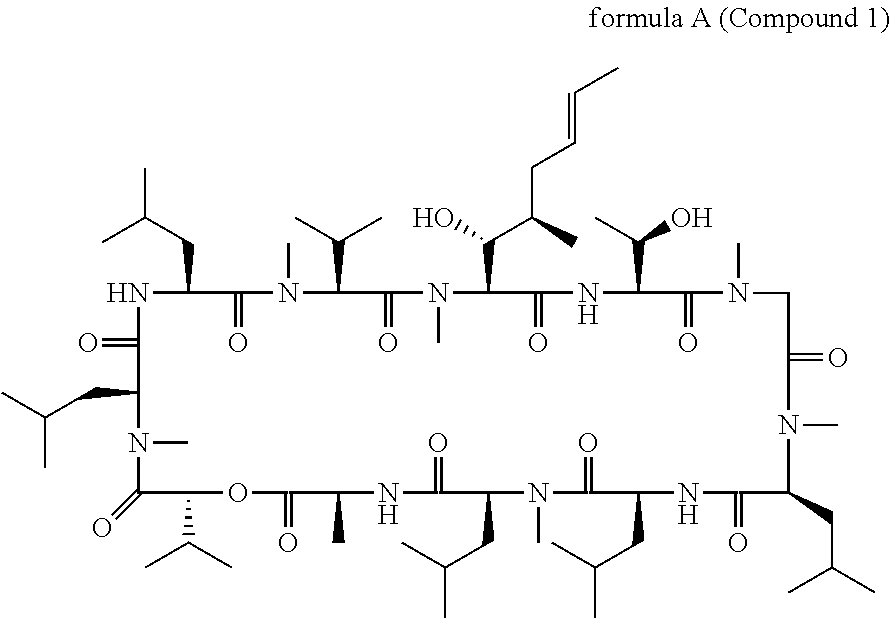
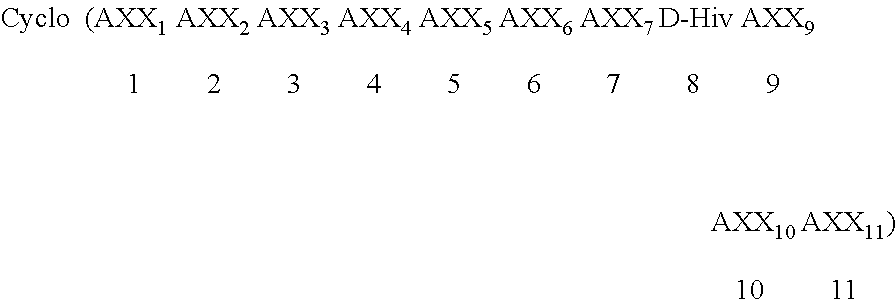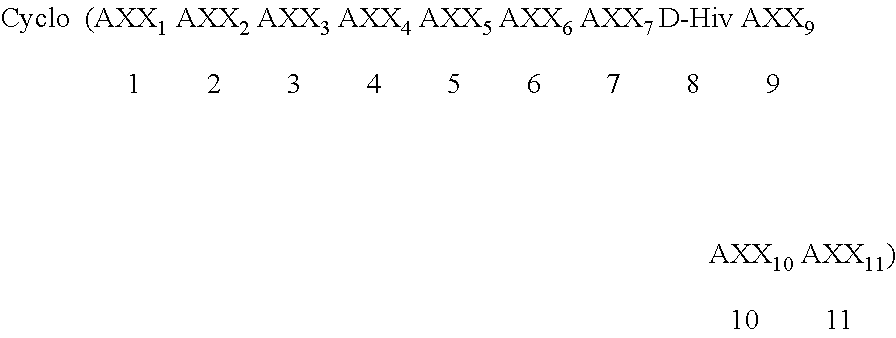Patents
Literature
100 results about "Cyclic depsipeptide" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Stick compositions
ActiveUS7347990B2Good storage stabilityCosmetic preparationsToilet preparationsFiberCosmetic ingredient
Many cosmetic stick compositions containing a continuous phase of a water-immiscible cosmetic oil structured by a fibre-forming amido structurant and an active cosmetic ingredient either exhibit poor physical stability when formed, or cannot be made readily using conventional processes for making stick compositions. The problem can be ameliorated or overcome by the use of a combination of amido structurants comprising in class (i) an N-acylaminoacid amide in which the N-acyl substituent has the formula —CO—RX in which RX represents a branched C6 to C11 alkyl group in combination with a further amido structurant, (class (ii), including a polyamido-substituted cyclohexane, an amido derivative of di or tricarboxylic acids or an hydroxystearamide and particularly employing an N-acylaminoacid amide in which the N-acyl substituent contains a linear alkyl group, or a cyclodipeptide.
Owner:UNILEVER HOME & PERSONAL CARE USA DIV OF CONOPCO IN C
Stick compositions
Anhydrous cosmetic stick compositions containing a continuous phase of a water-immiscible cosmetic oil structured by a fibre-forming amido structurant and containing a suspended particulate antiperspirant or deodorant can suffer from either somewhat poor physical stability when formed, or preparative difficulties using conventional processes for making stick compositions or opaqueness. The problem can be ameliorated or overcome by the use of a combination of amido structurants comprising (i) an N-acylaminoacid amide in which the N-acyl substituent has the formula -CO-R<X >in which R<X >represents a branched C6 to C11 alkyl group in combination with a further amido structurant (ii), selected from (iia) N-acylaminoacid amides other than (i), (iib) cyclodipeptides and (iic) a 1,2-di amido-substituted cyclohexane.
Owner:UNILEVER HOME & PERSONAL CARE USA DIV OF CONOPCO IN C
Biodegradable bio-absorbable material for clinical practice and method for producing the same
InactiveUS20050163822A1Deteriorate dynamic propertySuture equipmentsCyclic peptide ingredientsAbsorbable polymersUltimate tensile strength
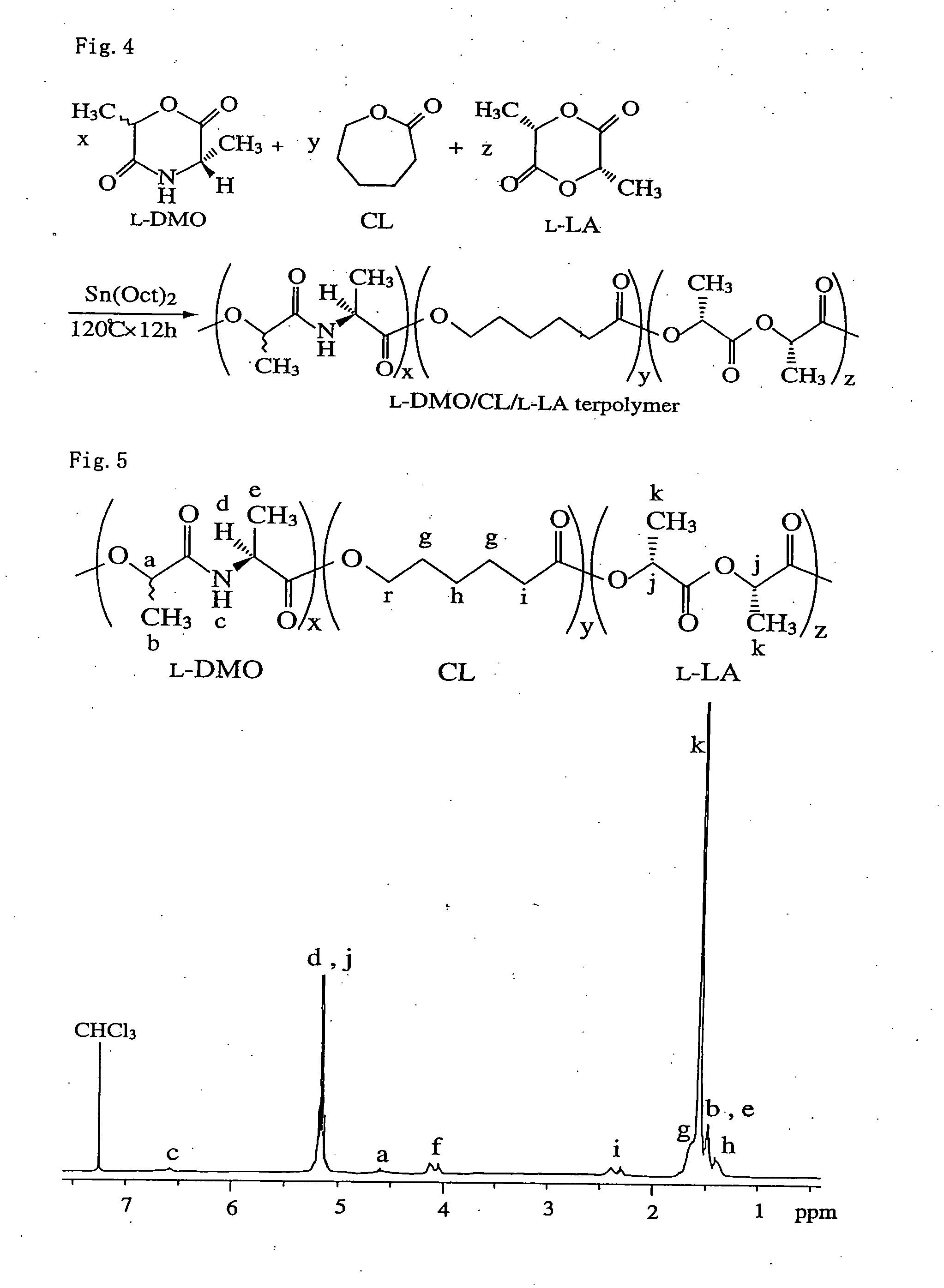
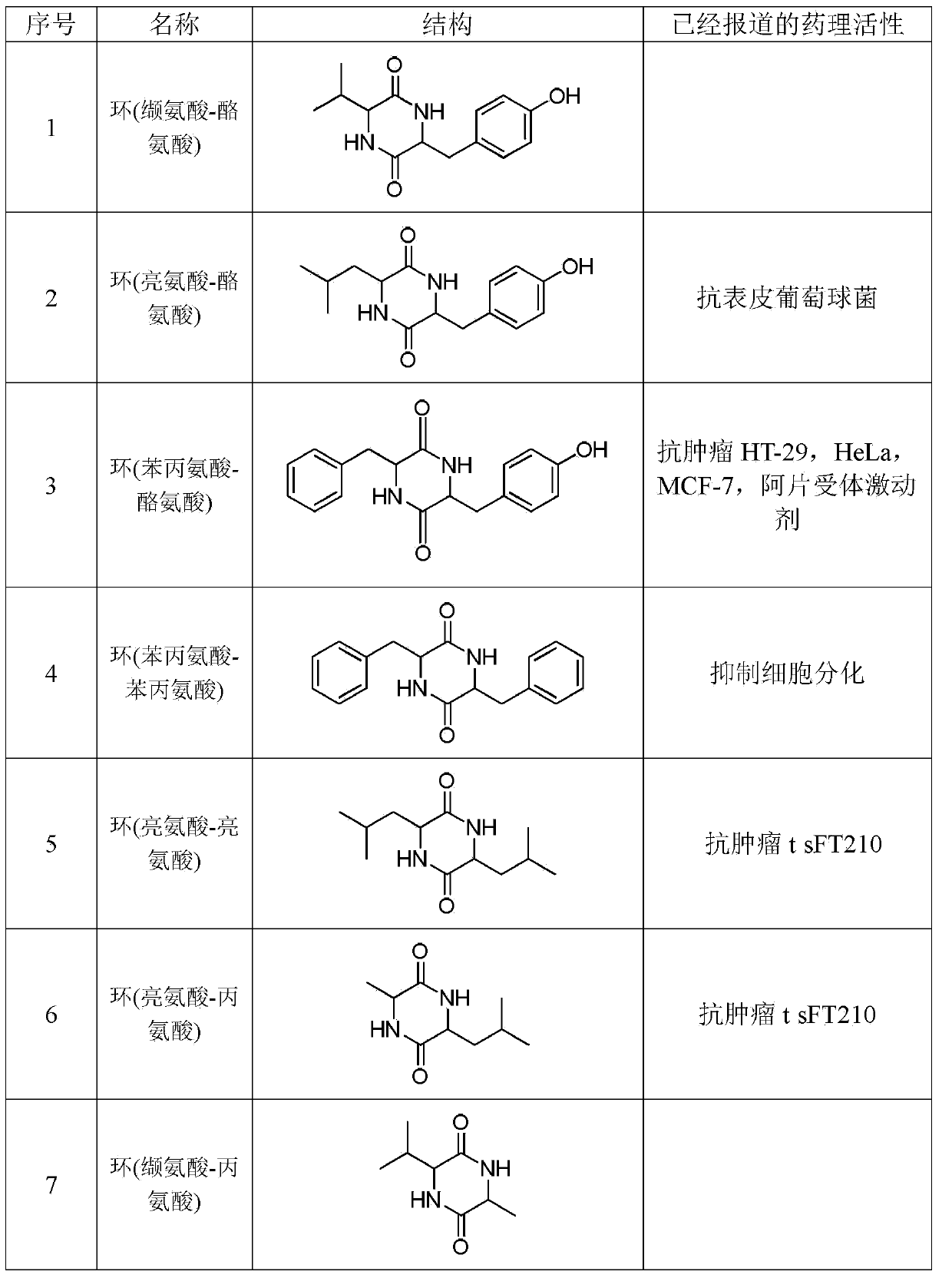
Bio-absorbable polymers such as vascular stent and suture thread for use as materials for clinical practice have almost definite dynamic properties such as tensile strength and degradation rate for absorption. When the dynamic properties thereof are elevated, therefore, the bio-absorbable polymers turn fragile, involving slower degradation rate. When the degradation rate is elevated, further, the dynamic properties are deteriorated. Disadvantageously, such bio-absorbable polymers have limited purposes for use and limited sites for use. Thus, copolymerization of bio-absorbable polymers with a cyclic depsipeptide to form a copolymer of the ring-opened and copolymerized depsipeptide can allow the adjustment of the dynamic properties and degradation rate of the resulting copolymer depending on the content of the depsipeptide.
Owner:GOODMAN & COMPANY
Hericium erinaceus mycelium and culture method thereof
ActiveCN104195052AEasy to controlImprove anti-tumor effectFungiMicroorganism based processesDipeptidePhenylalanine+Tyrosine
The invention discloses hericium erinaceus mycelium and a culture method thereof. The hericium erinaceus mycelium contains amino acids and vitamins of relatively high content as well as seven cyclic dipeptides, ergosterol and carboxymethylcellulose activities, wherein the seven cyclic dipeptides are respectively cyc(valine-tyrosine), cyc(leucine-tyrosine), cyc(phenylalanine-tyrosine), cyc(phenylalanine-phenylalanine), cyc(leucine-leucine), cyc(leucine-alanine) and cyc(valine-alanine). The culture method comprises the following steps: culturing a stock strain, culturing a seed strain, culturing a cultispecie strain, performing primary'orientated' biotransformation and performing secondary'orientated' biotransformation. The hericium erinaceus mycelium which is cultured by using the culture method is relatively good in anti-tumor curative effect.
Owner:HUNAN XINHUI PHARMA
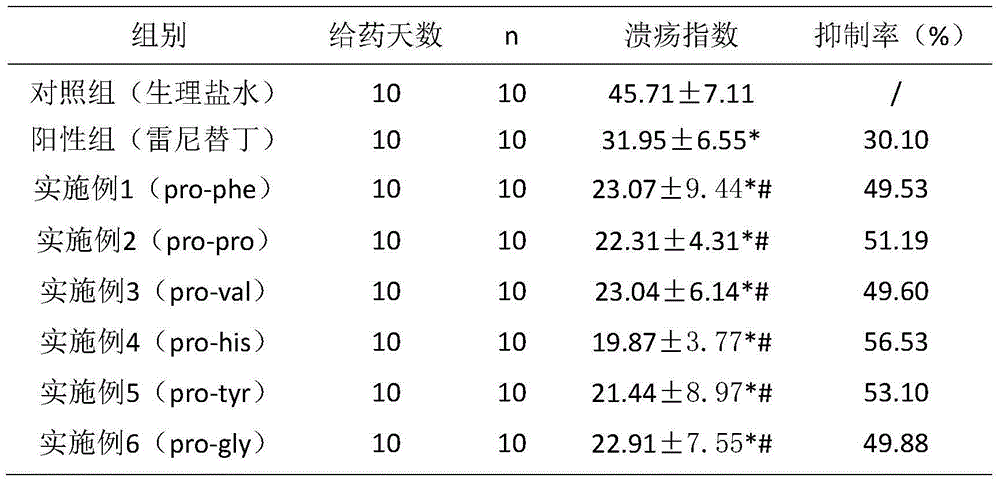
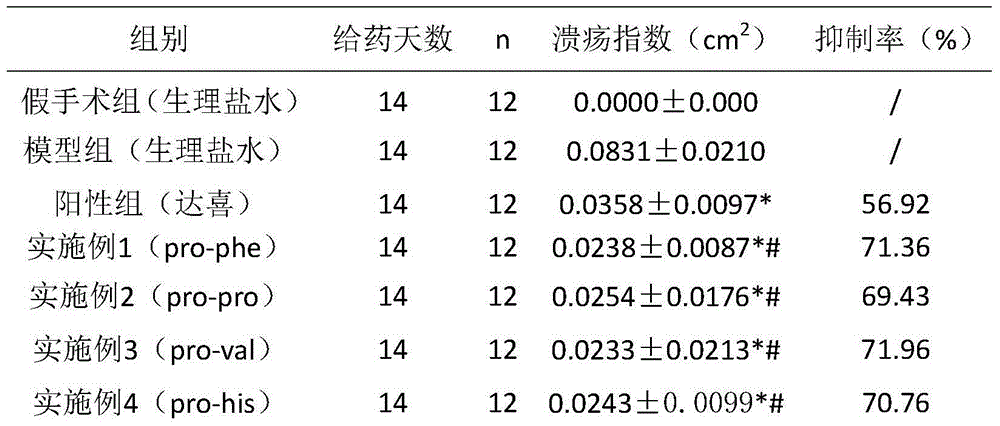
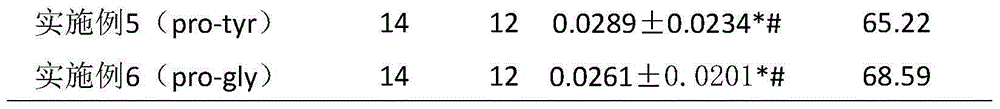
Six cyclic dipeptides used for wound healing
InactiveCN105622717AGood wound repair effectGood treatment effectDigestive systemPeptide preparation methodsWound healingDipeptide
The invention provides six cyclic dipeptides used for wound healing. The cyclic dipeptide is one of pro-phe, pro-pro, pro-val, pro-his, pro-tyr and pro-gly. The cyclic dipeptide has remarkable therapeutic effect on wound. Inhibition rate of acute and chronic gastric ulcer is high, wound healing speed of burns and scalds is fast, and scars are shallow.
Owner:SICHUAN GOODDOCTOR PHARMA GRP
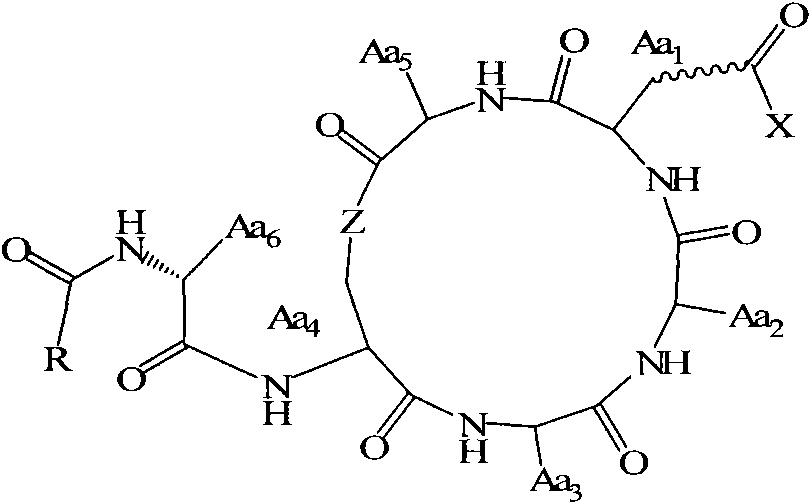
Use of cyclic depsipeptides to inhibit kallikrein 7
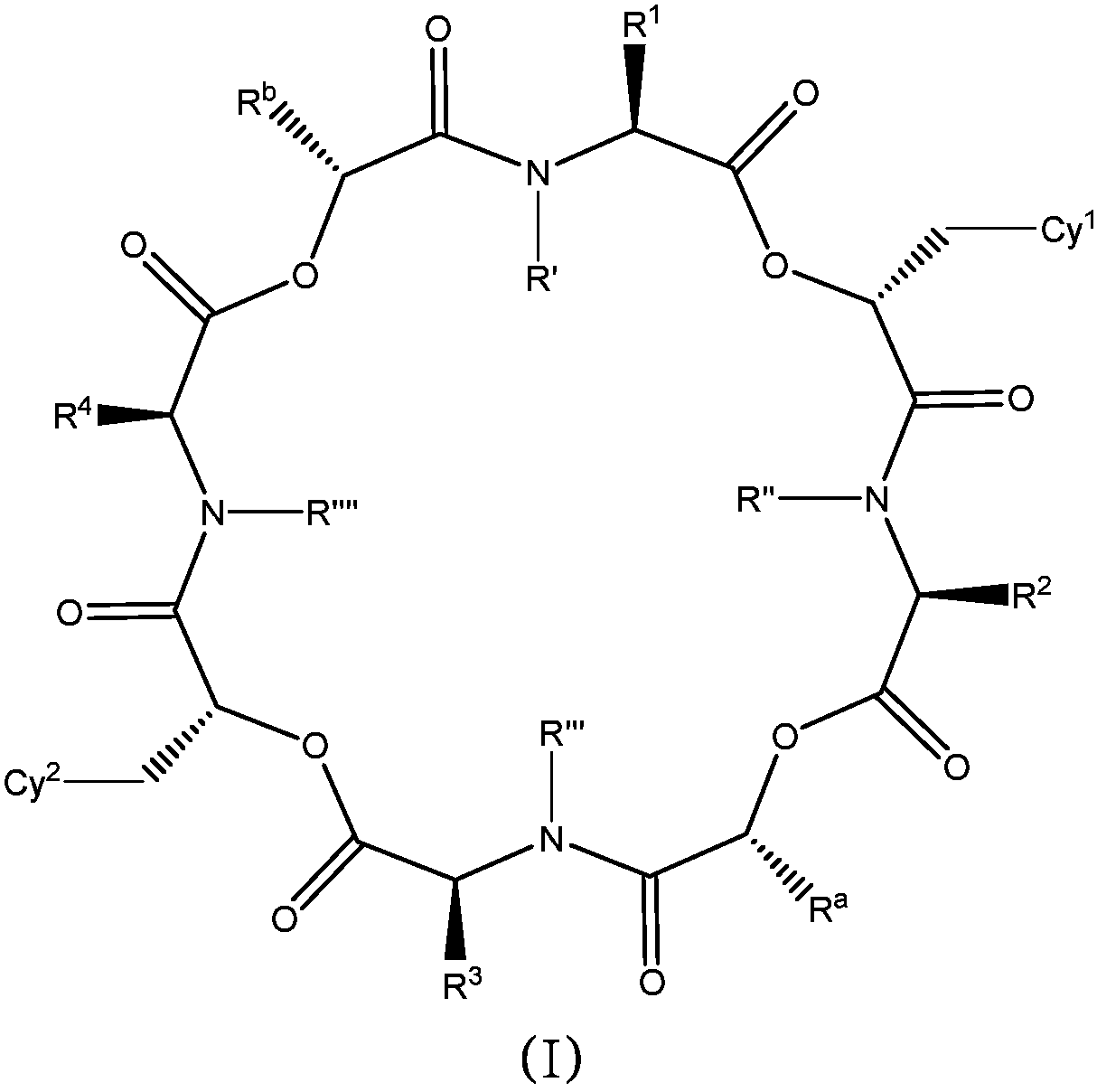
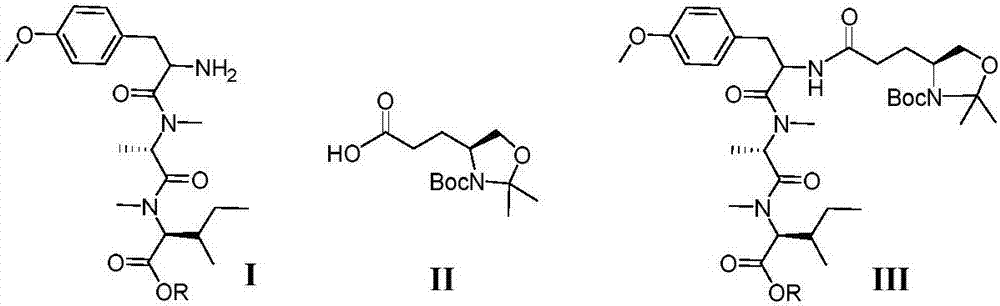
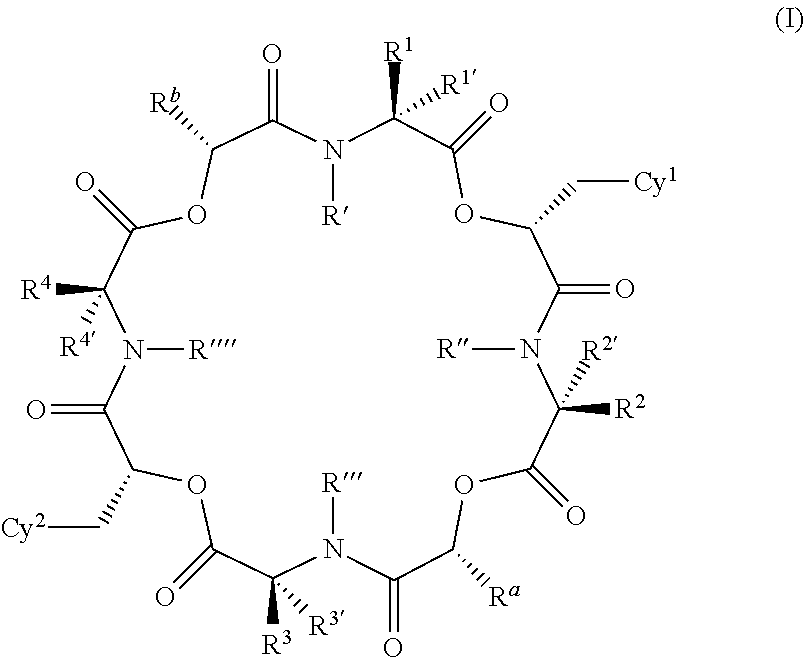
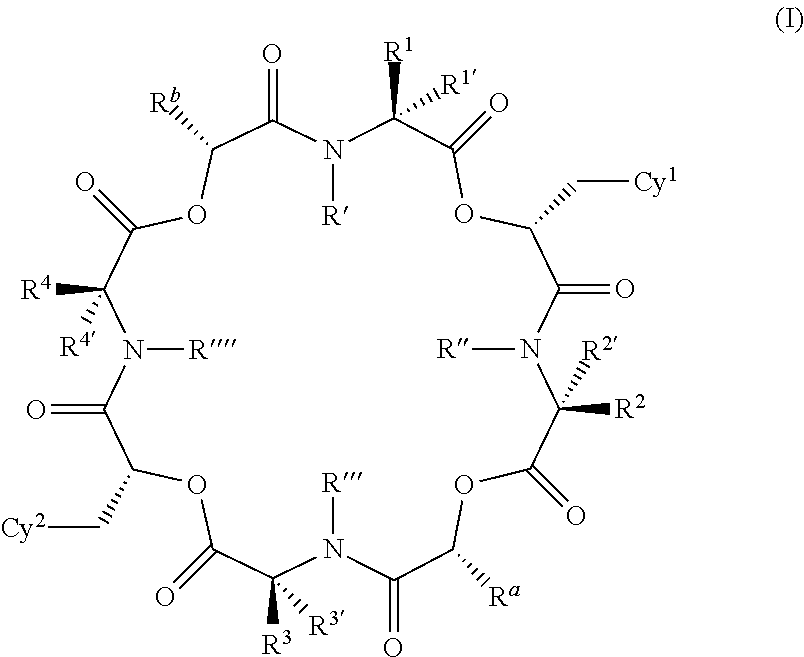
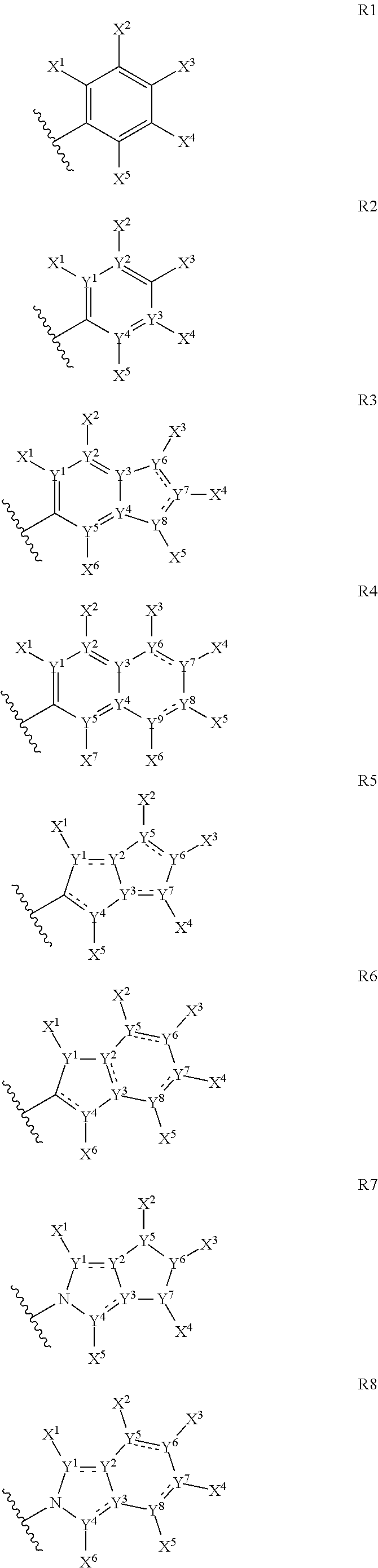
The present application relates to cyclic depsipeptides of Formula I: or derivatives thereof, wherein X, A1, A2, A3, A4, A5, A6 and A7 are defined herein. The cyclic depsipeptides of Formula I are inhibitors of kallikrein 7 and thus can be employed for the treatment of kallikrein-7 dependent diseases.
Owner:NOVARTIS AG
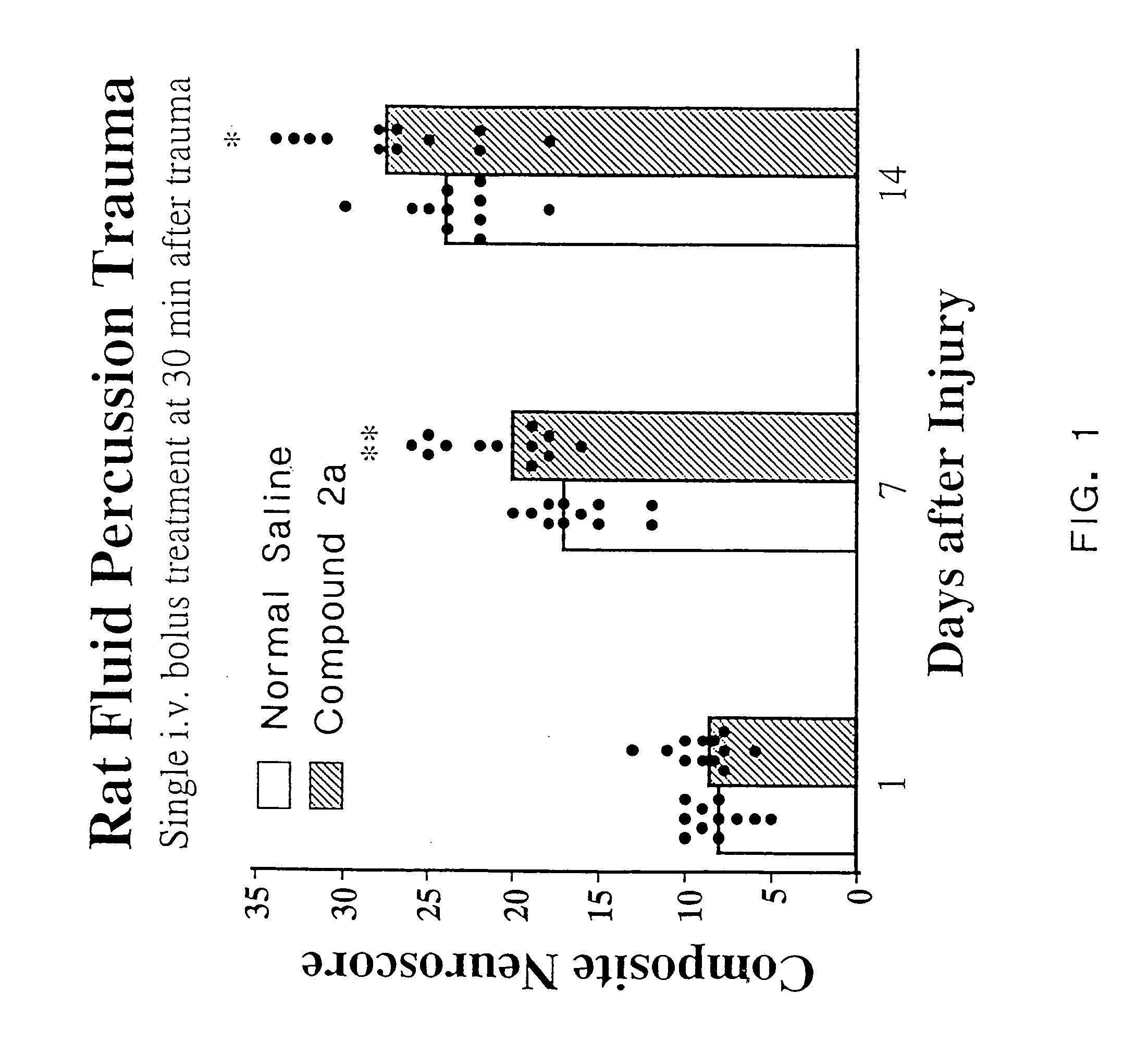
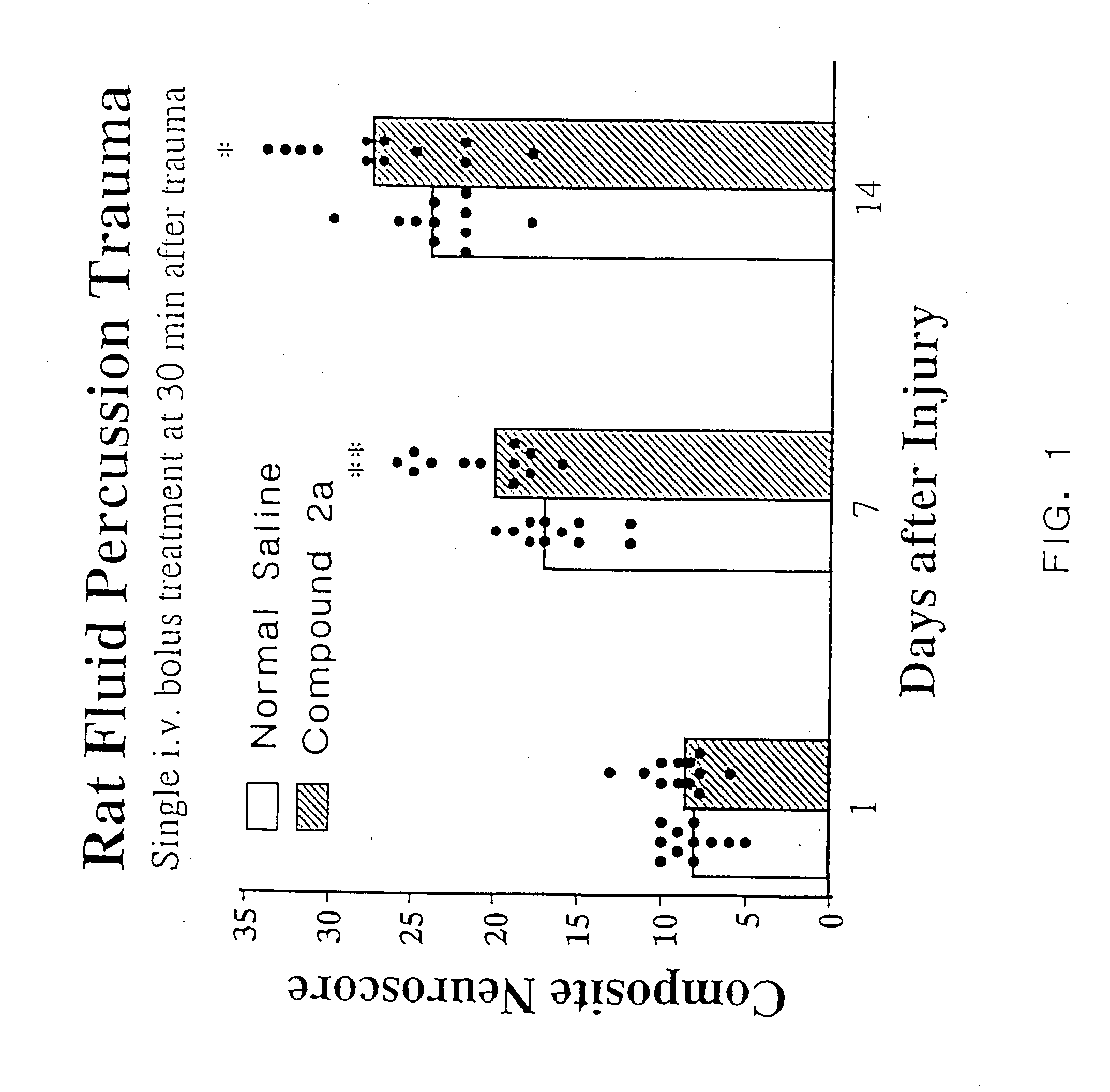
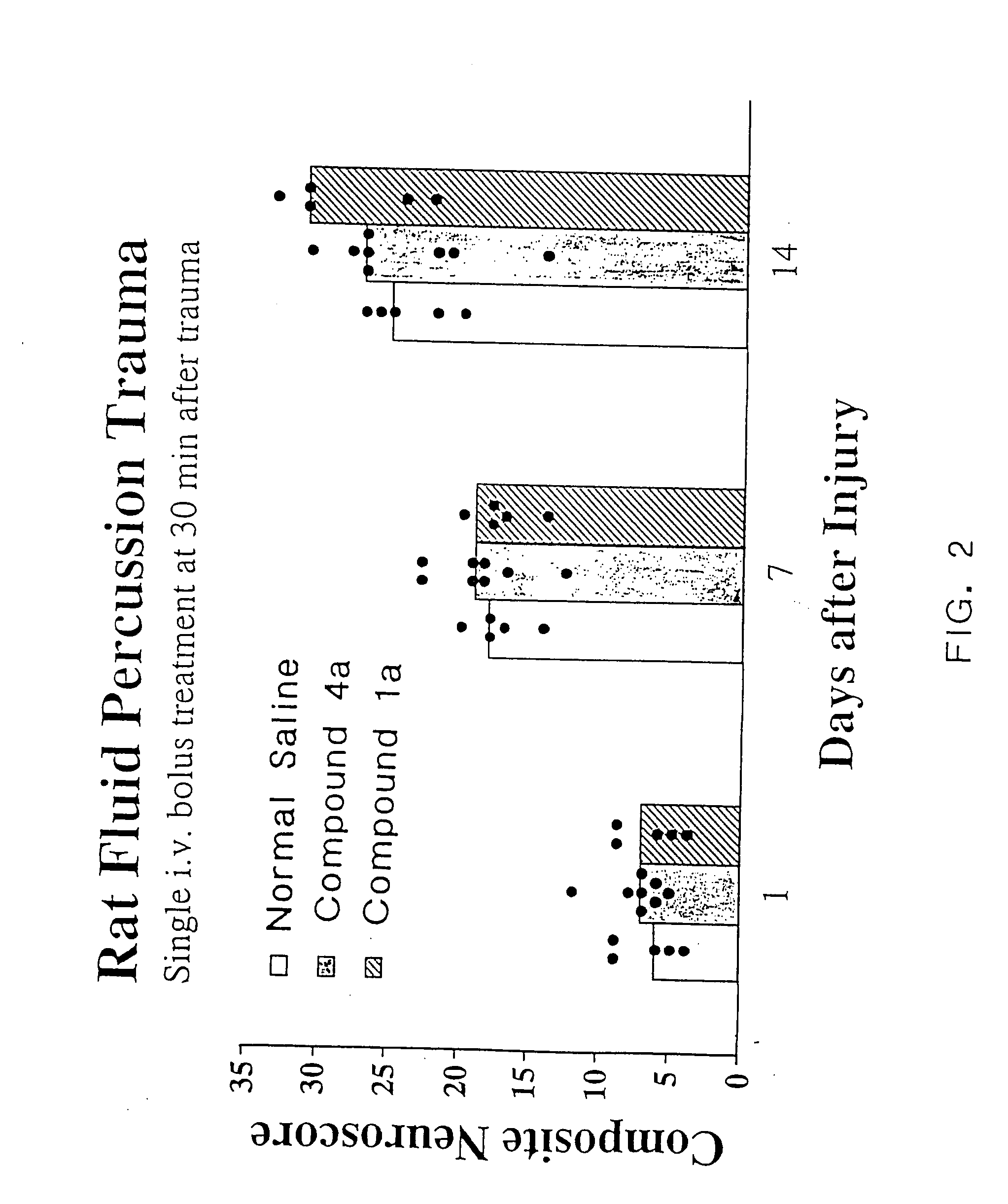
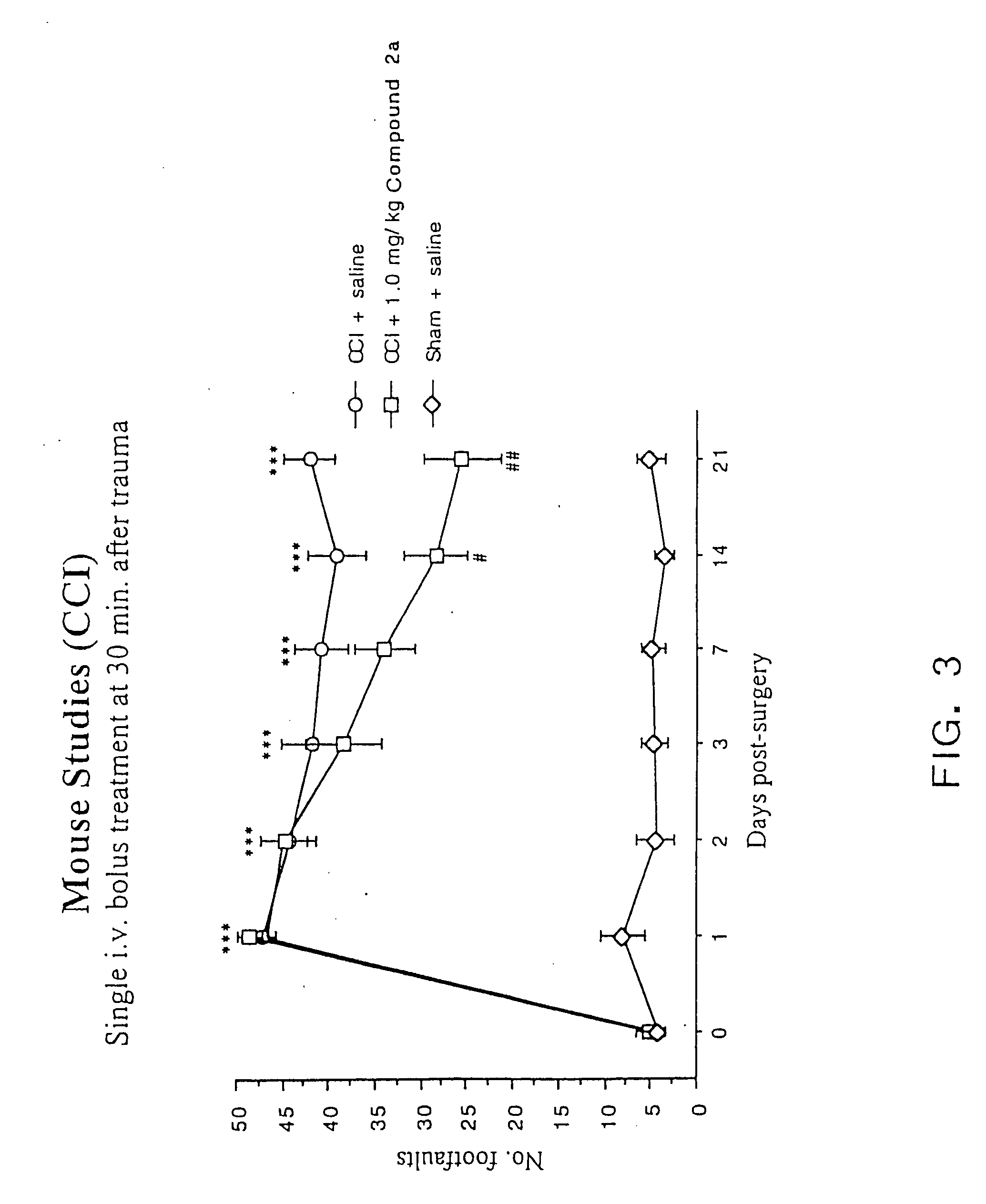
Cyclic dipeptides and azetidinone compounds and their use in treating CNS injury and neurodegenerative disorders
InactiveUS7202279B1Strong central nervous system (CNS) activityImprove cognitive functionBiocideNervous disorderDiseaseMedicine
The present invention provides 4-substituted-2-azetidinone compounds, bicyclic 2-5-diketopiperazine compounds, and pharmaceutical compositions thereof that are potent, safe and effective neuroprotective agents. Due to their strong central nervous system (CNS) activity, the compounds can be used to enhance memory and to treat a variety of neurological disorders. The compounds are particularly useful for treating neurological disorders caused by, or associated with, CNS trauma.
Owner:GEORGETOWN UNIV
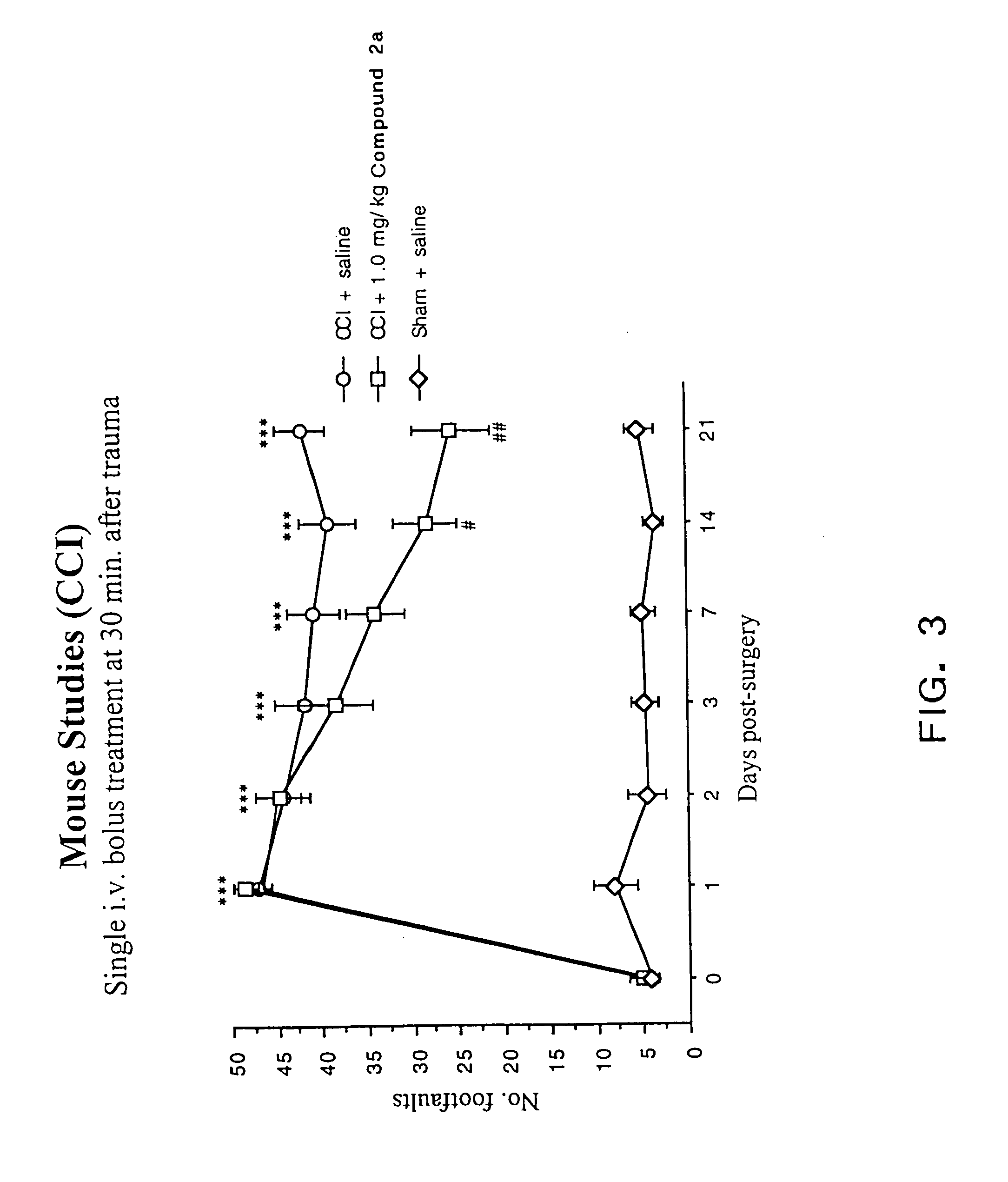
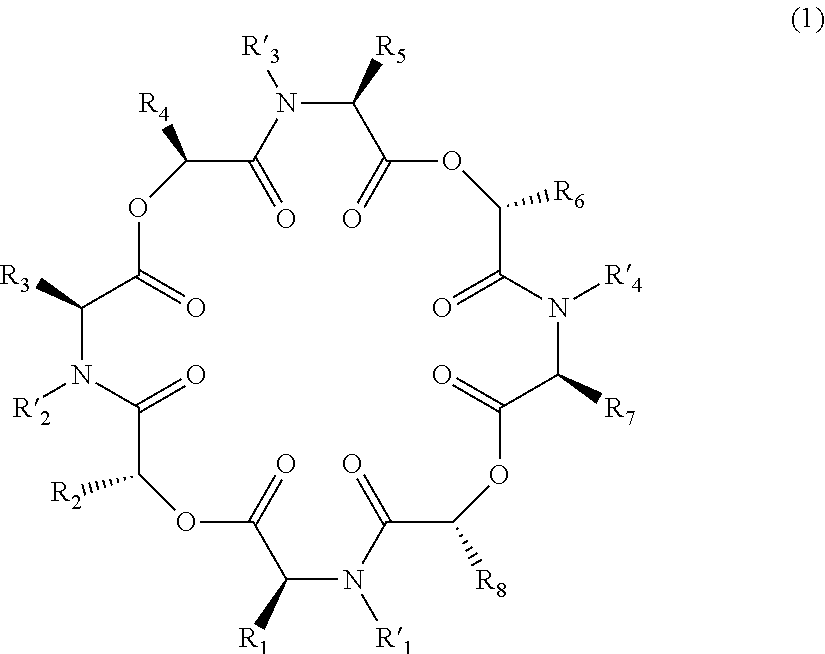
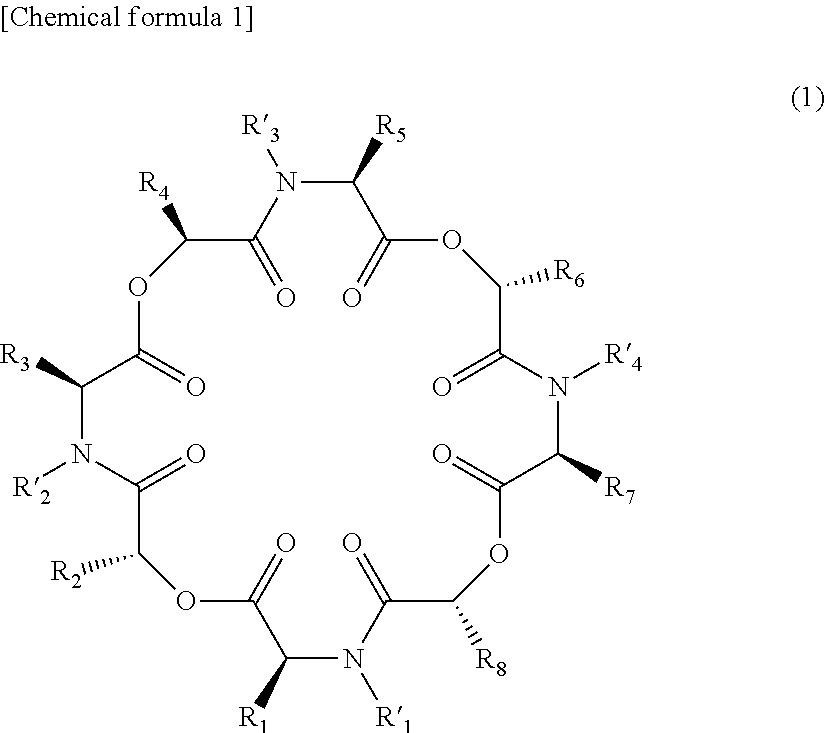
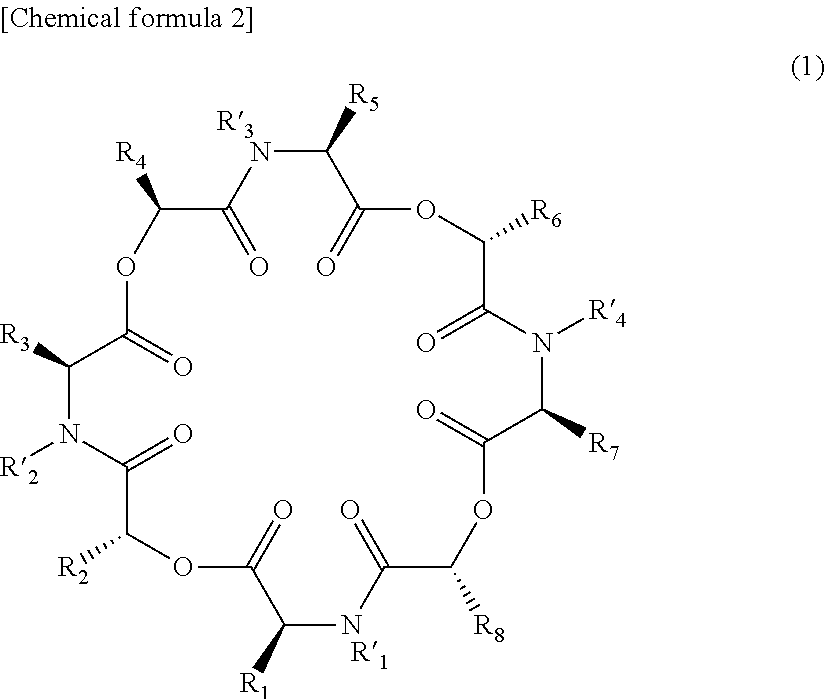
Novel cyclic depsipeptide derivatives and harmful organism control agents comprising the same
InactiveUS20150166608A1Easy to controlEffective controlBiocideCarbamic acid derivatives preparationOrganismStereoisomerism
An objective of the present invention is to provide novel cyclic depsipeptide derivatives and harmful organism control agents including the same as each other. Specifically, the present invention provides compounds represented by formula (1) or stereoisomers thereof, harmful organism control agents containing them, and a process for producing them.
Owner:THE KITASATO INST
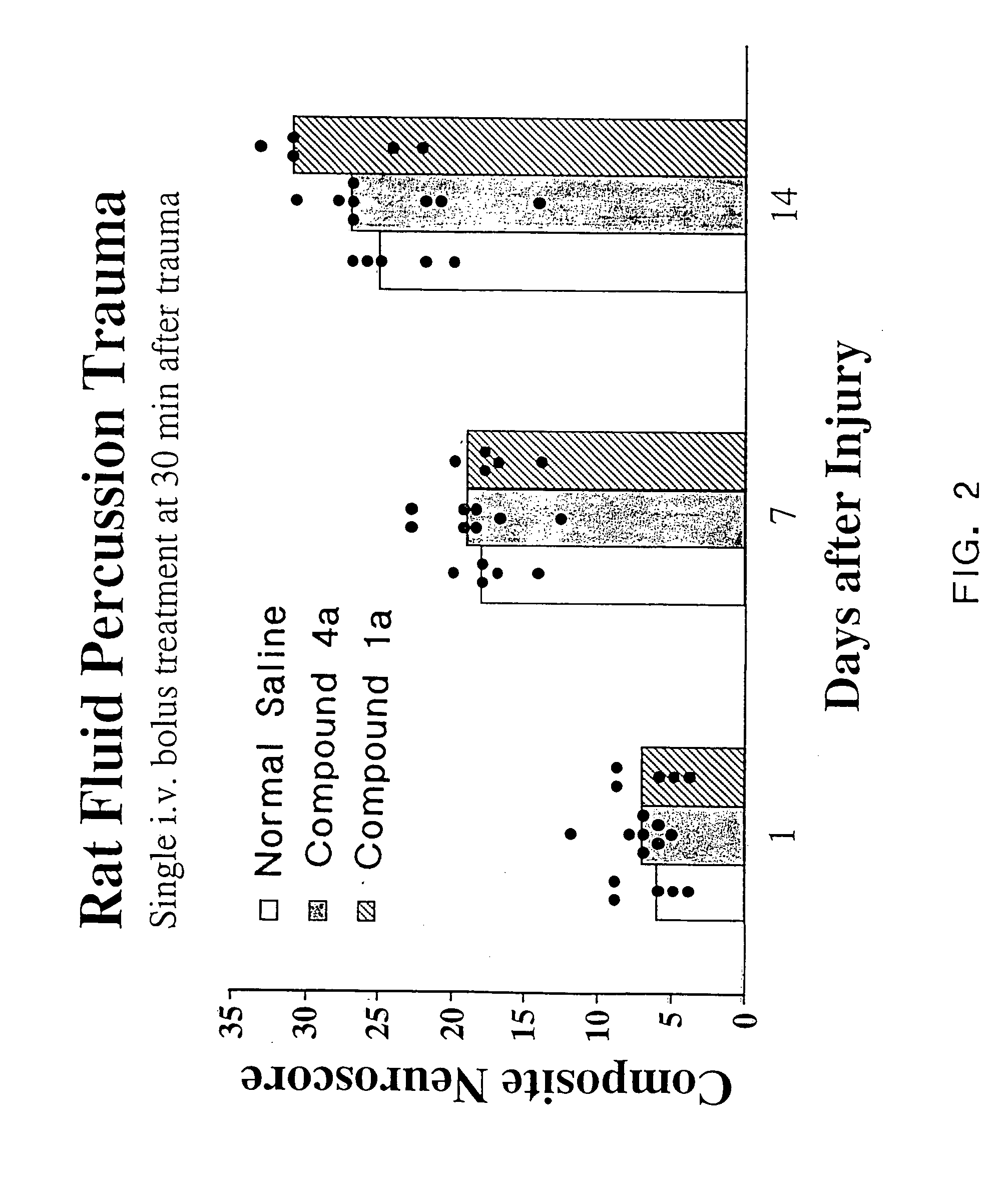
Cyclic dipeptides and azetidinone compounds and their use in treating CNS injury and neurodegenerative disorders
InactiveUS20070161640A1Improve cognitive functionIncrease awarenessBiocideNervous disorderDiseaseMedicine
The present invention provides 4-substituted-2-azetidinone compounds, bicyclic 2-5-diketopiperazine compounds, and pharmaceutical compositions thereof that are potent, safe and effective neuroprotective agents. Due to their strong central nervous system (CNS) activity, the compounds can be used to enhance memory and to treat a variety of neurological disorders. The compounds are particularly useful for treating neurological disorders caused by, or associated with, CNS trauma.
Owner:GEORGETOWN UNIV
Production method for cyclic dipeptide
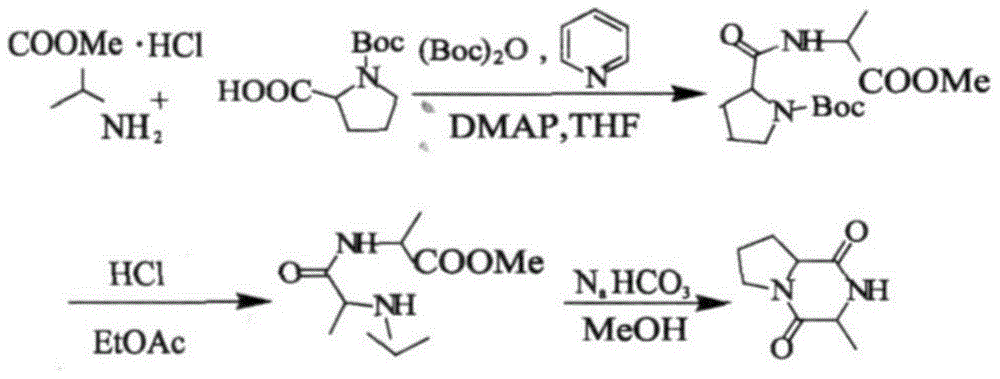
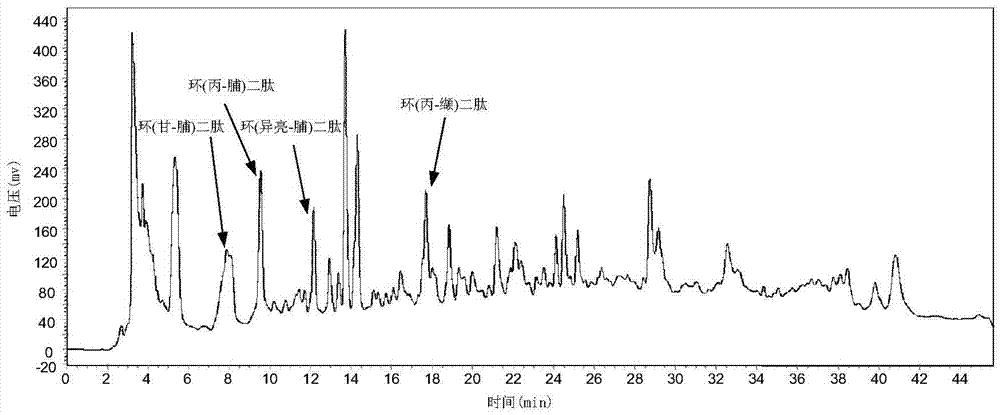
The invention relates to a production method for cyclic dipeptide, and belongs to a natural compound synthesis technology. Cyclic (histidine-proline) dipeptide is prepared by a cooling varying-temperature synthesis and high-pressure high-temperature water phase cyclizing technology. The production method comprises the following steps: taking hydrochloride of histidine proline dipeptide methyl ester as a raw material, and synthesizing high-quality and high-purity cyclic histidine-proline dipeptide by a high-pressure high-temperature assisted method in water containing strong basic and weak acidic salt. Compared with a conventional methanol reflux method, the production method is time-saving, efficient, high in product yield and free of racemization phenomenon.
Owner:JILIN AGRICULTURAL UNIV
A kind of solid phase synthesis method of cyclic lipopeptide orfamide A
InactiveCN103626848BEfficient productionMass productionPeptide preparation methodsBulk chemical productionCombinatorial chemistrySolid-phase synthesis
Owner:HYBIO PHARMA
Cyclic depsipeptides
The present application relates to cyclic depsipeptides, or derivatives thereof, having the structure of formula (I), and uses thereof, e.g. as inhibitors of kallikrein 7 and human neutrophil elastase.
Owner:NOVARTIS AG
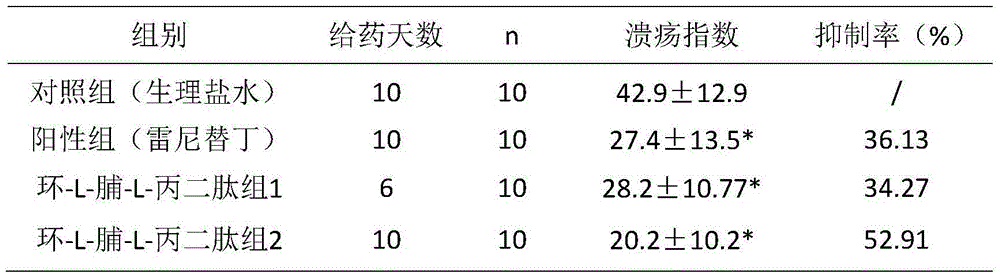
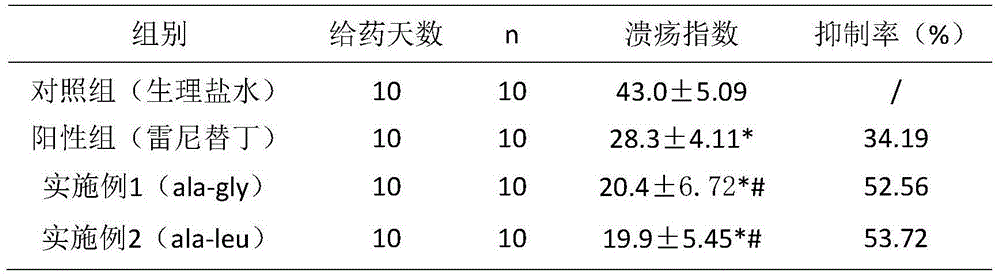
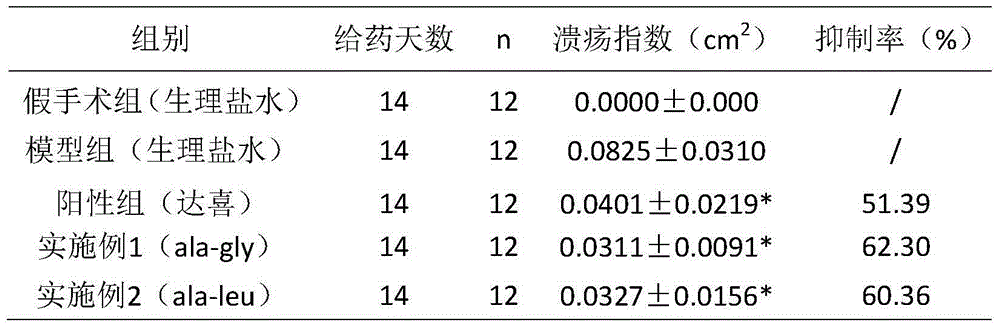
Application of cyclo-dipeptide in preparation of drug for treating gastrointestinal ulcer
InactiveCN105311619ASmall molecular weightSimple structureDigestive systemPeptide preparation methodsDipeptideTherapeutic effect
The invention provides an application of cyclo-dipeptide in preparation of a drug for treating gastrointestinal ulcer. The cyclo-dipeptide is cyclo-L-Pro-L-alanine dipeptide. The dipeptide is small in molecular weight and is simple in structure, has significant treatment effects on the gastrointestinal ulcer, can enter blood circulation quickly through mucous membrane tissue during treatment, can avoid first pass effect in liver and quickly arrive at lesion, achieves the best treatment effect on the gastrointestinal ulcer, is reduced in treatment time and use quantity and is also reduced in relapse rate.
Owner:陈光健
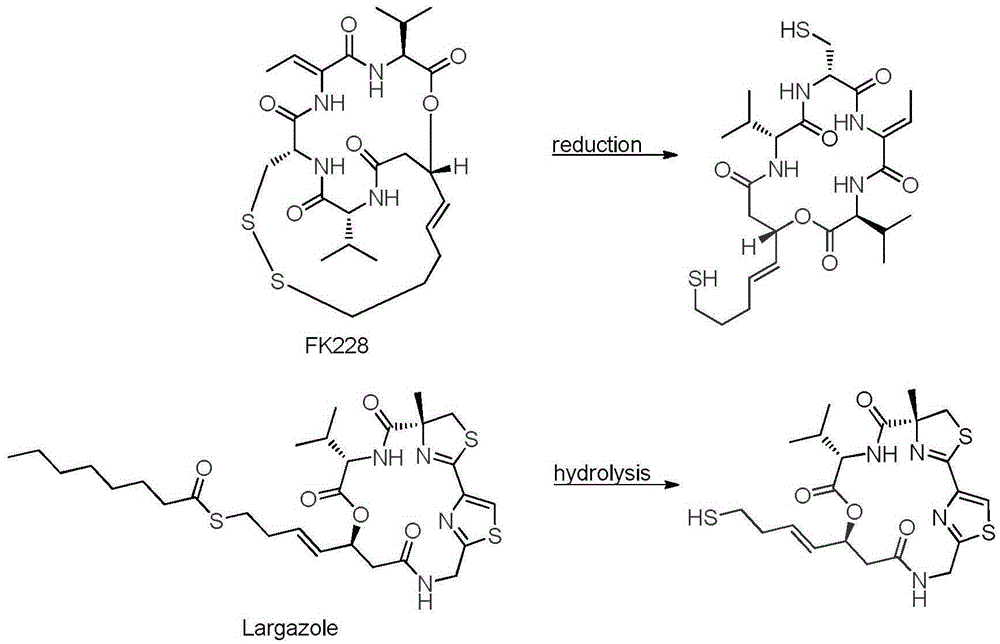
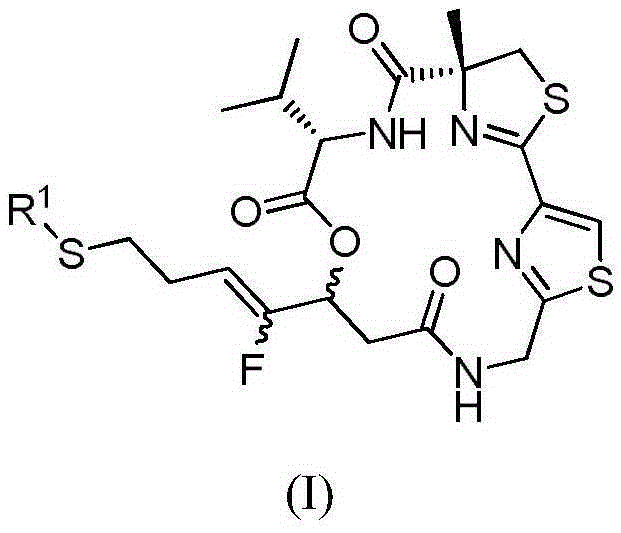
Fluorinated olefin analogue of marine natural product cyclic depsipeptide as well as preparation method and application of fluorinated olefin analogue
The invention belongs to the pharmaceutical field and relates to a novel fluorinated olefin analogue of marine natural product cyclic depsipeptide (namely marine natural product Largazole), in particular to a compound shown in the general formula (1) or salt and a preparation method of the compound as well as a drug containing the compound or application of the compound serving as an anti-tumor therapeutic agent.
Owner:FUDAN UNIV
Use of cyclic depsipeptides to inhibit kallikrein 7
The present application relate to cyclic depsipeptides, or derivatives thereof, having the formula (I), and uses thereof, e.g. as inhibitors of kallikrein 7.
Owner:NOVARTIS AG
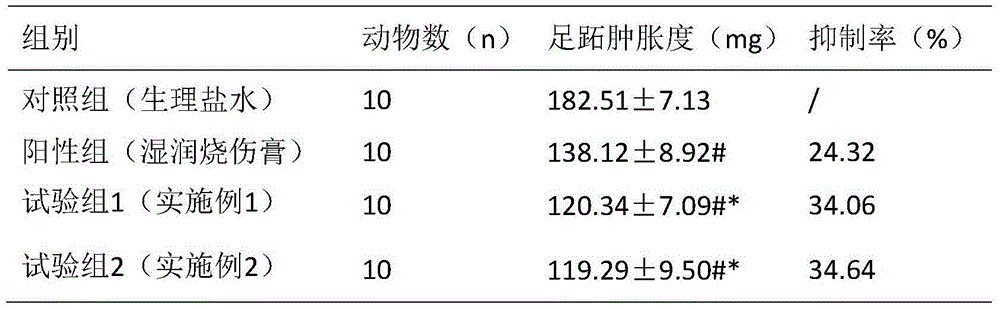
Cyclic dipeptide used for wound healing
InactiveCN105622716AGood wound repair effectGood treatment effectDigestive systemPeptide preparation methodsDipeptideMedicine
The invention provides cyclic dipeptide used for wound healing. The cyclic dipeptide is one of ala-gly and ala-leu. The cyclic dipeptide has remarkable therapeutic effect on wound. Inhibition rate of acute and chronic gastric ulcer is high, wound healing speed of burns and scalds is fast, and scars are shallow.
Owner:SICHUAN GOODDOCTOR PHARMA GRP
Synthesis method of cyclic dipeptide containing glutamine and asparagine
ActiveCN111875668AAvoid residueSynthetic operation is simplePeptide preparation methodsCyclic peptidesAmino acid side chainDipeptide
The invention discloses a synthesis method of a cyclic dipeptide. A cyclic dipeptide sequence contains L-asparagine or L-glutamine. The method comprises the following steps: by taking 2-Chlorotrityl Chloride Resin as a carrier, carrying out solid-phase synthesis to obtain a straight-chain dipeptide fragment H-Glu-AA-OH or H-Asp-AAOH, wherein AA is other alpha-amino acids except asparagine, glutamine, glutamic acid, aspartic acid and cysteine; then carrying out a methyl esterification reaction to obtain straight-chain dimethyl ester protected dipeptide H-Glu (OMe) -AA-OMe or H-Asp (OMe)- AA-OMe; then carrying out water-phase alkaline cyclization to obtain cyclic dipeptide Cyclo[ Glu (OMe)- AA] or Cyclo[ Asp (OMe)- AA] protected by side chain carboxyl methyl ester; and finally, carrying outammonolysis to obtain the cyclic dipeptide containing glutamine or asparagine. The method is simple in synthesis process and safe to operate, incomplete cyclization caused by steric hindrance of aminoacid side chain protecting groups in the cyclization process is effectively avoided, few byproducts are produced, purification is easy, and cyclic dipeptide containing Gln and Asn can be synthesizedin batches.
Owner:SHAANXI HUIKANG BIO TECH CO LTD
Use of cyclic depsipeptides to inhibit kallikrein 7
The present application relate to cyclic depsipeptides, or derivatives thereof, having the formula (I), and uses thereof, e.g. as inhibitors of kallikrein 7.
Owner:DR PY INST LLC +1
Preparation method of cyclic dipeptide compound
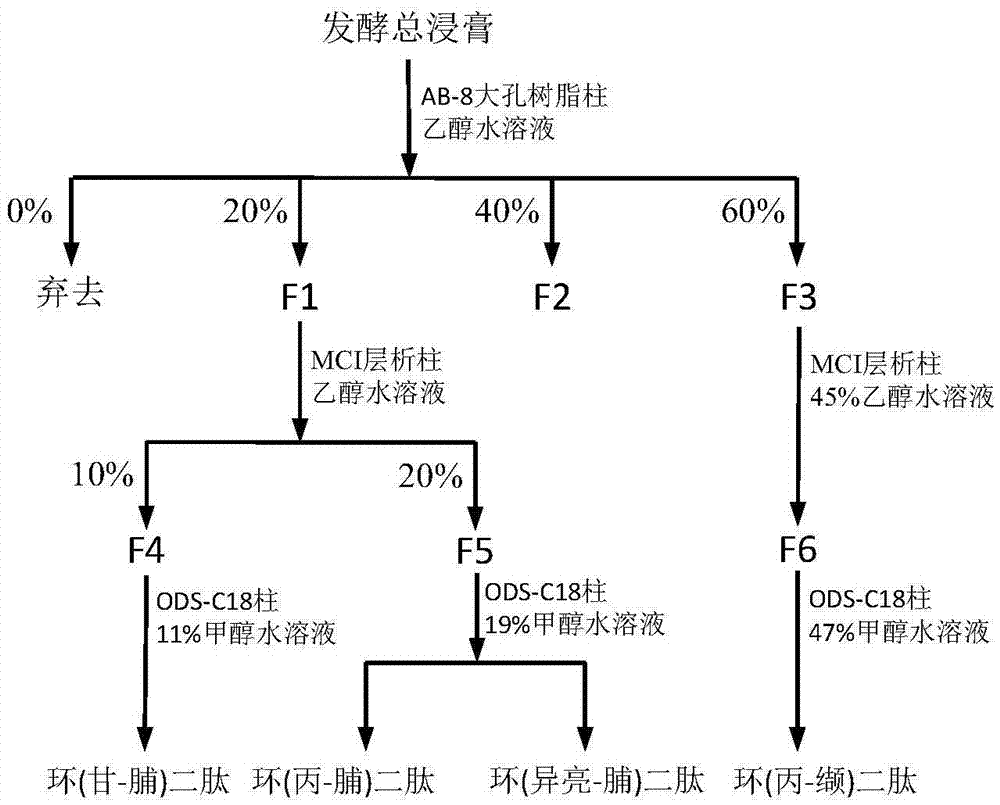
ActiveCN105441504AHas antitumor activitySimple compositionMicroorganism based processesFermentationPenicillium bilaiaeDipeptide
The invention provides a preparation method of a cyclic dipeptide compound. The method comprises the following steps: inoculating penicillium citrinum MNP12010101 into a fermentation medium containing cobalt ions; carrying out cultivation for 5-7d under a constant temperature vibration condition that the temperature is 25-30 DEG C and the speed is 200-250r / min; then standing for 4-5d at 25-30 DEG C; and separating and purifying the culture solution to obtain cyclic (gly-pro) dipeptide, cyclic (phe-pro) dipeptide, cyclic (isoleucine-pro) dipeptide and cyclic (phe-val) dipeptide compounds. According to the culture method provided by the invention, cyclic dipeptides which cannot be synthesized under a conventional culture condition are synthesized in penicillium citrinum MNP12010101 cells; the culture medium is simple in composition, the process is simple and the fermentation cost is low; and the four types of cyclic dipeptides synthesized in the penicillium citrinum MNP12010101 cells have antitumor activity.
Owner:杭州水芭莎生物医药科技有限公司
Anthelmintic depsipeptide compounds
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC
Cyclic dipeptide and application of cyclic dipeptide in preparation of bactericide
ActiveCN108586462ALess likely to cause harmEasy to degradeBiocideOrganic chemistryDipeptidePyricularia grisea
The invention discloses cyclic dipeptide and application of the cyclic dipeptide in the preparation of a bactericide and belongs to the field of plant protection. The cyclic dipeptide is 3-isopropyl-pyrrolopiperazine-2,5-dione. Pathogen controlled by the bactericide is one or more than one of a group consisting of Botrytis cinerea, corynespora cassiicola, Ascochyta citrullina, Colletotrichum orbiculare, Pyricularia grisea, Candida albicans, Pellicularia, Fusarium oxysporum, Rhizoctonia solani, xanthomonas oryzae, aspergillus flavus and Fusarium moniliforme. The invention reports bactericidal activity of cyclo(L-proline-L-valine) for the first time and provides a new thinking for research and development of the bactericide. As a bactericide, the cyclic dipeptide cyclo(L-proline-L-valine) isderived from a secondary metabolite of Pseudomonas syringae, belongs to a natural product, is easy to degrade in the environment, and is not easy to cause harm o the environment.
Owner:INST OF PLANT PROTECTION CHINESE ACAD OF AGRI SCI
Method for efficiently preparing cyclic depsipeptide, cyclic depsipeptide and application
The invention relates to the field of preparation of medicinal compounds, in particular to a method for efficiently preparing cyclic depsipeptide, the cyclic depsipeptide and application. The method comprises the following steps: inoculating a culture medium with the parasitic fungus Amphihorda guana LC5815 from the bat feces, and fermenting, so as to obtain a fermentation culture; and performing separating from the fermentation culture to obtain the cyclic depsipeptide compound.
Owner:INST OF MICROBIOLOGY - CHINESE ACAD OF SCI
Biosynthetic Systems Producing Fungal Indole Alkaloids
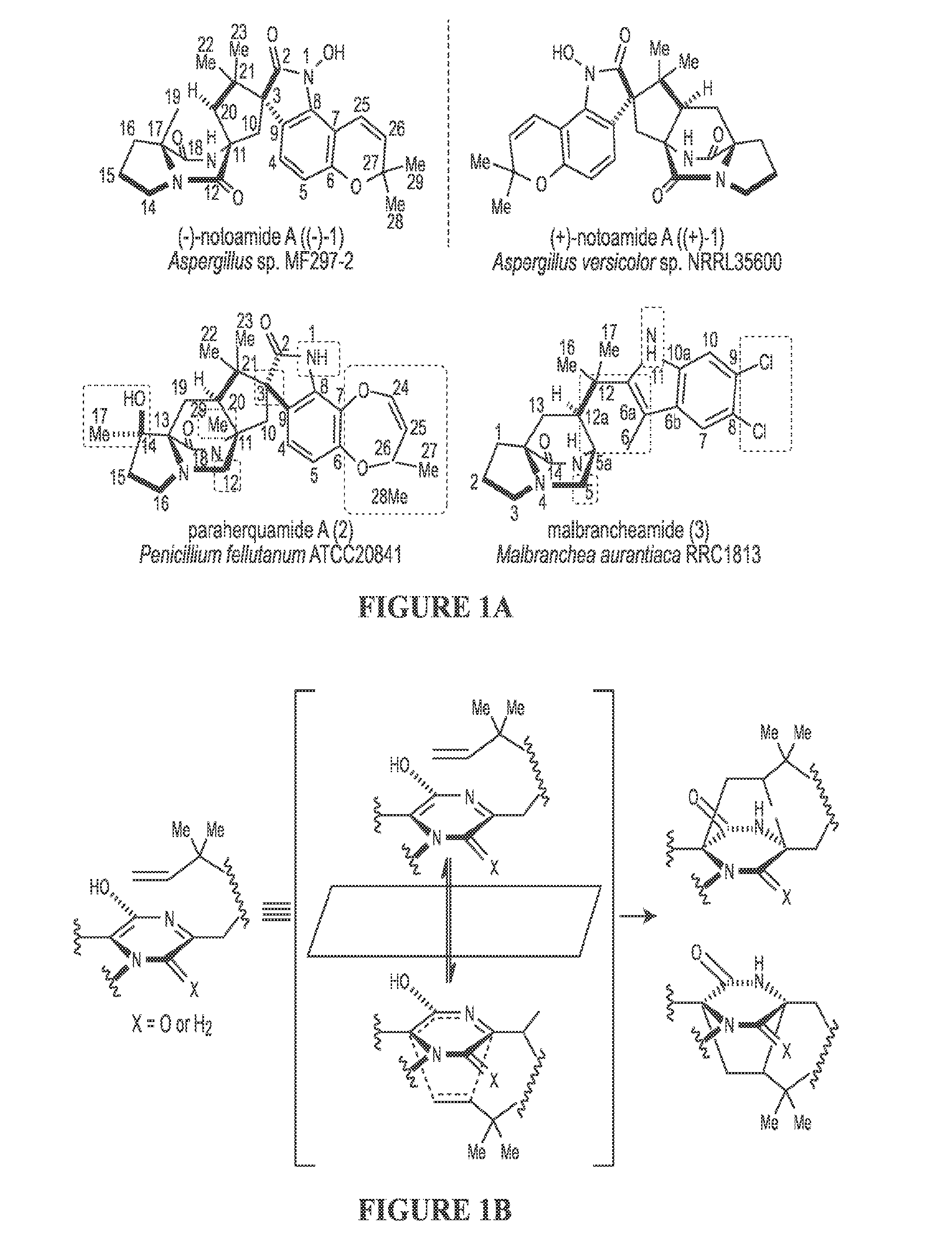
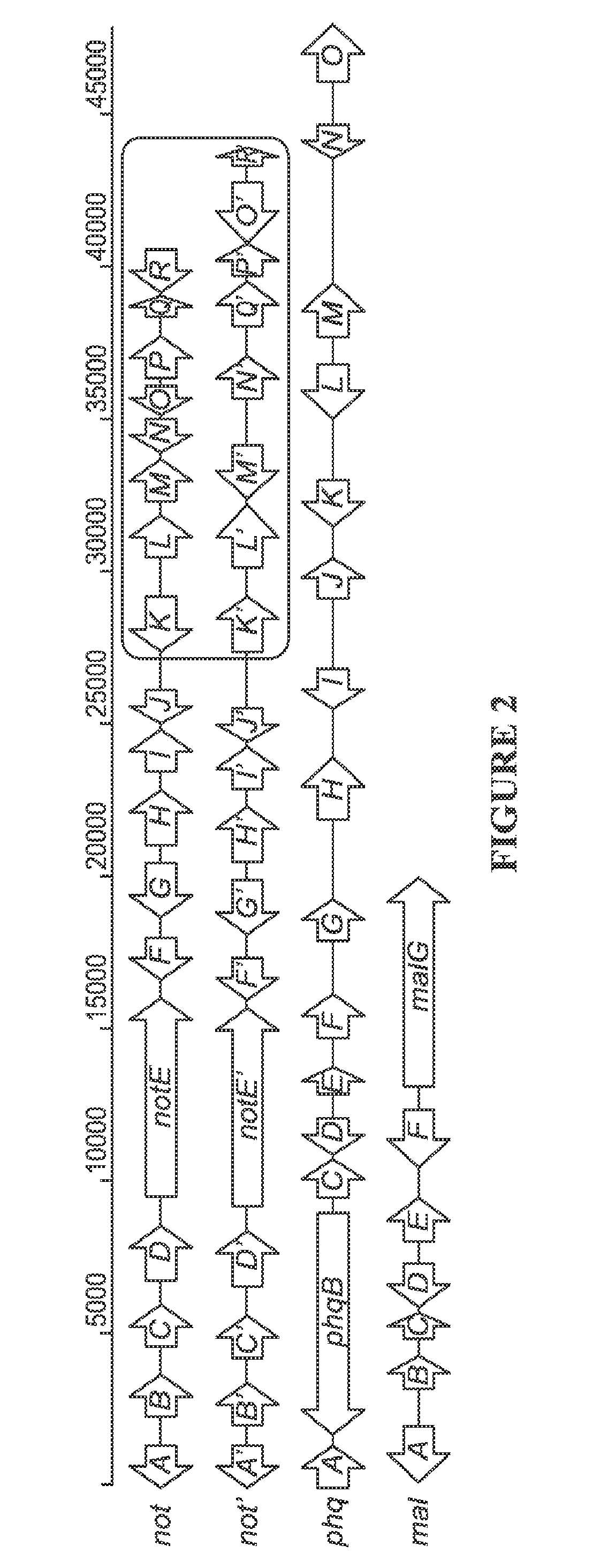
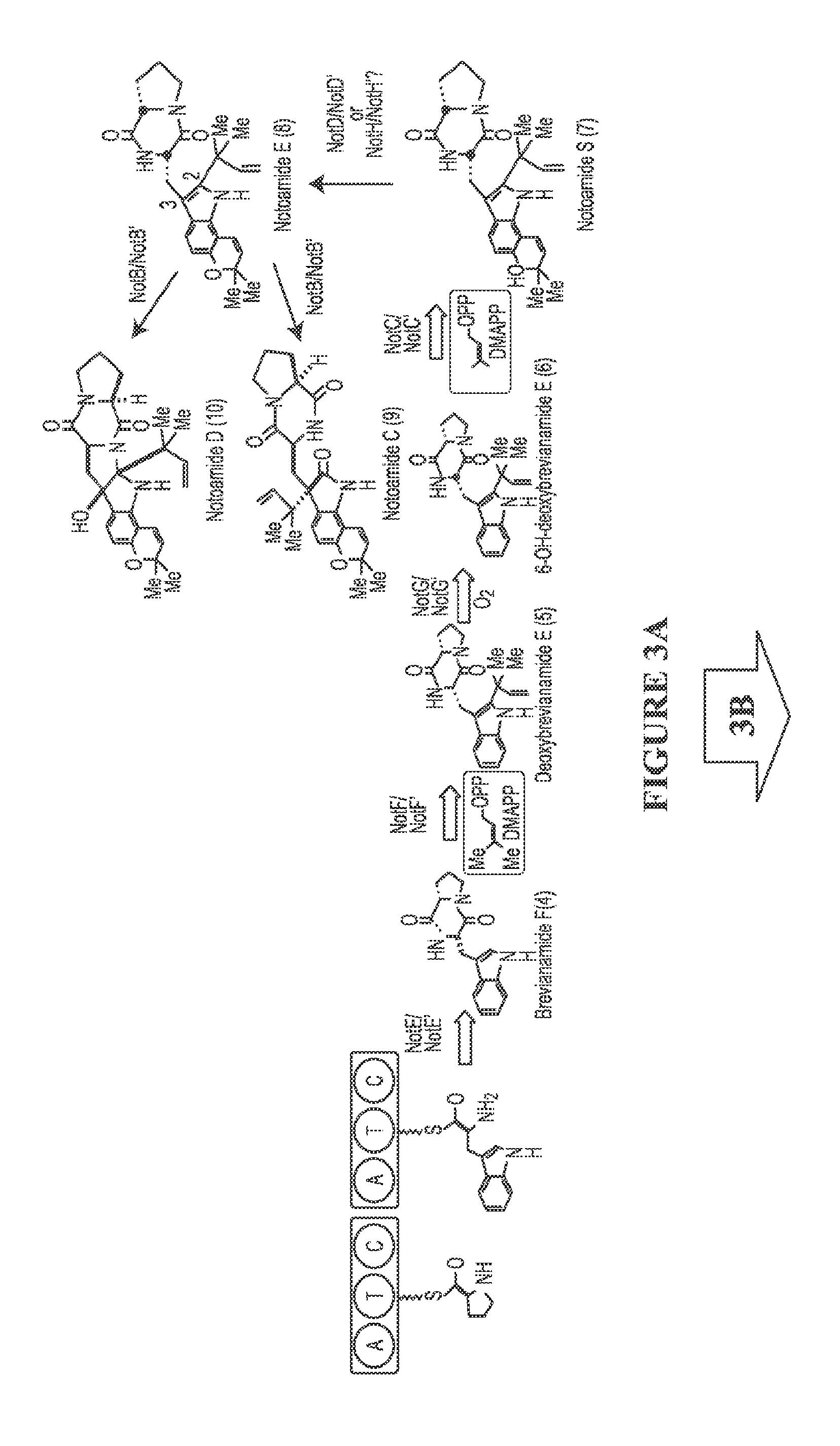
The biosynthesis of fungal bicyclo[2.2.2]diazaoctane indole alkaloids with a wide spectrum of biological activities have attracted increasing interest. Their intriguing mode of assembly has long been proposed to feature a non-ribosomal peptide synthetase, a presumed intramolecular Diels-Alderase, a variant number of prenyltransferases, and a series of oxidases responsible for the diverse tailoring modifications of their cyclodipeptide-based structural core. Until recently, the details of these biosynthetic pathways have remained largely unknown due to lack of information on the fungal derived biosynthetic gene clusters. Herein, we report a comparative analysis of four natural product metabolic systems of a select group of bicyclo[2.2.2]diazaoctane indole alkaloids including (+) / (−)-notoamide, paraherquamide and malbrancheamide, in which we propose an enzyme for each step in the biosynthetic pathway based on deep annotation and on-going biochemical studies.
Owner:COLORADO STATE UNIVERSITY +1
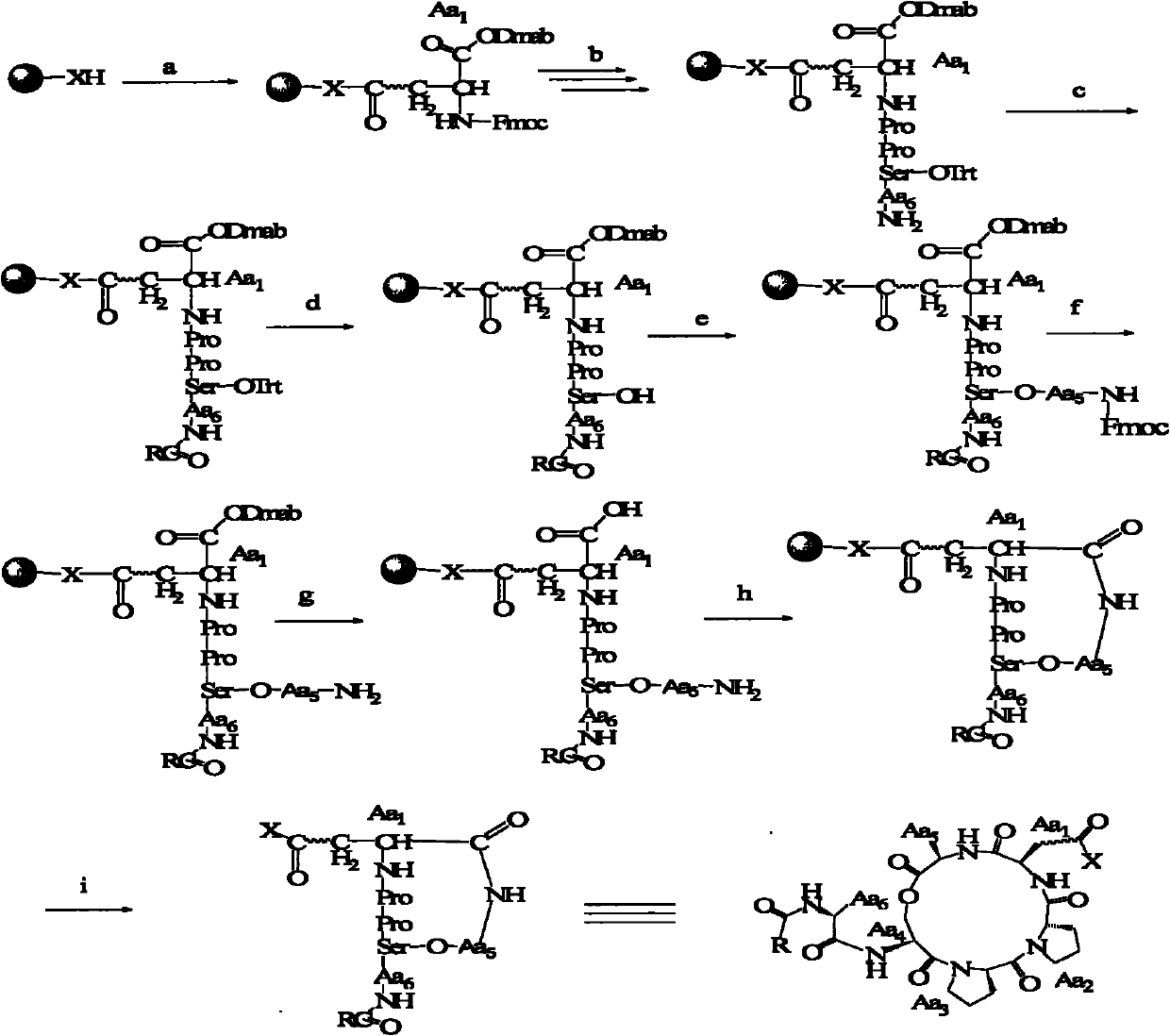
Method for preparing fatty acyl cyclic depsipeptide compounds by solid-phase synthesis
InactiveCN102020703ASynthetic fitSimple methodPeptide preparation methodsN dimethylformamideSide chain
The invention relates to a method for preparing fatty acyl cyclic depsipeptide compounds by solid-phase synthesis, which comprises the following steps of: adding amino resin from which fluorenl methoxy carbonyl (Fmoc) is removed, Fmoc-Asp-Odmab, an active agent and straight-chain polypeptide serving as a shrinking agent into a reactor; adding a detritylation reagent into the reactor to immerse a reactant, performing a reaction, and connecting side chain amino acid by an active ester method to obtain a protected cyclization precursor compound; adding a fluorenl methoxy carbonyl removing reagent into the resin for reacting, and washing the resin by using N,N-dimethylformamide; and adding a cyclization agent into the resin to obtain cyclic decapeptide products. By the method, the compounds and analogues thereof are synthesized successfully. The method is simple and high in synthesis yield, and is suitable for synthesizing cyclic depsipeptide with the similar structure.
Owner:NORTHWESTERN POLYTECHNICAL UNIV
Extractive purification of lipopeptide antibiotics
The present invention provides a rapid and inexpensive method for extractively isolating acidic lipopeptide antibiotics, such as those having a cyclic peptide or cyclic depsipeptide core, in high yield and purity. In particular, there is provided a method of extracting a variety of acidic lipopeptide antibiotics, directly or indirectly, into water immiscible organic solvents by using a divalent cation chelation procedure.
Owner:BIOSOURCE PHARM INC
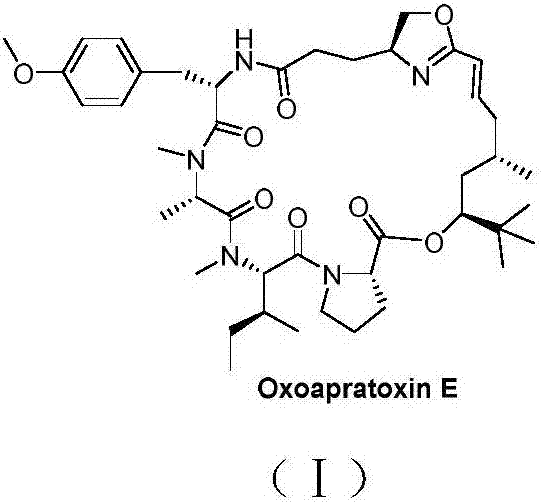
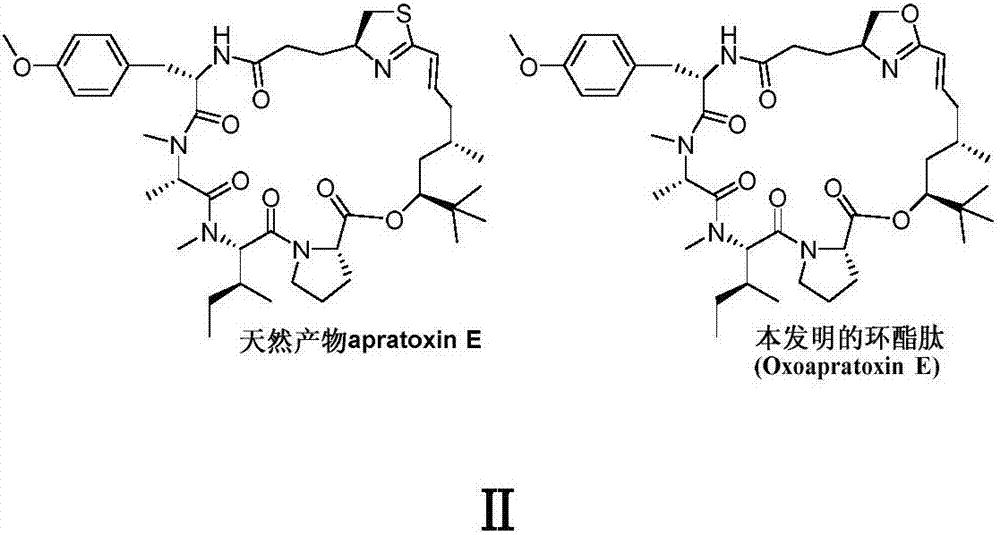
Cyclic depsipeptide as well as preparation method and application thereof
InactiveCN107344957AGood antitumor activityOvercome stabilityPeptide/protein ingredientsPeptide preparation methodsAntitumor activityReagent
The invention belongs to the field of medical chemistry and relates to cyclic depsipeptide Oxoapratoxin E with a structure shown as a formula (I) as well as a preparation method and application thereof. When the cyclic depsipeptide is prepared, a cyclized precursor is synthesized first; after N-end and C-end protective groups are removed, a large ring is closed; then a dehydration reagent is utilized to form the cyclic depsipeptide of the invention. The cyclic depsipeptide provided by the invention is subjected to in-vitro anti-tumor activity screening experiments and a result shows that the cyclic depsipeptide has relatively strong anti-tumor activity and has potential application value in the field of medicines, especially research and development of anti-tumor medicines. (The formula (I) is shown in the description.).
Owner:FUDAN UNIV
Anthelmintic depsipeptide compounds
The present invention provides cyclic depsipeptide compounds of formula (I) and compositions comprising the compounds that are effective against parasites that harm animals, including humans. The compounds and compositions may be used for combating parasites in or on animals including mammals and birds. The invention also provides for an improved method for eradicating, controlling and preventing parasite infestation in animals, including birds and mammals.
Owner:BOEHRINGER INGELHEIM ANIMAL HEALTH USA INC
Cyclic depsipeptide compounds and their uses
InactiveUS20150259386A1Reduced immunosuppressive activityHigh affinityAntimycoticsNervous disorderDiseaseSolubility
The present invention relates to novel cycloundecadepsipeptide compounds and their analogues which bind and inhibit cyclophilins, have reduced immunosuppressive activity and improved physicochemical properties including water solubility. The present invention further relates to pharmaceutical compositions containing said depsipeptide compounds and their analogues for use in the treatment or prevention of diseases and pathologies which may be ameliorated by the inhibition of cyclophilin activity.
Owner:CYPRALIS
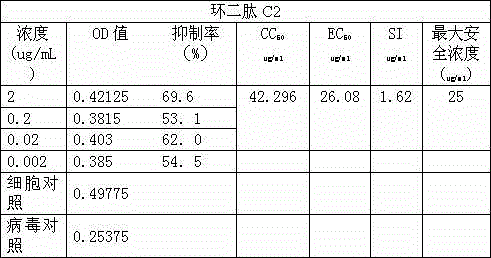
Application of cyclic dipeptide C2 in phellinus igniarius to resisting H5N1 avian influenza virus
The invention discloses a cyclic dipeptide C2 with an avian influenza-resisting activity, which is separated from fermentation broth and fruiting bodies of phellinus igniarius (LexFr) Quel (phellinus linteus (BerketCurt) Teng, phellinus baumii and phellinus hartigii (AlleschetSchnabl) Imaz). The invention firstly discloses a new activity of the cyclic dipeptide and proves that the cyclic dipeptide has an avian influenza-resisting action. Compared with the application of a general compound, the method has the advantages that the special new activity of the cyclic dipeptide is found, and the cyclic dipeptide has the action of resisting the avian influenza virus.
Owner:QINGDAO AGRI UNIV
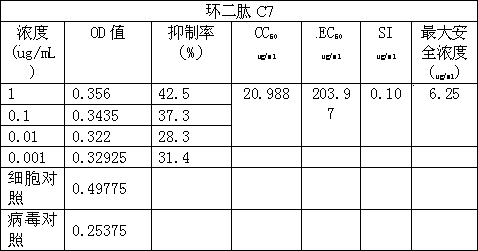
Application of cyclic dipeptide C7 in Phellinus igniarius in resisting avian influenza H5N1 virus
The invention discloses a cyclic dipeptide C7 with anti-avian influenza activity separated from Phellinus igniarius (LexFr) Quel (phellinus linteus (BerketCurt) Teng, Phellinus baumii, Phellinus hartigii (AlleschetSchnabl) Imaz) fermentation liquid and sporophores. The invention discloses new activity of cyclic dipeptide. The cyclic dipeptide is proved to have an anti-avian influenza action. Compared with the application of common compounds, the method detects the new activity of the cyclic dipeptide, and the cyclic dipeptide has the anti-avian influenza action.
Owner:QINGDAO AGRI UNIV
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com