Synthesis method of cyclic dipeptide containing glutamine and asparagine
A technology of glutamine and asparagine, which is applied in the field of cyclic dipeptide synthesis, can solve problems such as inability to cyclize sequences, surplus raw materials, and affect cyclization efficiency, so as to avoid raw material residues, increase the concentration of reactants, and avoid organic The effect of solvent use
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
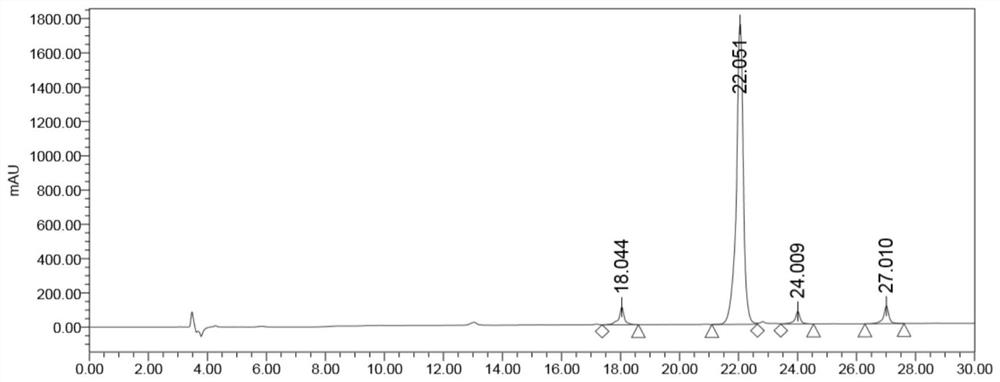
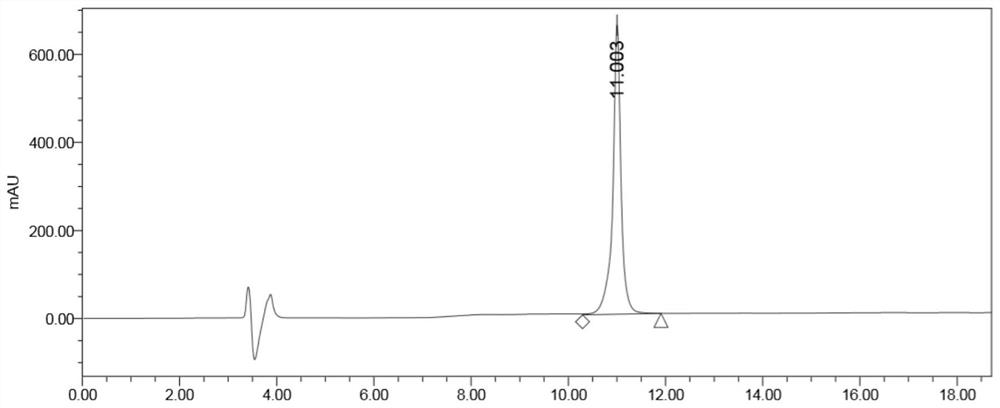
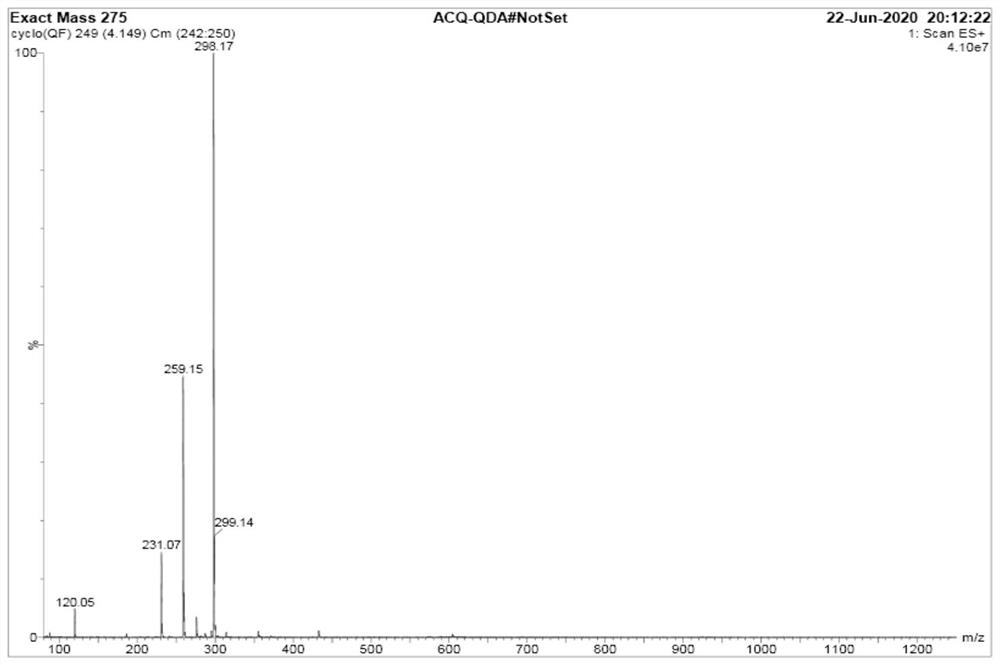
Image
Examples
Embodiment 1
[0044] Synthesis of glutamine-phenylalanine cyclic dipeptide
[0045] 1. Synthesis of trifluoroacetate of H-Glu-Phe-OH
[0046] Weigh 10g of 2-Chlorotrityl Chloride Resin (degree of substitution 1.6mmol / g) and swell in dichloromethane for 10 minutes, then add 6.82g (17.6mmol) of Fmoc-Phe-OH, 10.6mL (64mmol) of N,N′-di Isopropylethylamine, react at room temperature for 2 hours, then add anhydrous methanol to seal for 30 minutes, filter with suction, wash the filter cake with 50mL isopropanol, 50mL N,N-dimethylformamide in turn, and wash with 60mL volume concentration of 20 % piperidine in N,N-dimethylformamide solution to remove the Fmoc protecting group for 30 minutes, and then suction-filtered, the filter cake was washed twice with isopropanol and N,N-dimethylformamide successively, each 50mL each time, the resin is positive and black. Add the washed filter cake to 60mL N,N-dimethylformamide, and add 13.6g (32mmol) Fmoc-Glu(OtBu)-OH, 6.5g (48mmol) 1-hydroxybenzotriazole, 7....
Embodiment 2
[0055] Synthesis of glutamine-tyrosine cyclic dipeptide
[0056] In step 1 of this example, 8.1g (17.6mmol) of Fmoc-Tyr(tBu)-OH was added, and the other steps were the same as step 1 of Example 1, and solid-phase synthesis obtained 6.5g of H-Glu-Tyr-OH trifluoro Acetate.
[0057] In step 2 of this example, 6.5 g (15.3 mmol) of trifluoroacetic acid salt of H-Glu-Tyr-OH was added to 100 mL of methanol, and 3.2 mL (45.9 mmol) of di Thionyl chloride, other steps are the same as step 2 of Example 1, through methyl esterification, to obtain the aqueous solution of H-Glu(OMe)-Tyr-OMe hydrochloride.
[0058] In step 3 of this example, add sodium bicarbonate to the collected water phase, adjust pH=8, react at room temperature for 6 hours, the product is precipitated from water, filtered to obtain Cyclo[Glu(OMe)-Tyr] as a white solid, and dried 3.9g was obtained.
[0059] In step 4 of this example, 3.9 g (12.7 mmol) of Cyclo[Glu(OMe)-Tyr] was dissolved in 100 mL of 0.4 mol / L ammonia ...
Embodiment 3
[0061] Synthesis of glutamine-tryptophan cyclic dipeptide
[0062] In step 1 of this example, 7.5g (17.6mmol) Fmoc-Trp-OH was added, and other steps were the same as in step 1 of Example 1, and solid-phase synthesis obtained 6.9g of trifluoroacetic acid salt of H-Glu-Trp-OH .
[0063] In step 2 of this example, 6.9 g (15.8 mmol) of trifluoroacetic acid salt of H-Glu-Tyr-OH was added to 100 mL of methanol, and 2.3 mL (31.7 mmol) of di Thionyl chloride, other steps are the same as step 2 of Example 1, through methyl esterification, to obtain the aqueous solution of H-Glu(OMe)-Trp-OMe hydrochloride.
[0064] In step 3 of this embodiment, sodium bicarbonate was added to the collected water phase, adjusted to pH=8, reacted at room temperature for 8 hours, water was removed under reduced pressure, methanol was added to filter to remove inorganic salts, and the filtrate was concentrated to obtain 4.2 g of Cyclo[ Glu(OMe)-Trp].
[0065] In step 4 of this example, 4.2 g (12.8 mmol) ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com