Method for efficiently preparing cyclic depsipeptide, cyclic depsipeptide and application
A technology of cyclic depsipeptides and compounds, which is applied in the field of efficient preparation of cyclic depsipeptides, and can solve the problems of low yield of cyclic depsipeptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
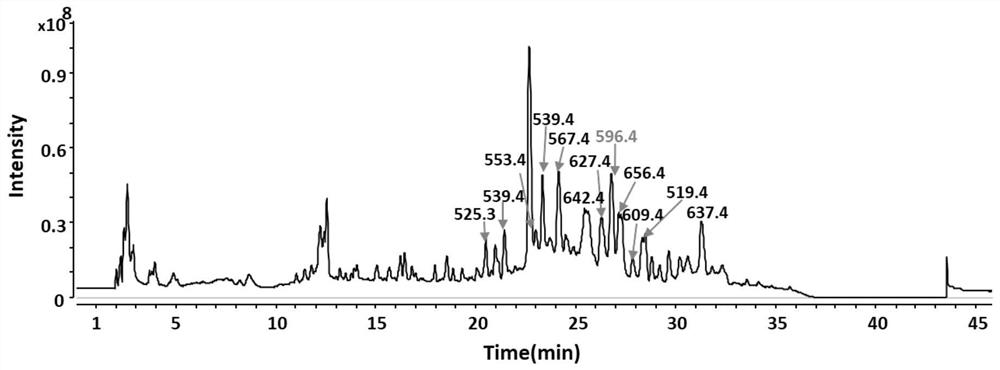
[0053] Example 1, Liquid Chromatography-Mass Spectrometry (LC-MS) Preliminary Analysis of Bat Feces Parasitic Fungus Amphichordaguana LC5815 Solid Fermentation
[0054] The bat feces parasitic fungus A.guana was inoculated on glucose agar medium (PDA) for strain activation, and the culture condition was 28°C for 5 to 7 days. Collect the spores of the activated bacterial strain with 0.1% (w / v) Tween-80, inoculate an appropriate amount of seed liquid to carry out solid fermentation, the medium used in the solid fermentation is composed of 20g rice and 30mL water, the culture conditions of the solid fermentation Incubate at 28°C for 7 days in the dark. For convenience of description, the culture medium composed of 30 mL of water per 20 g of rice can be called rice culture medium. The above experiments were repeated 3 times. Extract the fermentation product with ethyl acetate (specifically, extract three times with 7.5L ethyl acetate per 1kg of fermentation medium. After the fer...
Embodiment 2
[0060] Embodiment 2, separation and purification of active cyclic depsipeptide components
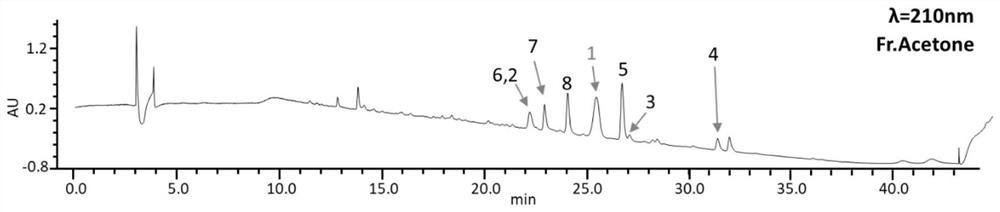
[0061] The preliminary analysis of the rice fermentation product of A.guana LC5815 by LC-MS in Example 1 has shown that it can produce abundant large molecular weight compounds. The bacterium was cultured in 20kg of rice culture medium (obtained by mixing 20kg of rice and 30L of water) for large-scale fermentation, and kept in the dark at 28°C for 30 days to obtain a rice fermentation culture. Ethyl acetate was added to the rice fermentation culture for extraction 3 times, soaked overnight each time to obtain an extract. Specifically, 7.5 L of ethyl acetate was used for extraction per 1 kg of rice, and ethyl acetate was directly added after the cultivation was completed. In the specific experiment, because the 250mL triangular flask used, each bottle of 20g rice, each bottle was extracted with 50mL ethyl acetate, repeated 3 times, a total of 150mL ethyl acetate was needed.
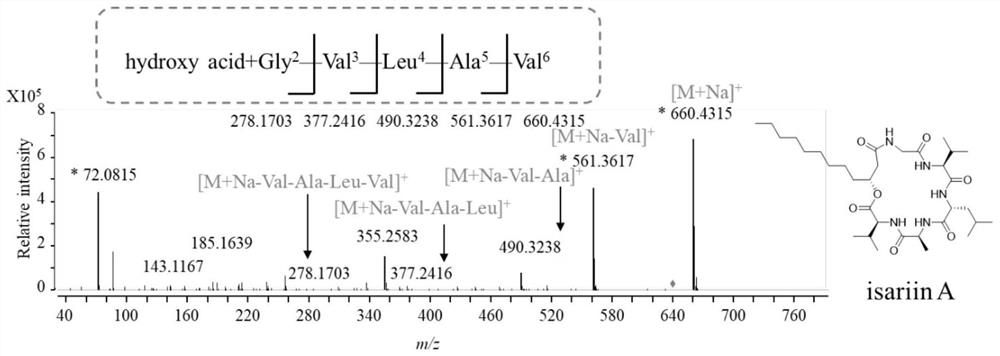
[0062] Th...
Embodiment 3
[0066] Example 3, verify the biological activity of the cyclic depsipeptide prepared in Example 2
[0067] 3.1, verify the anti-phytopathogenic fungal activity
[0068] Use a 90mm petri dish containing 10mL of PDA medium to measure its antifungal activity. Use the following three plant pathogenic fungi, Alternaria solani, Botrytis cinerea, and Fusarium oxysporum as indicator bacteria, and use PDA medium to cultivate plant pathogenic fungi. When the diameter grows to about 2cm Finally, a sterile blank filter paper sheet of the same size (diameter 0.625 cm) was placed 1 cm away from the edge of the mycelial colony. Distribution Dissolve the isaridin H, desmethylisaridin E, and isaridin E prepared in Example 2 with dimethyl sulfoxide (DMSO) to obtain samples with a final concentration of 1 mg / mL, respectively, and add 10 uL to the filter paper. in, Figure 6 Add isaridin H to sheet 1, add desmethylisaridin E to sheet 2, and isaridin E to sheet 3. Place the petri dish at 28°C a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com