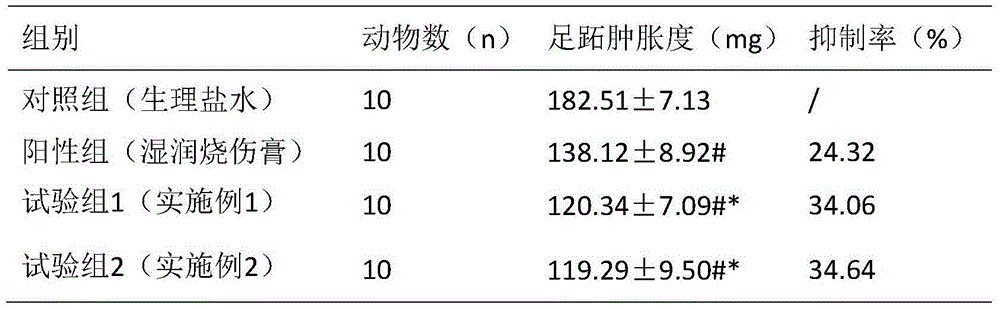
Cyclic dipeptide used for wound healing
A wound repairing and cyclic dipeptide technology, which is applied in the biomedical field, can solve problems such as the application of cyclic dipeptide not seen before, and achieve the effect of good wound repairing effect, obvious treatment effect and shallow scar.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1 The preparation method of the cyclic dipeptide (cyclopropyl-glycodipeptide) described in the present invention
[0016] Main pharmaceutical reagents: glycine, alanine protected by Boc amino group
[0017] Process for preparing cyclic ala-gly dipeptide:
[0018] (1) Preparation of glycine methyl ester hydrochloride: Add 10 g of glycine to a mixture of an appropriate amount of methanol and thionyl chloride, and stir at room temperature for 20 h. The completion of the reaction was tracked by TLC, and the solvent was spin-dried under reduced pressure to obtain 13.91 g of glycine methyl ester hydrochloride.
[0019] (2) Neutralization and condensation reaction: Add glycine methyl ester hydrochloride obtained in step (1), 20 g of alanine protected by Boc amino group, 20 ml of pyridine, DMAP (4-dimethylaminopyridine) in 100 ml of anhydrous tetrahydrofuran ), after all mixing, under the protection of argon, add the (Boc) of anhydrous tetrahydrofuran 2 O, stirred...
Embodiment 2
[0022] Embodiment 2 The preparation method of the cyclic dipeptide (cyclopropan-leupeptide) described in the present invention
[0023] Main pharmaceutical reagents: leucine, alanine protected by Boc amino group
[0024] Process for preparing cyclic ala-leu dipeptide:
[0025] (1) Preparation of leucine methyl ester hydrochloride: add 10 g of leucine to a mixture of an appropriate amount of methanol and thionyl chloride, and stir at room temperature for 24 hours. TLC traced the completion of the reaction, and the solvent was spin-dried under reduced pressure to obtain 14.34 g of leucine methyl ester hydrochloride.
[0026] (2) Neutralization and condensation reaction: add leucine methyl ester hydrochloride obtained in step (1), 20 g of alanine protected by Boc amino group, 20 ml of pyridine, DMAP (4-dimethyl aminopyridine), after all mixing, add anhydrous tetrahydrofuran (Boc) under the protection of argon 2 O, stirred at room temperature for 12 hours. The completion of th...
Embodiment 3
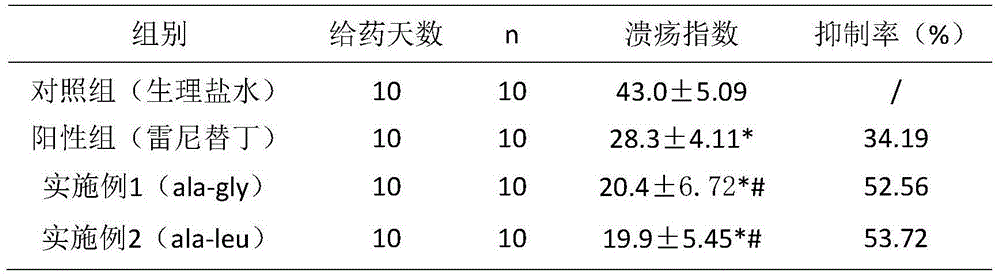
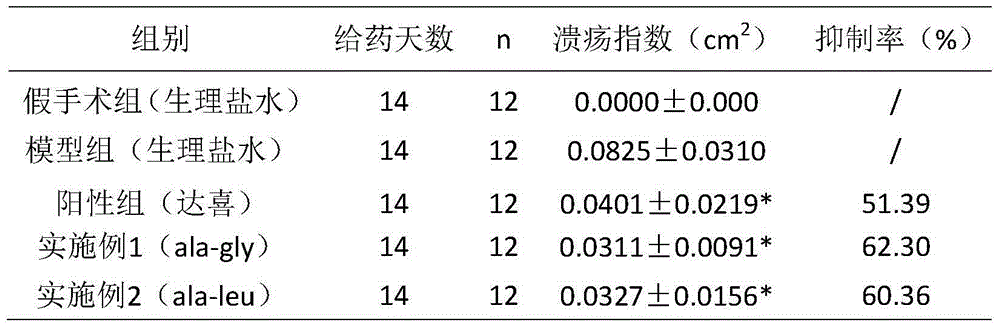
[0030] Example 3 Application of the cyclic dipeptide of the present invention in ulcers
[0031] 1. Experimental materials
[0032] 1.1 Samples: Samples prepared according to Example 1-2: Cyclopropane-Glycine dipeptide (ala-gly), Cyclopropane-Leu dipeptide (ala-leu)
[0033] 1.2 Experimental animals: KM mice, 18-22 g, 120, half male and half male. SD rats, 200-220g, 180, half male and half male.
[0034] 1.3 Experimental reagents: ranitidine hydrochloride capsules, Daxi (aluminum magnesium carbonate tablets)
[0035] 2. Method for evaluating the protective function of samples on gastric ulcer mucosa
[0036] 2.1 Absolute ethanol-induced gastric mucosal injury model test in mice
[0037] 40 KM mice were randomly divided into groups according to sex and body weight (see Table 1 for details), and each group was given the corresponding drug. The mice in each group were given the drug for 10 consecutive days. After 3 hours of the last administration of the mice in each group, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com