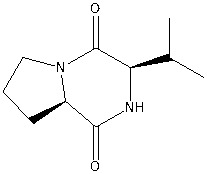
Cyclic dipeptide and application of cyclic dipeptide in preparation of bactericide
A technology of cyclic dipeptide and bactericide, applied in the field of cyclic dipeptide and its application in the preparation of bactericide, to achieve the effect of easy degradation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0019] Embodiment 2 loop (L-proline-L-valine) inhibits pathogenic bacteria growth activity assay
[0020] The test bacteria with consistent activity were inoculated into PDA plates containing 200ppm, 100ppm, 50ppm, 25ppm, and 12.5ppm ring (L-proline-L-valine) respectively.
[0021] Three replicates were set up for each group of experiments, and three blank controls were set up.
[0022] Cultivate at 25°C until the blank control colonies cover two-thirds of the petri dish, then measure the experimental results.
[0023] Table 1 shows the results of the determination of the growth inhibitory activity of cyclic dipeptide (L-proline-L-valine) on pathogenic bacteria.
[0024] Table 1
[0025] strain
Embodiment 3
[0026] The control of embodiment 3 cucumber target spot disease
[0027] Spray once in the early stage of the disease, and 3 parallel treatment plots, each with an area of 20 square meters. The incidence was investigated before and 10 days after the application, and 5 points were randomly selected in each plot for sampling, and the percentage of the lesion area on each leaf of the whole plant to the leaf area was investigated, and the disease index and control effect were calculated.
[0028] The soluble reagent was formulated according to the following proportions: cyclic (L-proline-L-valine) 15%, lignosulfonic acid 25%, and dibutyl phthalate 60%.
[0029] The preparation made according to the above ratio, diluted 100 times and sprayed, can reach 83% of the control effect of cucumber target spot disease; do contrast spraying with 30% cyclic (L-proline-L-valine) , its control efficiency can only reach 46%.
Embodiment 4
[0030] Embodiment 4 rice flax leaf spot
[0031] Spray once in the early stage of the disease, and 3 parallel treatment plots, each with an area of 20 square meters. The incidence was investigated before and 10 days after the application, and 5 points were randomly selected in each plot for sampling, and the percentage of the lesion area on each leaf of the whole plant to the leaf area was investigated, and the disease index and control effect were calculated.
[0032] Prepare the soluble reagent according to the following proportions: cyclic (L-proline-L-valine) 10%, niclosamide 10%, quality sulfonic acid 20%, and dibutyl phthalate 60%.
[0033] The preparation made according to the above ratio, diluted 100 times and sprayed, can reach 75% to the control effect of flax leaf spot of rice; implementation, its control efficiency can only reach 37%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com