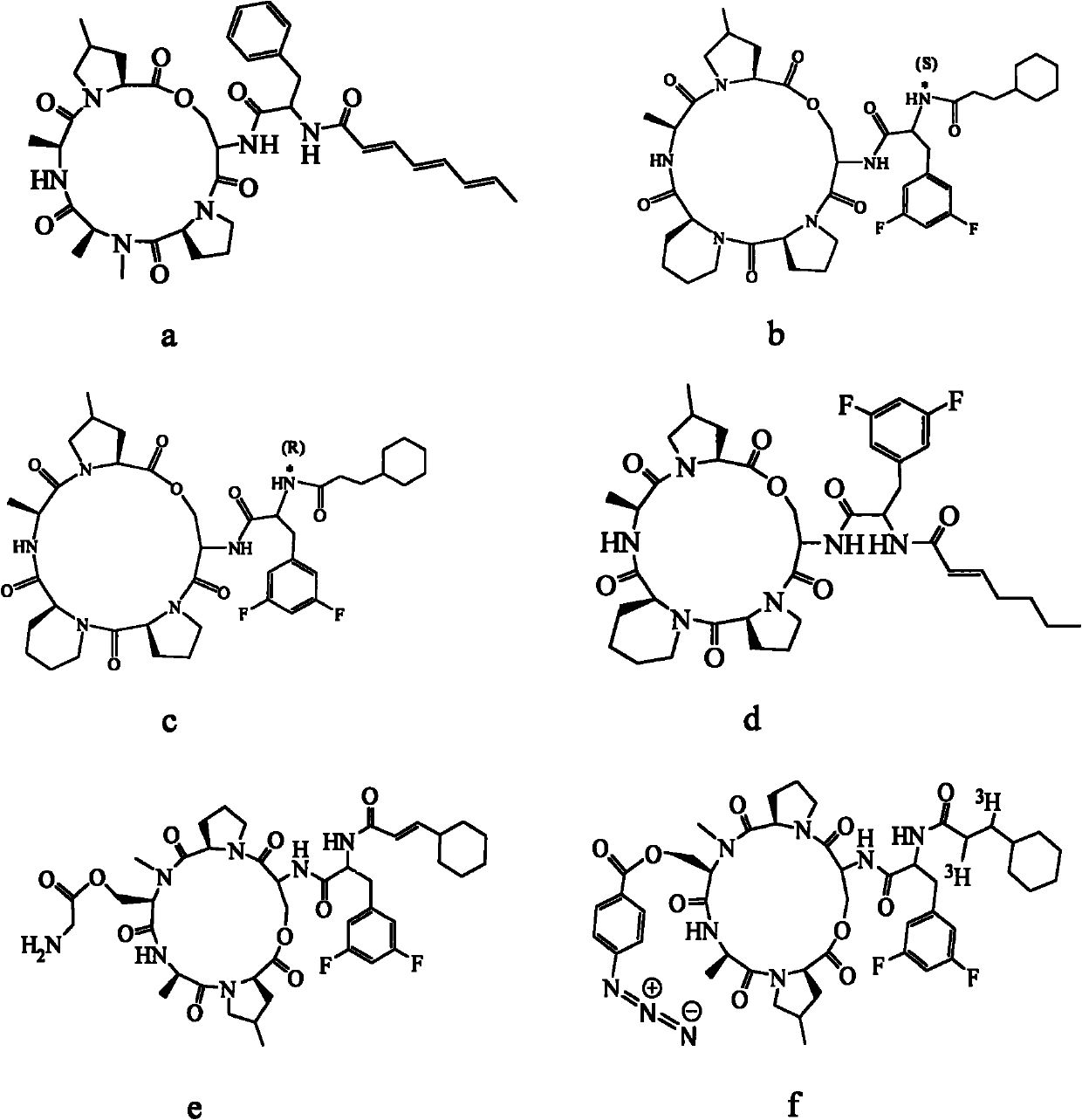
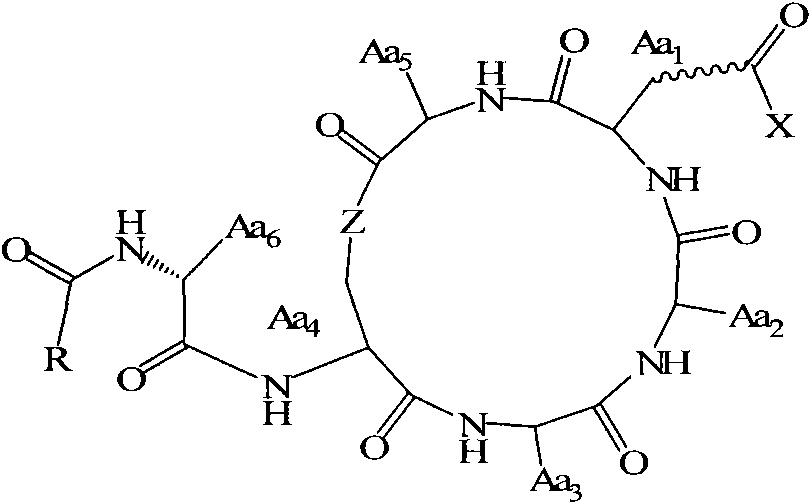
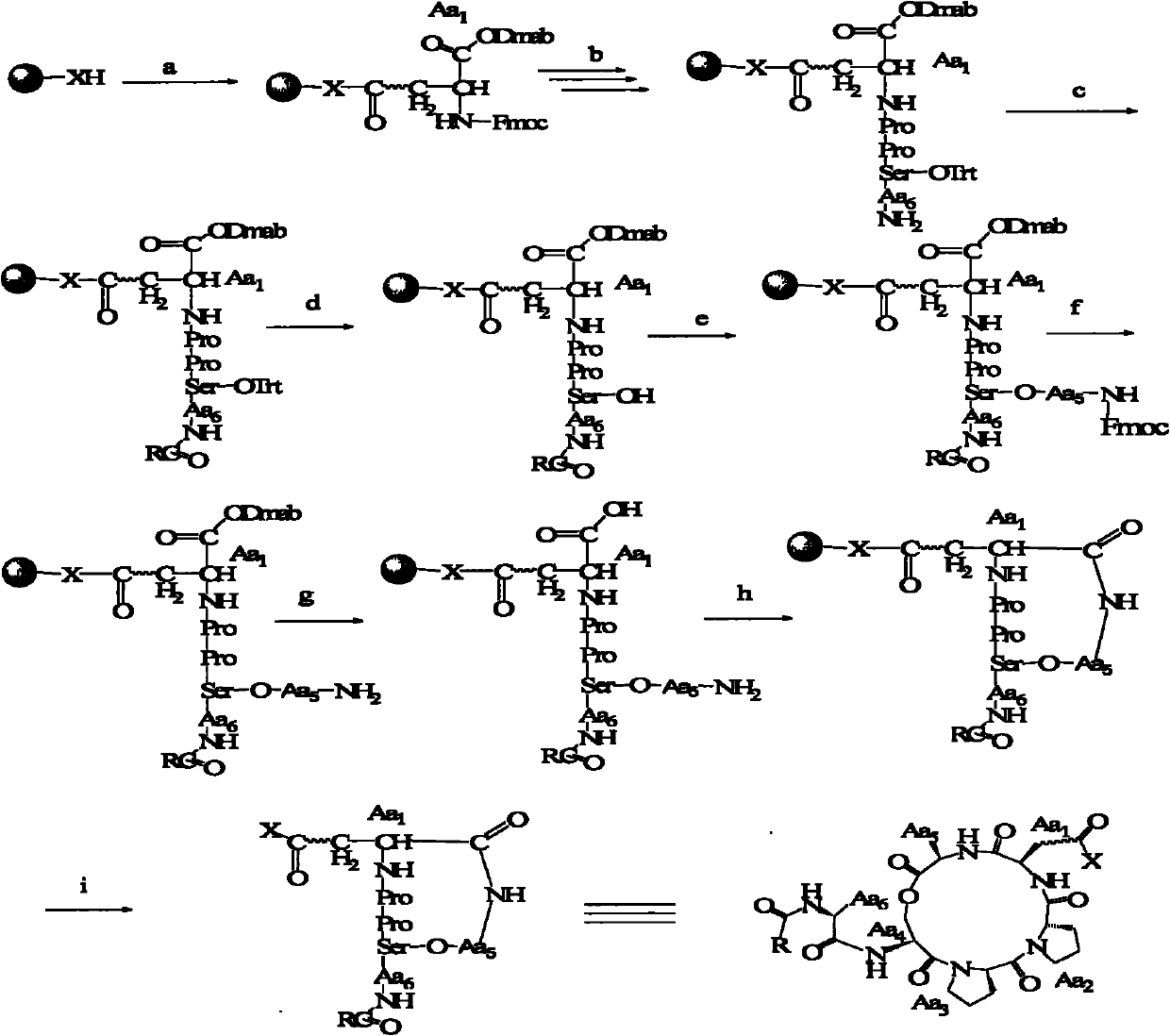
Method for preparing fatty acyl cyclic depsipeptide compounds by solid-phase synthesis
The technology of a fatty acyl cyclic ester and a synthesis method is applied in the field of solid-phase synthesis to prepare fatty acyl cyclic ester peptide compounds, which can solve the problems of incomplete esterification of fatty acyl cyclic ester peptides, difficult cyclization and the like, and achieves high synthetic yield, simple method effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] 1.1 Solid-phase synthesis of linear part of fatty acylcyclic ester peptide
[0072] Weigh 50mg of Wang resin (0.2-0.3mmol / g) onto the empty Bio-Spin column, add dichloromethane, fully swell the resin for 0.5-1.0 hours, drain and wash twice with DMF. Take 112mg of Fmoc-Asp-ODmab and 24mg of HOBT in a 1.5ml centrifuge tube, add 1ml of DMF, put it on a rotary mixer and rotate it to dissolve for 10 minutes, then add the pre-reacted solution into the resin, and finally add 27ul DIC to react for 3 hours , washed with DMF for more than three times, drained, added 1ml of 20% pyridine in DMF, reacted twice for 15 minutes, and washed with DMF for more than three times. At this point, the first amino acid, Asp, has been attached to the resin.
[0073] In the same way, Fmoc-Pro-OH, FmocPro-OH, Fmoc-Ser(Trt)-OH, Fmoc-Arg(Pbf)-OH are connected in turn in the same way. After the protection is removed, the reaction conditions agreed to connect the fatty acid RCOOH. After the connectio...
Embodiment 2
[0080] Example 2 attached image 3 The reaction conditions:
[0081] R is a fatty acid, X=O (king resin) or NH (amino resin); a. HOBT / DIC, 3h; b. 20% piperidine (DMF, 15min), 2 times; HOBT / DIC, 2h; repeated connection; c. RCOOH / HOBT / DIC, 3h; d. 1% TFA, 5% TIS (DCM), 3min, 3 times; e. HOBT, DMAP, DCC; f. 20% piperidine (DMF), 15min, 2 times ;g.2%N 2 h 4 (DMF), 2min, 3 times; h.PYBOP / HOBT / DIEA / NMP, 48h; i.TFA / TIS / phenol / H 2 O(95 / 1 / 2 / 2), 3h.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com