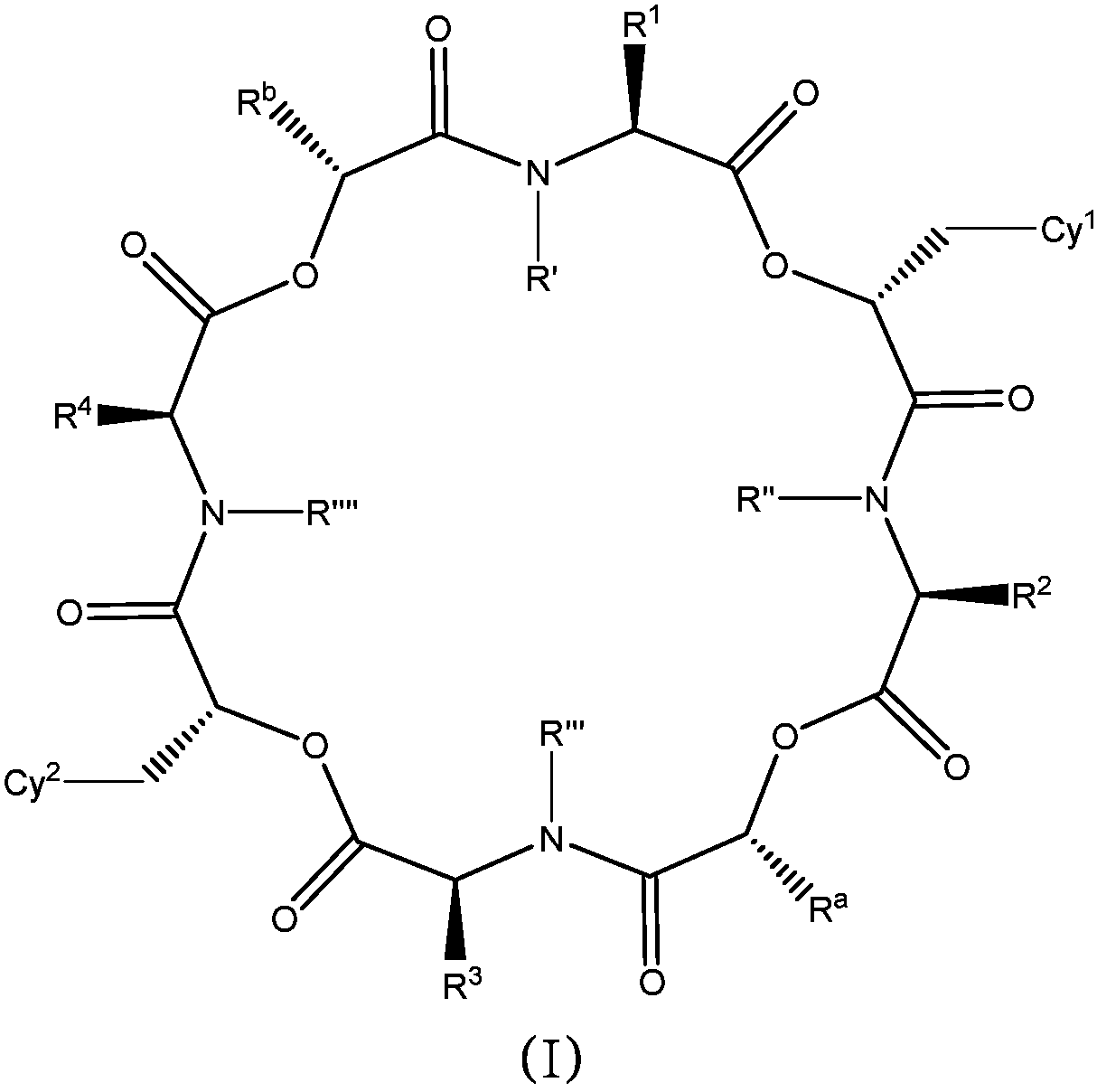
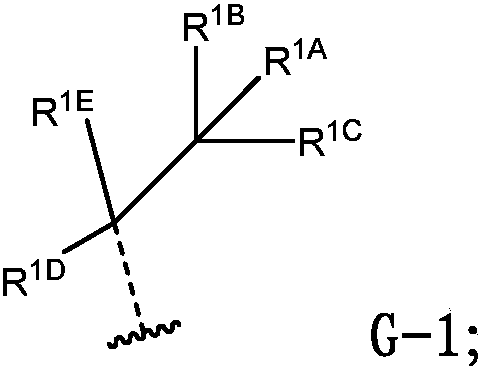
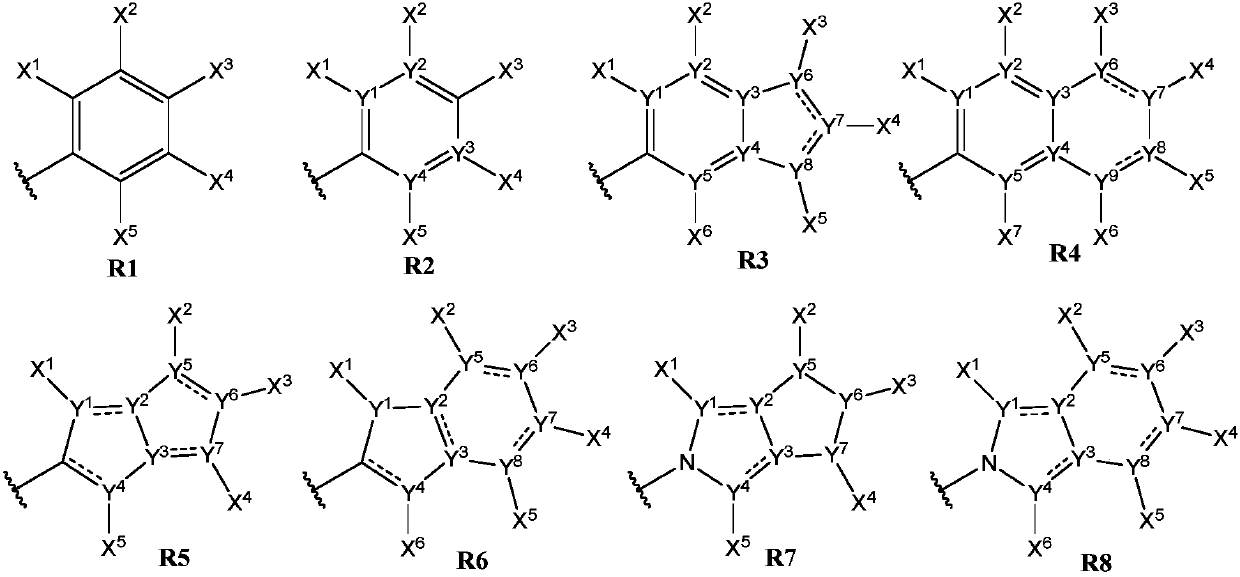
Anthelmintic depsipeptide compounds
一种缩酚酸肽、化合物的技术,应用在缩肽、肽、药物组合等方向,能够解决防寄生虫活性不太令人满意等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[1054] Preparation Example 1: Preparation of monomer M1.
[1055] The monomer M1 was prepared by the method shown in Scheme 2 below.
[1056] Scenario 2
[1057]
[1058] Experimental details
[1059]
[1060] (2S)-2-[[(tert-butoxy)carbonyl]amino]succinic acid 1-benzyl 4-methyl ester (butanedioate): To a 20-L 3-neck circle that is purged and maintained with an inert nitrogen atmosphere Put (2S)-2-[[(tert-butoxy)carbonyl]amino]-4-methoxy-4-oxobutanoic acid (150g, 606.69mmol, 1.00 equivalent) in the bottom flask in N, N- Solution in dimethylformamide (5L), Cs 2 CO 3 (396g, 1.22mol, 2.00 equivalents), BnBr (124g, 725.02mmol, 1.20 equivalents). The resulting solution was stirred at room temperature for 2 hours. The resulting solution was diluted with 10 L of EA. Use 3×5L of H 2 O wash. The resulting mixture was washed with 3×5 L brine. The mixture was dried over anhydrous sodium sulfate. The solid is filtered out. The resulting mixture was concentrated under vacuum. This gave 170...
preparation Embodiment 2
[1073] Preparation Example 2: Preparation of monomer M2.
[1074] The monomer M2 was prepared by the method shown in Scheme 3 below.
[1075] Scheme 3
[1076]
[1077] Experimental details
[1078]
[1079] (R)-2-Hydroxy-3-[4-(trifluoromethyl)phenyl]propionic acid: Put (R)-2-amino-3-[4 in a 500-mL 3-neck round bottom flask -(Trifluoromethyl)phenyl]propionic acid (20g, 85.77mmol, 1.00 equivalent), sulfuric acid (0.5M) (340mL). Next, add NaNO dropwise at 0°C 2 (35.5 g, 514.49 mmol, 6.00 equivalents) in water (80 mL) while stirring. The resulting solution was stirred for 1 hour at 0°C. The resulting solution was allowed to react, while stirring, overnight at room temperature. The solid was collected by filtration. This gave 17.5 g (87%) of (R)-2-hydroxy-3-[4-(trifluoromethyl)phenyl]propionic acid as a white solid. MS(ES,m / z):233(M-H); 1 H NMR (DMSO, 300MHz) δ: 7.63 (d, J = 3.9 Hz, 2H), 7.46 (d, J = 4.0 Hz, 2H), 4.21-4.17 (m, 1H), 3.09-3.03 (m, 1H) ,2.91-2.84(m,1H).
[1080]
[108...
preparation Embodiment 3
[1082] Preparation Example 3: Preparation of monomer M3.
[1083] Monomer M3 was prepared by the method shown in Scheme 4 below.
[1084] Scheme 4
[1085]
[1086] Experimental details
[1087]
[1088] (2R)-3-(4-Fluorophenyl)-2-hydroxypropionic acid: Put (2R)-2-amino-3-(4-fluorophenyl) into a 500-mL 4-neck round bottom flask Propionic acid (10 g, 54.59 mmol, 1.00 equivalent), sulfuric acid (218.6 mL, 2.00 equivalent). Next, add NaNO dropwise at 0°C 2 (23 g, 333.33 mmol, 6.00 equivalents) in water (15 mL) while stirring. The resulting solution was stirred at 5°C for 3 hours. The resulting solution was extracted with 3×30 mL of ethyl acetate, and the organic layers were combined. The resulting mixture was washed with 5×40 mL of sodium chloride. The mixture was dried over anhydrous sodium sulfate, and concentrated under vacuum. This gave 12 g (crude material) of (2R)-3-(4-fluorophenyl)-2-hydroxypropionic acid as a white solid. MS (ES, m / z): 183 (M-H).
[1089]
[1090] (2R)-3-(4-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com