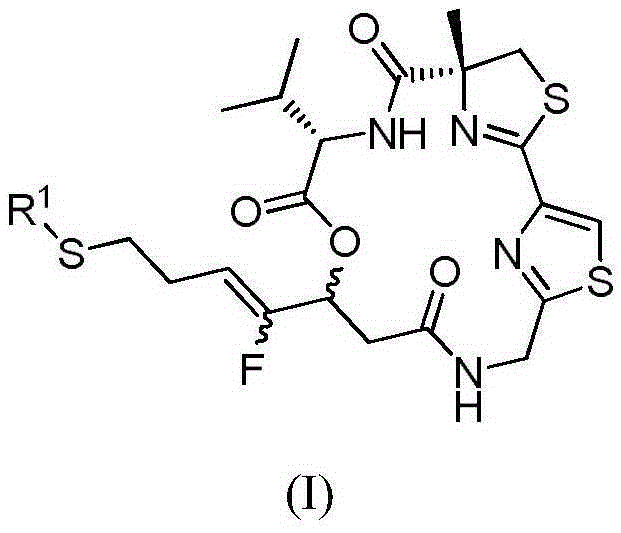
Fluorinated olefin analogue of marine natural product cyclic depsipeptide as well as preparation method and application of fluorinated olefin analogue
A kind of technology of fluorinated olefin and compound, applied in the field of antitumor therapeutic agent
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 117
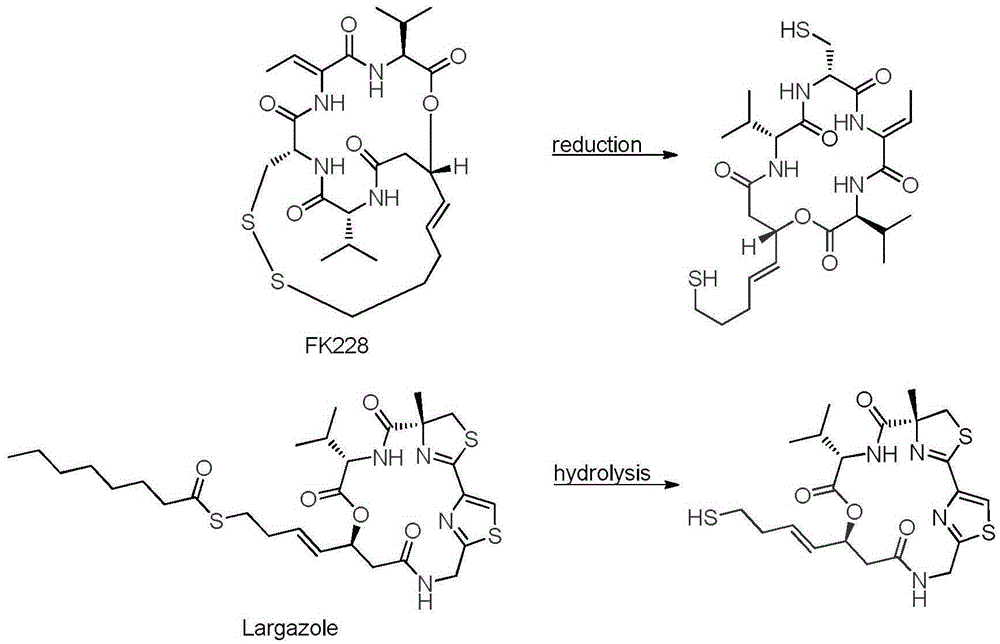
[0076] Example 1 Synthesis of Largazole Fluorinated Analogs in 17R-Z Configuration
[0077]
[0078] The first step prepares compound 2:
[0079]
[0080] Add the compound trityl mercaptan (1.28g, 4.62mmol) and 20ml of anhydrous dichloromethane into a 100ml dry reaction flask, stir and dissolve at room temperature, add triethylamine (0.9ml, 6.50mmol), and then add acrolein dropwise (0.43ml, 6.50mmol), stirred at room temperature for 1h (TLC tracking). The stirring was stopped, and the solvent was spin-dried to obtain a white crude product, which was directly used for the preparation of compound 2 without further purification. R f =0.13(PE:EA=40:1). 1 H-NMR (400MHz, CDCl3): δ9.56 (brs, 1H), 7.23–7.43 (m, 15H), 2.47 (t, J = 7.0Hz, 2H), 2.37 (t, J = 6.7Hz, 2H) .
[0081] The second step prepares compound 3:
[0082]
[0083] Add PPh to a 100ml dry reaction vial 3 (3.15g, 12.0mmol), ethyl dibromofluoroacetate (0.83ml, 6.0mmol) and 30ml of anhydrous THF were stirred...
Embodiment 2
[0110] Example 2 Synthesis of Largazole Fluorinated Analogs in 17S-Z Configuration
[0111]
[0112] Prepare and synthesize Largazole fluorinated analogues of 17S-Z configuration according to the method and experimental procedures of Example 1, wherein, in the fifth step, chiral alcohol S of corresponding configuration is prepared with the chiral prosthetic raw material of the opposite configuration -5, or the S-5 isomer separated and purified in the fifth step, other experimental methods are the same; the Largazole fluorinated analogue of the prepared 17S-Z configuration: HRMS-ESI(M / Z):[M+Na] + Calcd.for C 40 h 41 FN 4 o 4 S 3 Na:663.2115,found:663.2139.
Embodiment 3
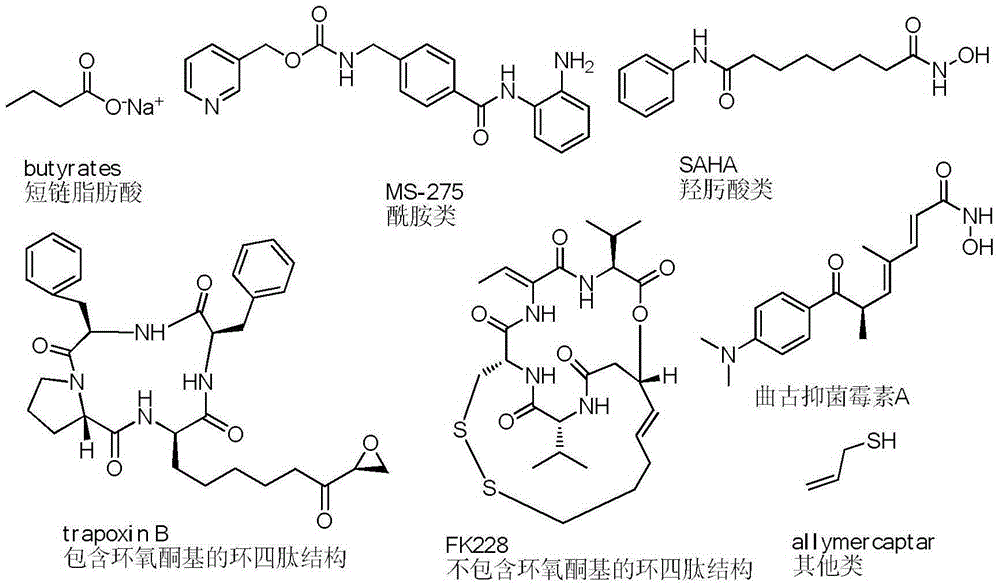
[0114] Some compounds of the present invention use the SRB method to EBC-1 (human lung cancer cell line, c-Met gene amplification), EBC-1 / SR (human lung cancer cell line, c-Met gene amplification, SGX-523 drug resistance strain) and NCI-H3122 (human lung cancer cell line, EML4-ALK (variant1)), the effect on cell proliferation (72 hours) was investigated respectively, and compared with the clinically used drug SAHA.
[0115] It includes the steps: cells in the logarithmic growth phase are inoculated to a 96-well culture plate at an appropriate density, 90 μL per well, and after overnight culture, different concentrations of drugs are added for 72 hours, and each concentration is set in triplicate. Vehicle control and cell-free zero wells. After the effect, the adherent cells poured out the culture medium, added 10% (w / v) trichloroacetic acid (100 μL / well) and fixed at 4°C for 1 hour, then washed five times with distilled water, dried at room temperature, and added SRB solution ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com