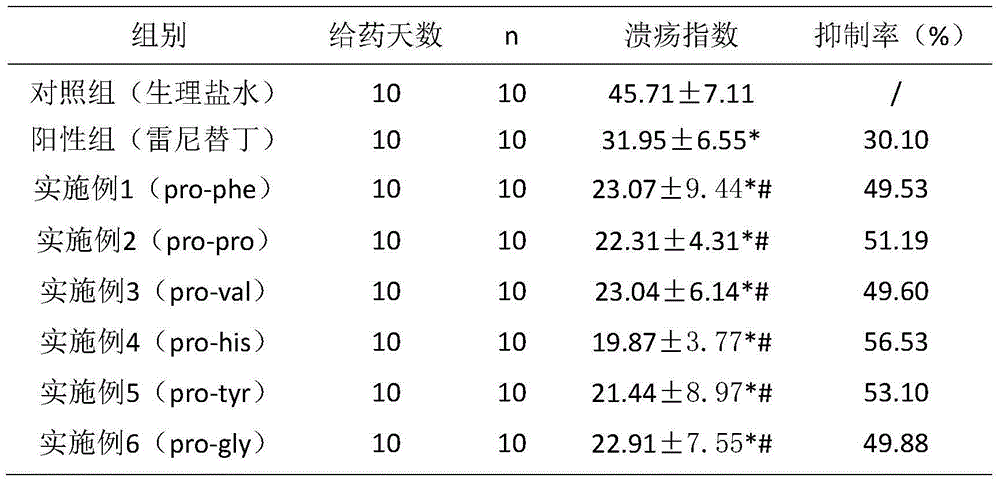
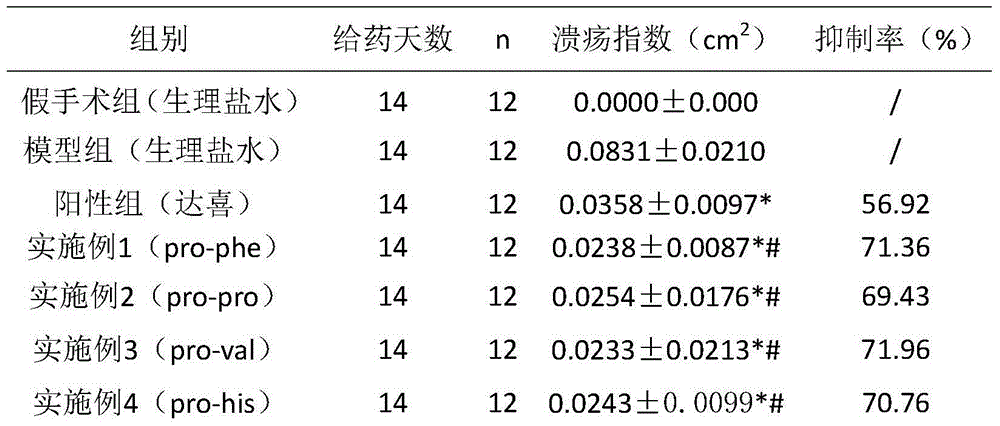
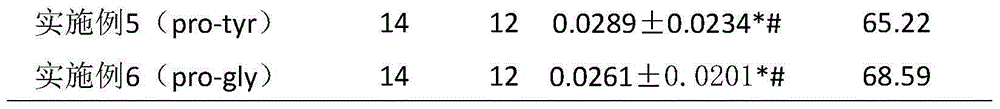
Six cyclic dipeptides used for wound healing
A wound repair and cyclic dipeptide technology, applied in the biological medicine field, can solve problems such as the application of cyclic dipeptide not seen before, and achieve the effects of good wound repair effect, obvious treatment effect and fast wound healing speed.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Embodiment 1 The preparation method of the cyclic dipeptide (cycloproline-phenylpropane dipeptide) described in the present invention
[0016] Main pharmaceutical reagents: phenylalanine, proline protected by Boc amino group
[0017] Process for preparing cyclic pro-phe dipeptide:
[0018] (1) Preparation of phenylalanine methyl ester hydrochloride: Add 10 g of phenylalanine to a mixture of an appropriate amount of methanol and thionyl chloride, and stir at room temperature for 24 hours. TLC traced the completion of the reaction, and the solvent was spin-dried under reduced pressure to obtain 14.5 g of phenylalanine methyl ester hydrochloride.
[0019] (2) Neutralization and condensation reaction: add phenylalanine methyl ester hydrochloride obtained in step (1), 20 g of proline protected by Boc amino group, 20 ml of pyridine, DMAP (4-di methylaminopyridine), after being completely mixed, under the protection of argon, add the (Boc) of anhydrous tetrahydrofuran 2 O, ...
Embodiment 2
[0022] Embodiment 2 The preparation method of the cyclic dipeptide (cyclic pro-pro dipeptide) described in the present invention
[0023] Main pharmaceutical reagents: proline, proline protected by Boc amino group
[0024] Process for preparing cyclic pro-pro dipeptide:
[0025] (1) Preparation of proline methyl ester hydrochloride: add 11 g of proline to a mixture of an appropriate amount of methanol and thionyl chloride, and stir at room temperature for 20 h. The completion of the reaction was tracked by TLC, and the solvent was spin-dried under reduced pressure to obtain 15.19 g of proline methyl ester hydrochloride.
[0026] (2) Neutralization and condensation reaction: add proline methyl ester hydrochloride obtained in step (1), proline 22g, pyridine 20ml, DMAP (4-dimethyl aminopyridine), after all mixing, add anhydrous tetrahydrofuran (Boc) under the protection of argon 2 O, stirred at room temperature for 12 hours. The completion of the reaction was tracked by TLC, ...
Embodiment 3
[0029] Embodiment 3 The preparation method of the cyclic dipeptide (cycloproline-valeric dipeptide) described in the present invention
[0030] Main pharmaceutical reagents: valine, proline protected by Boc amino group
[0031] Process for preparing cyclic pro-val dipeptide:
[0032] (1) Preparation of valine methyl ester hydrochloride: Add 10 g of valine to a mixture of an appropriate amount of methanol and thionyl chloride, and stir at room temperature for 18 hours. The completion of the reaction was tracked by TLC, and the solvent was spin-dried under reduced pressure to obtain 16.81 g of valine methyl ester hydrochloride.
[0033] (2) Neutralization and condensation reaction: Add valine methyl ester hydrochloride obtained in step (1), 20 g of proline protected by Boc amino group, 20 ml of pyridine, DMAP (4-dimethyl aminopyridine), after all mixing, add anhydrous tetrahydrofuran (Boc) under the protection of argon 2 O, stirred at room temperature for 10 hours. The compl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com