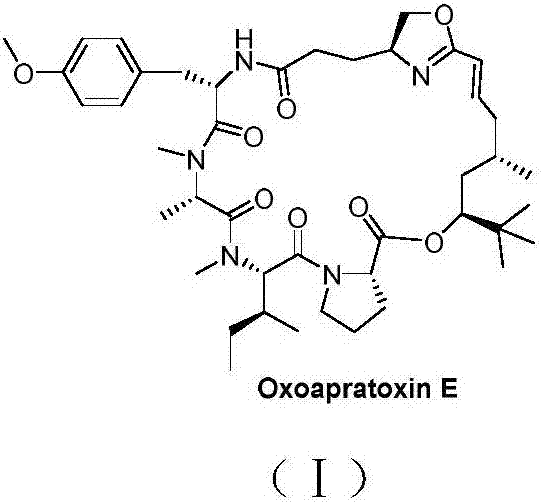
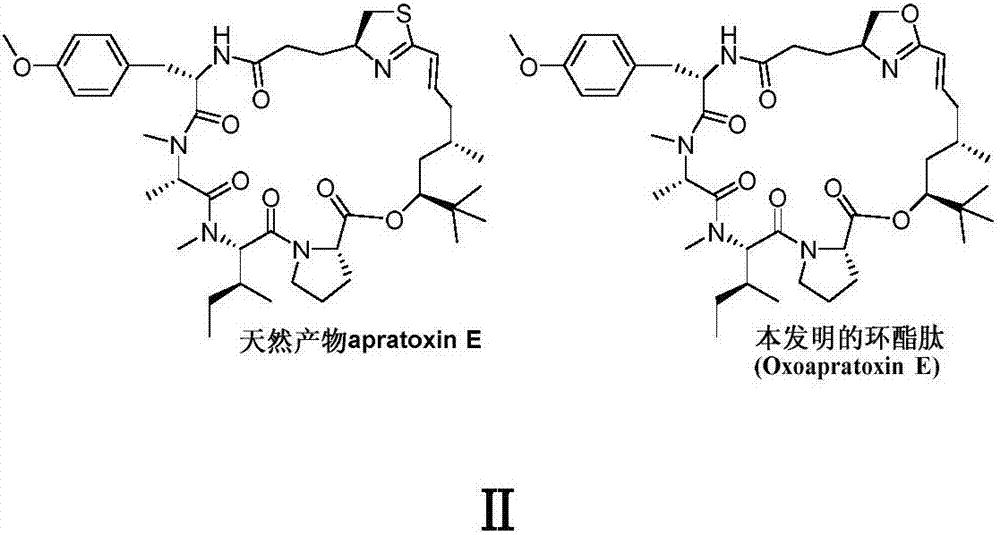
Cyclic depsipeptide as well as preparation method and application thereof
A cyclic lipopeptide and reaction technology, applied in the field of medicinal chemistry, can solve problems such as difficult chemical preparation, instability, and limited application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
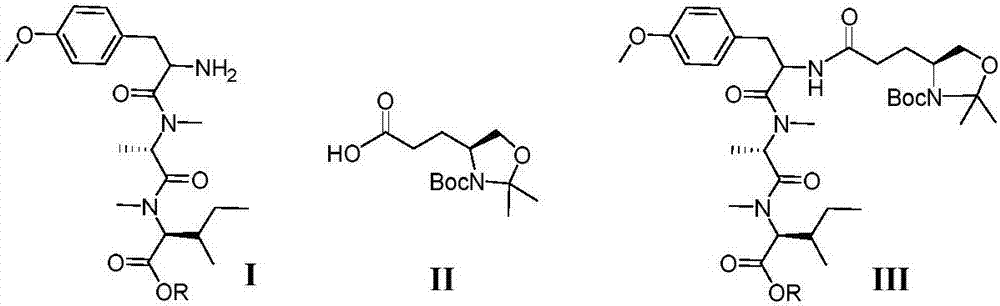
[0020] Embodiment 1 prepares cyclic lipopeptide, specifically comprises the following steps:
[0021] 1. Preparation of linear tetrapeptide (taking R=allyl as an example)
[0022]
[0023] Take compound I (prepared according to literature method, see: Doi, T.; Numajiri, Y.; Munakata, A.; Takahashi, T.Org.Lett.2006,8,531-534.) 492mg (1.1mmol) was dissolved in 10mL dry In dichloromethane, compound II (301.2 mg, 1.1 mmol) and HATU (627.4 mg, 1.65 mmol) were added, and DIPEA (576.0 μL, 3.3 mmol) was added dropwise in ice bath. Stir at room temperature under nitrogen protection for 12 h, LC-MS showed that the reaction was complete. Quenched with saturated ammonium chloride, diluted with dichloromethane, washed several times with 5% aqueous potassium bisulfate solution until the aqueous phase was nearly colorless, washed with saturated sodium chloride, dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The crude product was subjected to silica gel col...
Embodiment 2
[0033] Example 2 In Vitro Antitumor Activity Screening Experiment
[0034] In this example, the in vitro anti-tumor activity screening experiment of cyclic ester peptide Oxoapratoxin E was carried out. Staurosporine was used as a positive control, tetrazolium salt reduction method and sulforhodamine B protein staining method were used as screening methods; four tumor cell lines were used A549, HCT 116, Hela, MCF-7 (the tumor cell line is commercially available) have carried out activity test, the result shows (as shown in table 1), cyclic lipopeptide Oxoapratoxin E of the present invention has stronger antitumor activity , has potential application value in the field of medicine, especially in the research and development of antitumor drugs.
[0035] Table 1 compound Oxoapratoxin E antitumor activity (IC 50 / μM)
[0036]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com