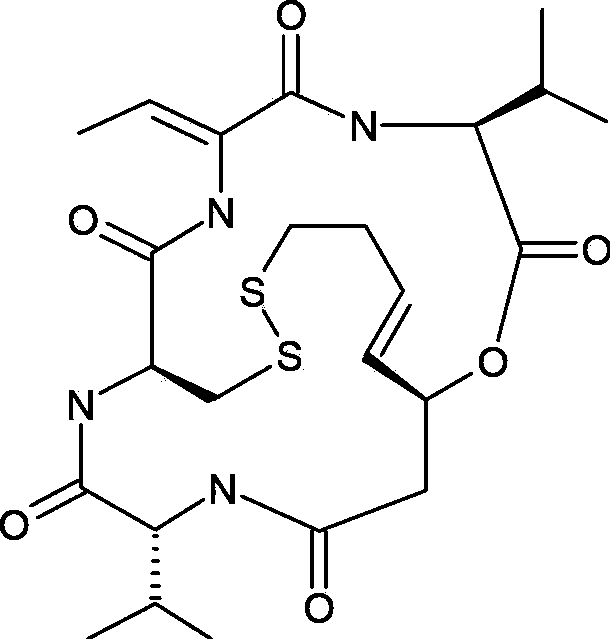
Preparation method for romidepsin
A technology for romidepsin and a compound, which is applied in the field of preparation of romidepsin, can solve problems such as affecting the production efficiency of romidepsin, is not simple enough, and has complicated steps, and achieves low cost, reduced synthesis steps, and simple operation. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0050] The invention discloses a preparation method of romidepsin, and those skilled in the art can refer to the content of this article and appropriately improve the process parameters to realize it. In particular, it should be pointed out that all similar replacements and modifications are obvious to those skilled in the art, and they are all considered to be included in the present invention. The method of the present invention has been described through preferred embodiments, and the relevant personnel can obviously make changes or appropriate changes and combinations to the compounds and preparation methods described herein without departing from the content, spirit and scope of the present invention to achieve and Apply the technology of the present invention.
[0051] In the specific embodiment of the present invention, all amino acids coupled with protecting groups can be obtained commercially. The protected amino acids in the present invention are purchased from Jill ...
Embodiment 1
[0055] Embodiment 1: the preparation of formula 1 compound
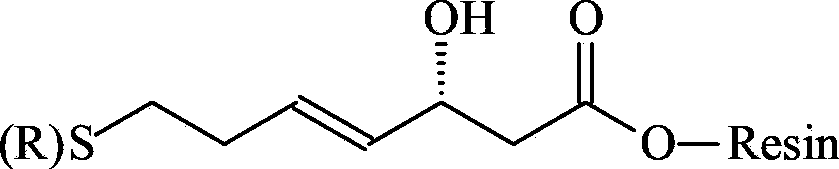
[0056] Weigh 2g of CTC Resin with a substitution degree of 0.5mmol / g (synthesis scale: 1mmol), add it to a solid-phase reaction column, wash it twice with DMF, and swell the resin with DMF for 30 minutes, then weigh 1.26g of 3-hydroxyl-7-( Trityl)mercapto-4-heptenoic acid was dissolved in DMF, activated by adding 0.6mL DIPEA in an ice-water bath, then added to the above-mentioned reaction column equipped with resin, reacted for 2 hours, and the reaction was completed, washed with DMF for 6 times to obtain formula 1 compound.
Embodiment 2
[0057] Embodiment 2: the preparation of formula 2 compound
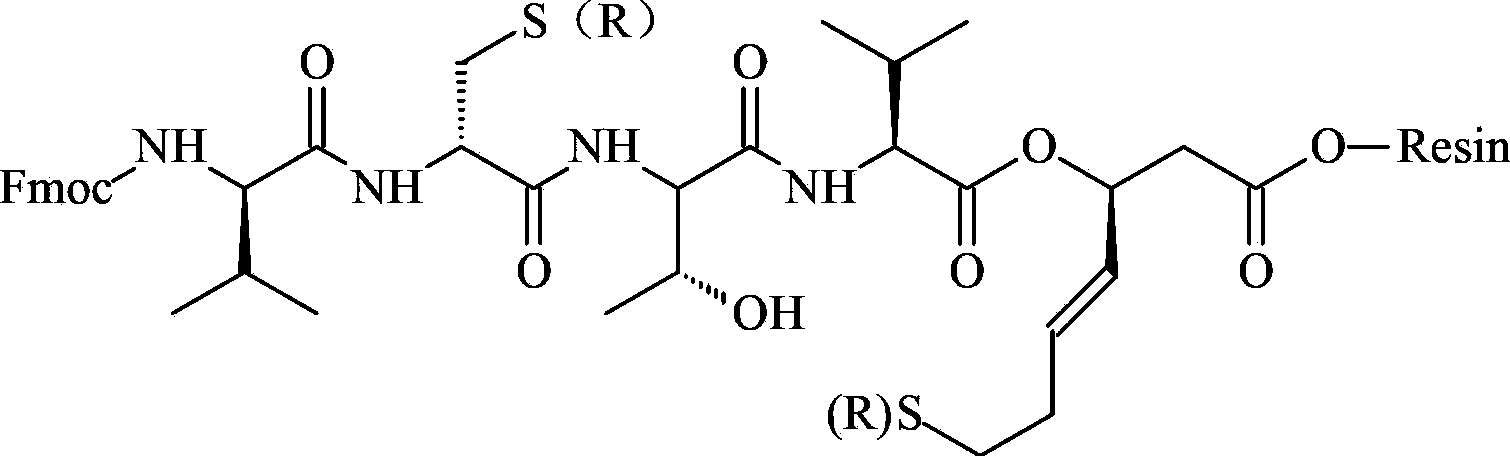
[0058] Dissolve 1.01g of Fmoc-Val-OH, 0.38g of HOBt, and 0.03g of DMAP in a mixed solution of DMF and NMP with a volume ratio of 1:1, add 0.3mL of DIC to activate it under an ice-water bath, and then add it to the solid-phase reaction column in Example 1 React with the compound of formula 1, and react at room temperature for 2 hours (the end point of the reaction is determined by the ninhydrin method. If the resin is colorless and transparent, the reaction is complete, and the resin develops color, indicating that the reaction is incomplete, and another coupling reaction is required for 1 hour). The Fmoc protecting group was then removed with DBLK and washed 6 times with DMF.
[0059] Then repeat the above steps of adding coupling agent and DMAP, adding amino acid and removing Fmoc protecting group, and complete Fmoc-L-Thr-OH, Fmoc-D-Cys(Trt)-OH, Fmoc-D-Val-OH one by one Coupling of the polypeptide chain extension t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com