A kind of romidepsin lipid microsphere preparation and preparation method thereof
A technology of romidepsin and lipid microspheres, which is applied in the directions of powder delivery, emulsion delivery, antineoplastic drugs, etc., can solve the problems of no reports of romidepsin lipid microsphere preparations, etc., so as to improve drug safety and increase Targeting, reducing toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
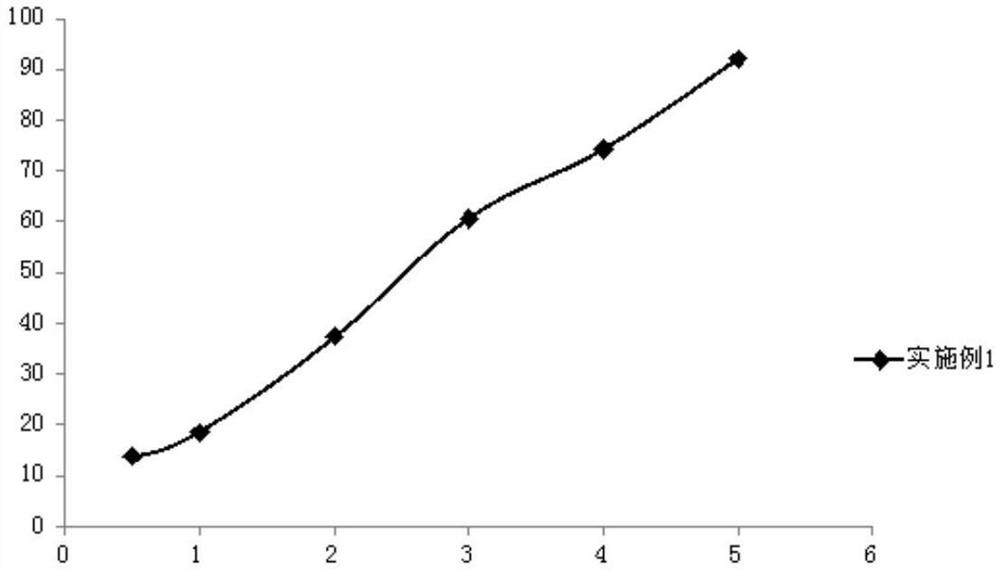
Embodiment 1
[0050] (1) Weigh 1.0g romidepsin, 5.0g soybean oil, 5.0g medium-chain fatty acid triglycerides, 1.5g soybean lecithin, 0.4g propylene glycol and 0.05g ethanol in a beaker, and use a high-speed shearing machine Shear at high speed for 3 minutes, heat and stir in a magnetic stirring water bath at 60°C at a stirring speed of 500rpm to completely dissolve and obtain an oil phase;
[0051] (2) Weigh 0.9g of sodium chloride and place it in a beaker, add 86.2g of water for injection, heat and stir in a magnetic stirring water bath at 60°C, and stir at a stirring speed of 500rpm to completely dissolve it to obtain an aqueous phase;
[0052] (3) under high-speed stirring, the oil phase and the water phase are mixed, and the pH value is adjusted to 7.0 to obtain colostrum;
[0053] (4) transfer the colostrum to a high-pressure homogenizer, and use 500bar pressure for high-pressure homogenization to obtain a submicron emulsion;
[0054] (5) Nitrogen-filled sub-package, after autoclaving...
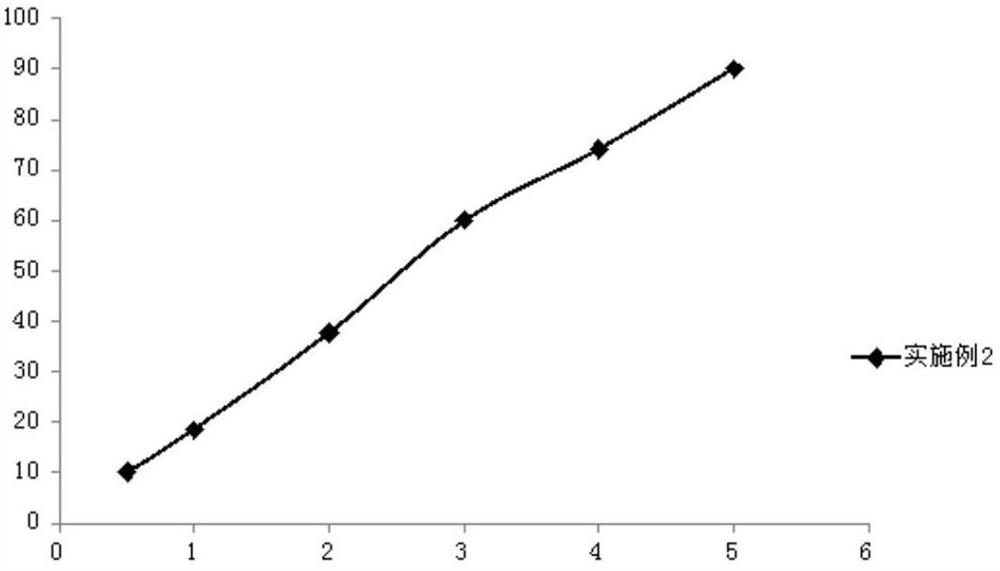
Embodiment 2
[0056] (1) Weigh 1.5g romidepsin, 6.5g soybean oil, 10.5g medium-chain fatty acid triglycerides, 2.0g egg yolk lecithin, 0.8g propylene glycol and 0.2g ethanol in a beaker, and use a high-speed shearing machine Shear at high speed for 5 minutes, heat and stir in a magnetic stirring water bath at 65°C at a stirring speed of 500rpm to completely dissolve it to obtain an oil phase;
[0057] (2) Weigh 0.9g of sodium chloride and put it in a beaker, add 77.4g of water for injection, heat and stir in a magnetic stirring water bath at 65°C, and stir at a speed of 400rpm, so that it is completely dissolved to obtain an aqueous phase;
[0058] (3) under high-speed stirring, the oil phase and the water phase are mixed, and the pH value is adjusted to 7.2 to obtain colostrum;
[0059] (4) transfer the colostrum to a high-pressure homogenizer, and use 350bar pressure for high-pressure homogenization to obtain a submicron emulsion;
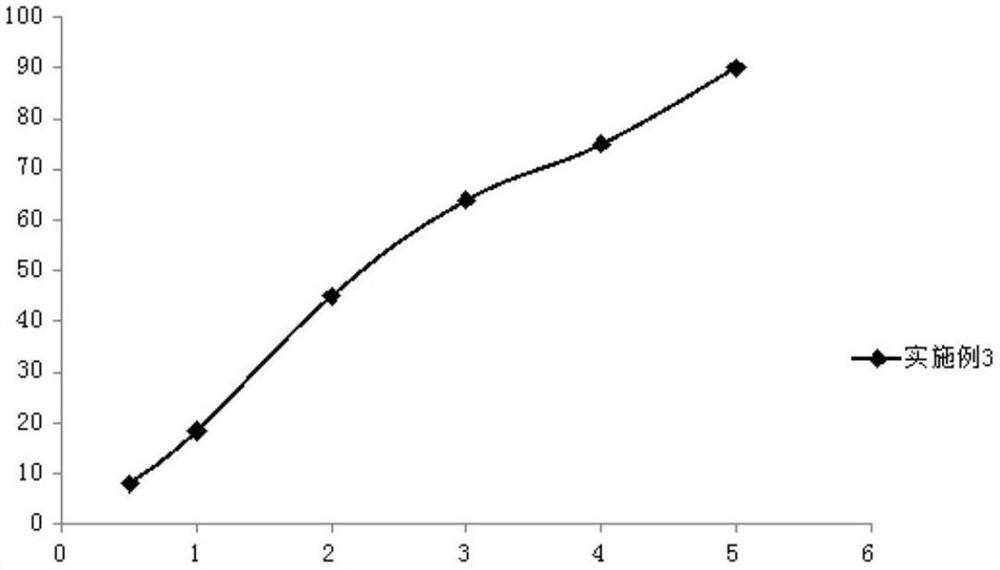
Embodiment 3
[0062] (1) Weigh 2.5g romidepsin, 4.5g safflower oil, 15.5g medium-chain fatty acid triglycerides, 2.0g hydrogenated egg yolk lecithin, and 1.0g propylene glycol in a beaker, and use a high-speed shearing machine to perform high-speed shearing. Cut for 8 minutes, heat and stir at 45°C in a magnetic stirring water bath at a stirring speed of 700rpm to completely dissolve and obtain an oil phase;
[0063] (2) Weigh 4.5g of mannitol and place it in a beaker, add 70g of water for injection, heat and stir at 45°C in a magnetic stirring water bath at a stirring speed of 700rpm, and dissolve it completely to obtain an aqueous phase;
[0064] (3) under high-speed stirring, the oil phase and the water phase are mixed, and the pH value is adjusted to 6.7 to obtain colostrum;
[0065] (4) transfer the colostrum to a high-pressure homogenizer, and use 650bar pressure for high-pressure homogenization to obtain a submicron emulsion;
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com