New crystal form of romidepsin, and preparation method and application thereof
A kind of technology of romidepsin and crystal form, applied in the field of pharmacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
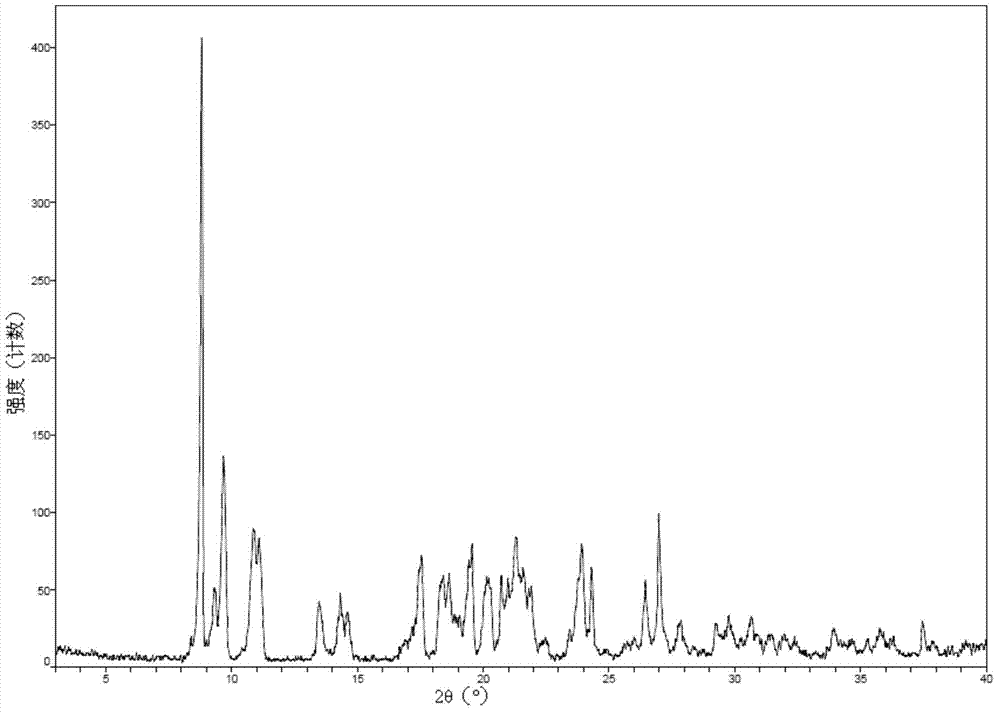
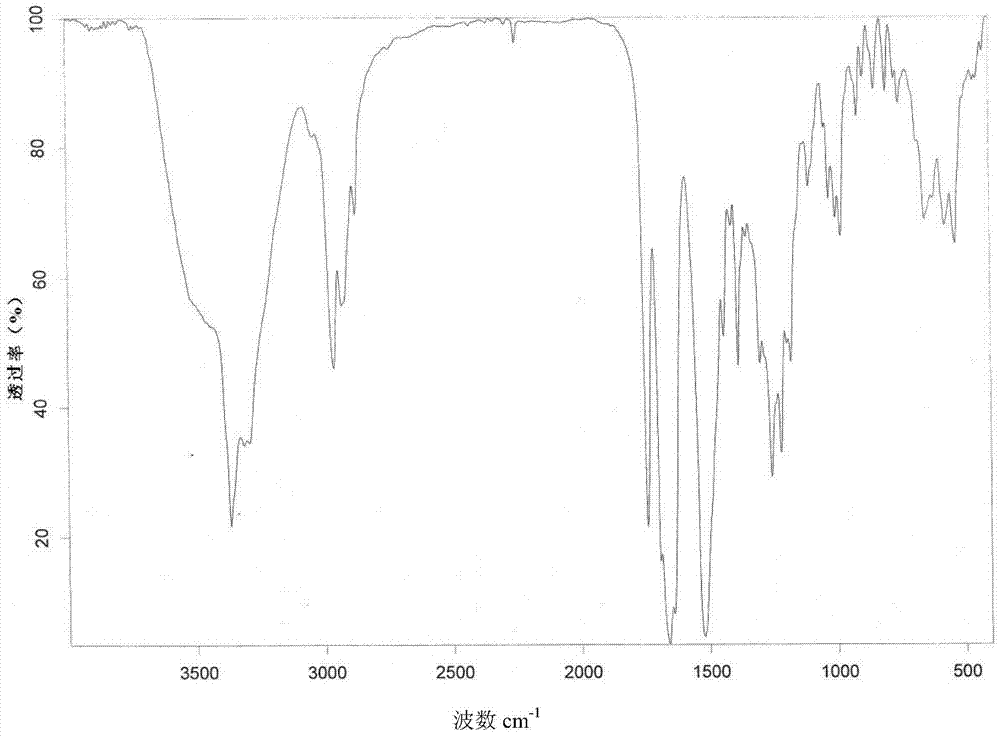
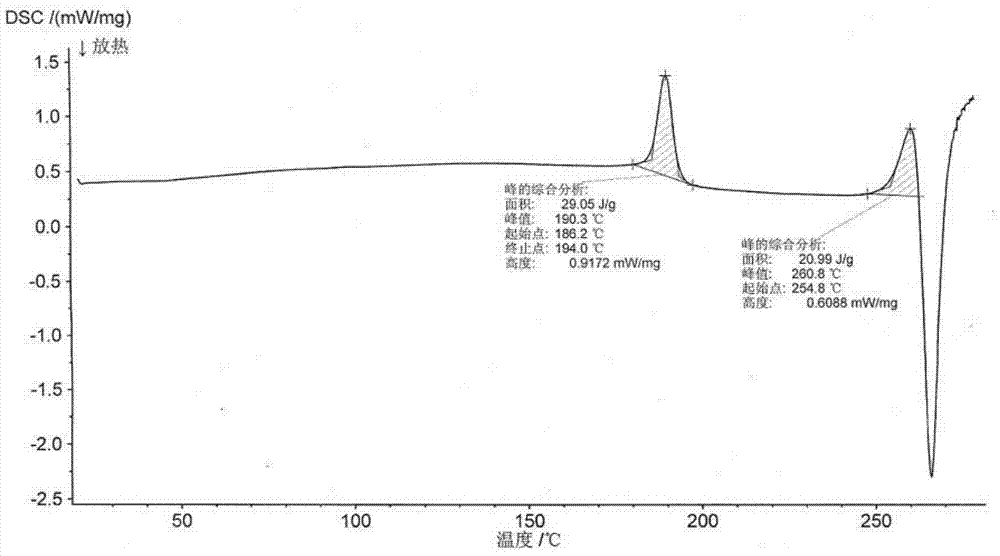
[0045] 2.0 g romidepsin (HPLC purity greater than 99%) was dissolved in 5 ml of a mixed solvent of chloroform:methanol = 9 / 1 (volume ratio) to form a saturated solution (crystals were not completely dissolved), and the filtrate was obtained by filtration. Take 3ml of the filtrate, and then add 27ml of acetonitrile to the filtrate, mix well and place in a refrigerator at 4°C to avoid light. After 72h, the crystal is filtered and dried at 45°C under vacuum for 48h to obtain romidepsin crystal form O. Take a sample for testing, and its X-ray powder diffraction pattern is as follows figure 1 As shown, the infrared absorption spectrum is as figure 2 As shown, the DSC spectrum is as image 3 As shown, the TGA map is as Figure 4 Shown.
Embodiment 2
[0047] 10.0 g of romidepsin (HPLC purity greater than 99%) was dissolved in 25 ml of a mixed solvent of chloroform: methanol = 9 / 1 (volume ratio) to form a saturated solution (crystals were not completely dissolved), and the filtrate was obtained by filtration. Take 11 parts of 1ml filtrate, and then add 1ml, 2ml, 3ml, 4ml, 5ml, 6ml, 7ml, 8ml, 9ml acetonitrile to the filtrate, mix well and place in a refrigerator at 4℃ to avoid light, and filter to obtain crystals after 48h The crystals were dried in vacuum at 45°C for 48 hours. After testing, the crystal forms obtained by each solvent system are shown in the table below.
[0048] Acetonitrile (m1)
Embodiment 3
[0050] 1.0g romidepsin (HPLC purity greater than 99%) was dissolved in 2.5ml dichloromethane: ethanol = 9 / 1 (volume ratio) mixed solvent, then 35ml acetonitrile was added, stirred overnight at room temperature, filtered, and vacuum at 45°C After drying for 48 hours, 0.92 g of solid was obtained with a yield of 92%. After testing, it was confirmed that the crystalline form O of romidepsin was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com