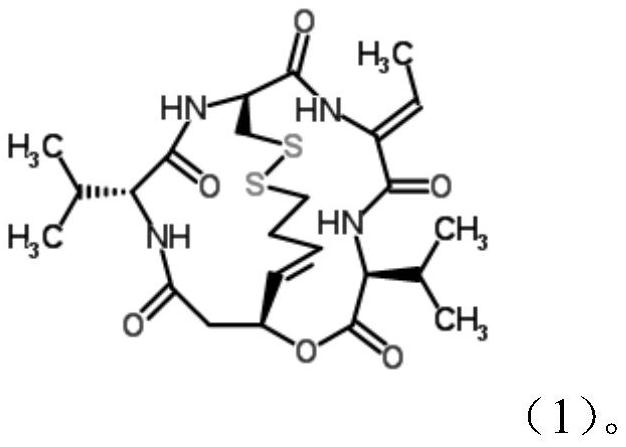
Application of romidepsin in preparation of medicine for preventing and treating novel coronavirus of COVID-19
A technology of romidepsin and coronavirus, applied in the direction of antiviral agents, drug combinations, pharmaceutical formulas, etc., can solve the problems of poor curative effect, achieve low effective concentration, high therapeutic index, and increase the effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1. Virus amplification
[0026] African green monkey kidney cells (VeroE6) were divided into 3×10 5 Each well was inoculated into a 96-well plate in Eagle’s minimum basal medium (minimum Eagle’s medium, MEM; GibcoInvitrogen) containing 10% fetal bovine serum (FBS; GibcoInvitrogen) at 37°C, 5% CO 2 Cultivate until the monolayer grows. Inoculate a 100-fold dilution of the clinical isolate of the novel coronavirus pneumonia into a 96-well plate full of monolayer cells, place at 37°C, 5% CO 2 Culture for two days (including the normal control group).
[0027] Two days later, the lesion degree reached more than 75%. Put it in a -80°C ultra-low temperature refrigerator, freeze and thaw once repeatedly, collect the virus liquid amplified by the cells, centrifuge at 3000r / min for 30 minutes, remove the sediment, put it in a small tube and put it in a -80°C ultra-low temperature freezer Long-term preservation.
Embodiment 2
[0028] Embodiment 2 Romidepsine Drug Toxicity Evaluation
[0029] After dissolving romidepsin powder in DMSO, add culture medium to dilute to 20 mg / mL, the final concentration of DMSO is 1%, filter through a 0.22 μm filter membrane and store at 4°C; filter and store at 4°C. Press about 2.5×10 per hole 4 Cells were seeded into a 96-well plate, and after 24 to 48 hours, when the cells had grown into a single layer, the culture medium was discarded, and 100 μL / well of drugs of different dilutions were added, and 100 μL / well of MEM was added to the control wells of normal cells, at 37°C in 5% CO 2 Continue culturing for 2 to 5 days, add 20 μL of CCK8 solution (5 mg / mL) to each well, and place at 37°C in 5% CO 2 Continue to incubate for 4 hours in the incubator. Discard the culture supernatant, add 100 μL dimethyl sulfoxide (DMSO) to each well, and shake at low speed for 10 minutes to fully dissolve the crystals. Select a wavelength of 490nm, and measure the light absorption val...
Embodiment 3
[0030] Example 3 Romidepsin anti-new coronary pneumonia novel coronavirus efficacy evaluation
[0031] To evaluate the antiviral efficacy of the drug, VeroE6 cells were grown at a density of 5×10 4 Cells / well were cultured overnight in a 48-well cell culture dish. Add virus (MOI 0.05) to infect for 2 hours. Then add 2-fold serial dilutions of the drug, set 4 replicate wells for each concentration, take the maximum non-toxic concentration as the initial concentration of the drug, at 34°C, 5% CO 2Incubate for 2 days in the incubator. Cytopathogenic Effect (CPE) was recorded. The occurrence of CPE in cells is recorded according to the 6-level standard. After recording the CPE, staining with CCK8, the OD value was determined. Efficacy evaluation of romidepsin against novel coronavirus pneumonia The efficacy of romidepsin was evaluated by the CCK8 method, and the half effective concentration (EC) against novel coronavirus pneumonia (SARS-Cov-2) 50 ) is 200nM, and the half eff...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com