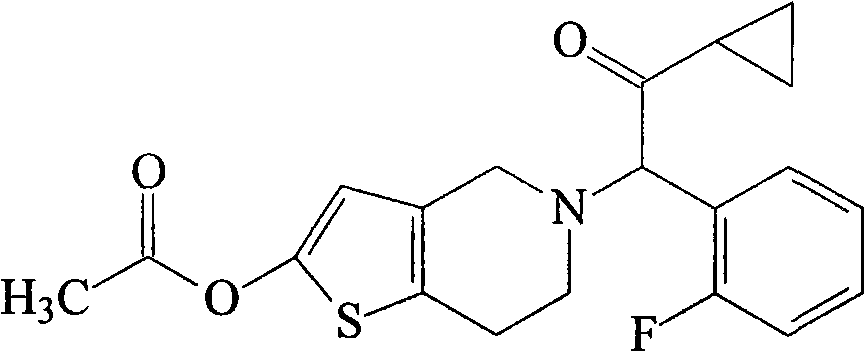
Prasugrel liposome solid preparation
A technology of liposomes and liposomes, applied in the field of liposome solid preparations, can solve problems such as not easy to dissolve, unfavorable preparation of pharmaceutical preparations, low solubility, etc., to achieve improved stability, easy removal by decompression evaporation, and small side effects Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
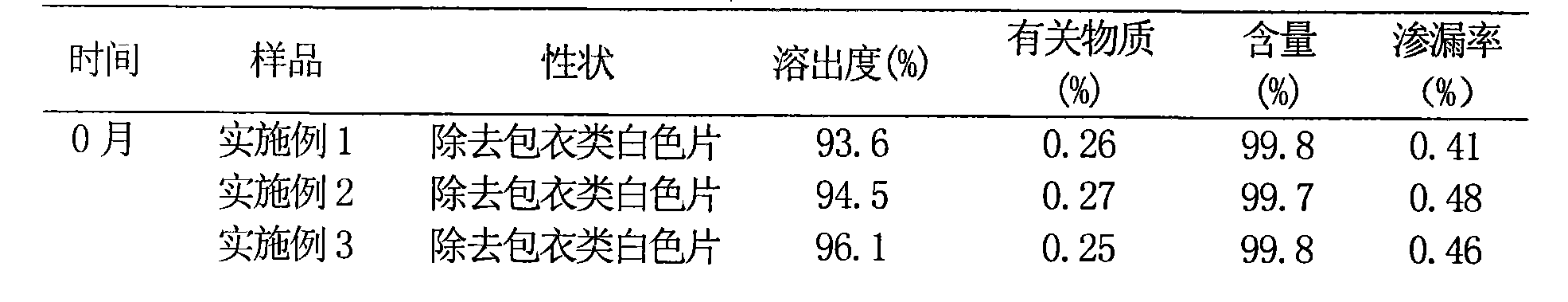
Embodiment 1
[0059] The preparation of embodiment 1 prasugrel liposome sheet
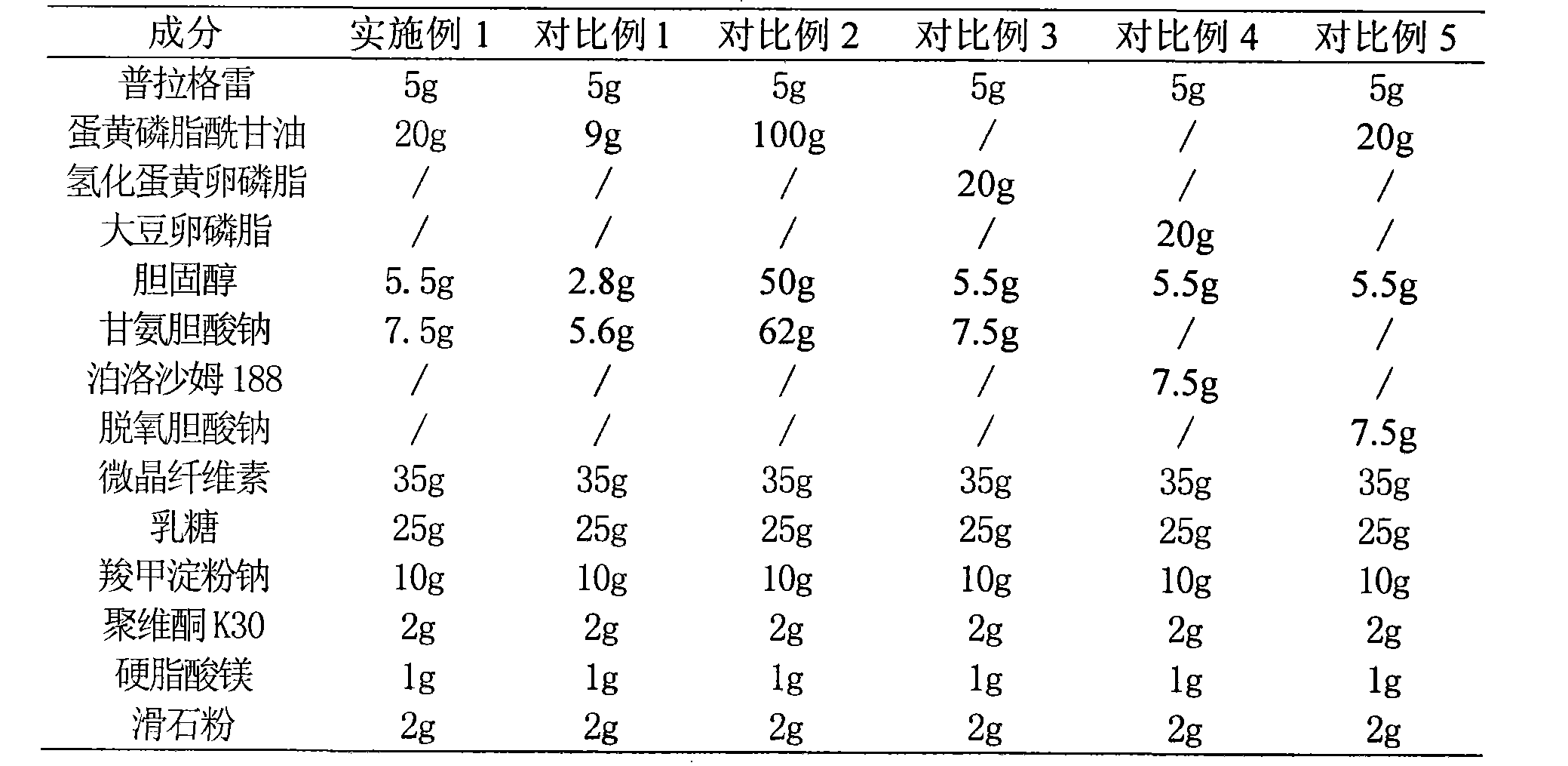
[0060] prescription:
[0061] Prasugrel 5g
[0062] Egg Yolk Phosphatidylglycerol 20g
[0063] Cholesterol 5.5g
[0064] Sodium Glycocholate 7.5g
[0065] Microcrystalline Cellulose 35g
[0066] Lactose 25g
[0067] Carboxymethyl Starch Sodium 10g
[0068] Povidone K30 2g
[0071] Preparation Process
[0072] (1) 20g egg yolk phosphatidylglycerol, 5.5g cholesterol, 7.5g sodium glycocholate are dissolved in the mixed solvent of isopropanol and acetone with a volume ratio of 5:2 in 500ml, mix well, and reduce Remove the mixed solvent under pressure to obtain a phospholipid film;
[0073] (2) Add 300ml of phosphoric acid-sodium hydrogen phosphate buffer solution with a pH value of 5.2, shake and stir to completely hydrate the phospholipid membrane, emulsify at a high speed, and filter through a 0.45 μm microporous membrane to prepare a blank liposome...
Embodiment 2
[0079] The preparation of embodiment 2 prasugrel liposome sheet
[0080] prescription:
[0081] Prasugrel 10g
[0082] Egg Yolk Phosphatidylglycerol 120g
[0083] Cholesterol 50g
[0084] Sodium Glycocholate 80g
[0085] Microcrystalline Cellulose 240g
[0086] Lactose 100g
[0087] Carboxymethyl Starch Sodium 30g
[0088] Povidone K30 10g
[0091] Preparation Process
[0092] (1) Dissolve 120g egg yolk phosphatidylglycerol, 50g cholesterol, and 80g sodium glycocholate in a mixed solvent of isopropanol and acetone with a volume ratio of 5:2 in 1500ml, mix well, and remove under reduced pressure on a rotary thin film evaporator Mixing solvents to prepare a phospholipid film;
[0093] (2) Add 600ml of phosphoric acid-sodium hydrogen phosphate buffer solution with a pH value of 5.2, shake and stir to completely hydrate the phospholipid membrane, emulsify at a high speed, and filter through a 0.45 μm microporous me...
Embodiment 3
[0099] The preparation of embodiment 3 prasugrel liposome sheet
[0100] prescription:
[0101] Prasugrel 5g
[0102] Egg Yolk Phosphatidylglycerol 40g
[0103] Cholesterol 150g
[0104] Sodium Glycocholate 25g
[0105] Starch 64g
[0106] Lactose 50g
[0107] Crospovidone 20g
[0108] Hypromellose 2g
[0109] Micronized silica gel 10g
[0111] Preparation Process
[0112] (1) Dissolve 40g of egg yolk phosphatidylglycerol, 150g of cholesterol, and 25g of sodium glycocholate in a mixed solvent of isopropanol and acetone with a volume ratio of 5:2 in 500ml, mix well, and remove under reduced pressure on a rotary thin film evaporator. Mixing solvents to prepare a phospholipid film;
[0113] (2) Add 300ml of phosphoric acid-sodium hydrogen phosphate buffer solution with a pH value of 5.2, shake and stir to completely hydrate the phospholipid membrane, emulsify at a high speed, and filter through a 0.45 μm microporous membrane to prepare a ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com