Edaravone lipid microsphere formulation and preparation method
A technology of edaravone fat and microspheres, which is applied in the field of medicine, can solve problems such as poor stability, achieve the effects of improving stability, reducing side effects of drugs, and ensuring product quality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
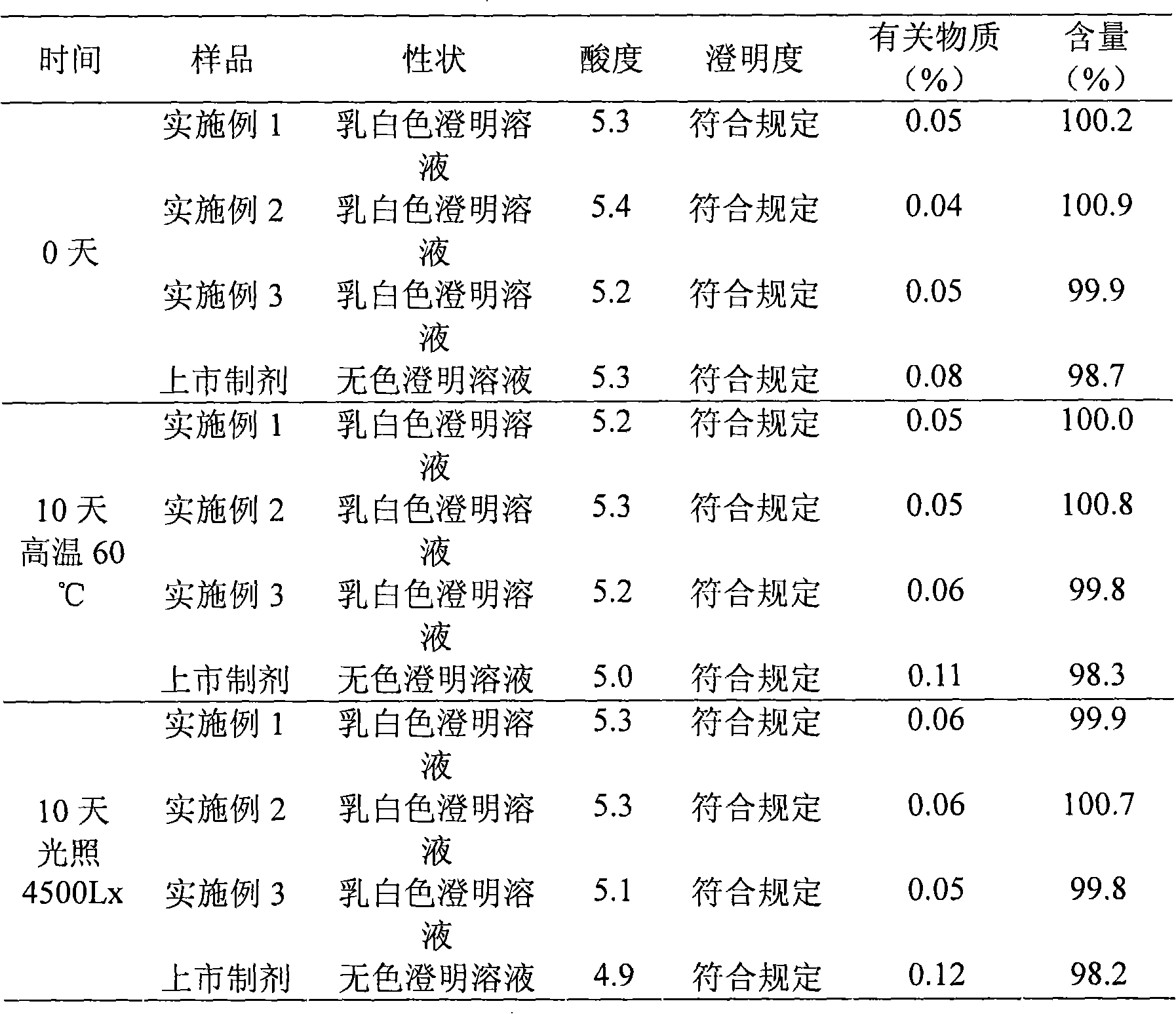
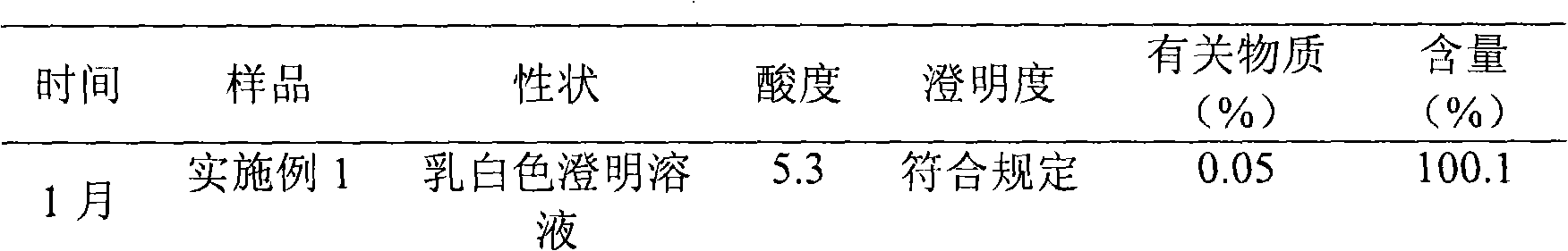
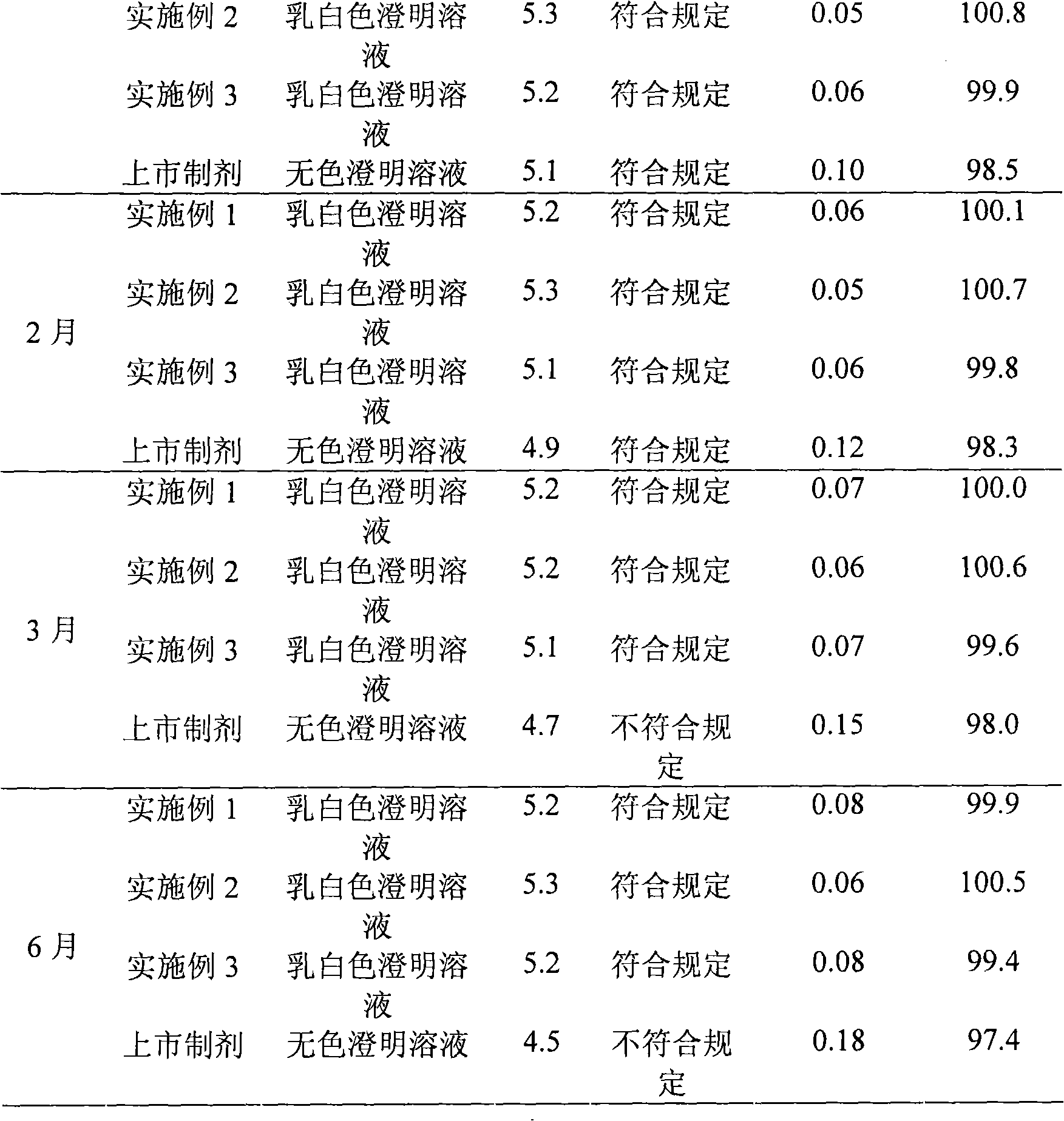
Embodiment 1
[0031] The preparation of embodiment 1 Edaravone lipid microsphere preparation
[0032] Prescription (1000 bottles): Edaravone 10g
[0033] Soy Lecithin 50g
[0034] Poloxamer 188 20g
[0035] Linoleic acid 8g
[0037] Soybean oil for injection 180g
[0038] Water for injection 1500g
[0039] Preparation Process
[0040] (1) 10g of edaravone was dissolved in 30ml of ethanol, and added in 180g of soybean oil to obtain an oil phase;
[0041] (2) Dissolve 50 g of soybean lecithin, 20 g of poloxamer 188, 8 g of linoleic acid, and 15 g of sodium bisulfite in water to obtain an aqueous phase;
[0042] (3) Mix the oil phase and the water phase, transfer to a tissue grinder, rotate at 10,000r / min, stir at high speed for 10 minutes, filter through a microporous membrane, fill with nitrogen, potting, and autoclave at 115°C to obtain the product.
Embodiment 2
[0043] The preparation of embodiment 2 Edaravone lipid microsphere preparation
[0044] Prescription (1000 bottles): Edaravone 15g
[0045] Soy Lecithin 120g
[0046] Poloxamer 188 50g
[0047] Palmitic acid 20g
[0048] Ascorbyl Palmitate 60g
[0049]Soybean oil for injection 400g
[0050] Water for injection 2500g
[0051] Preparation Process
[0052] (1) 15g of edaravone and 60g of ascorbyl palmitate are dissolved in 100ml of ethanol, and added in 400g of soybean oil to obtain an oil phase;
[0053] (2) 120g of soybean lecithin, 50g of poloxamer 188, and 20g of palmitic acid were dissolved in water to obtain an aqueous phase;
[0054] (3) Mix the oil phase and the water phase, transfer to a tissue grinder, rotate at 12000r / min, stir at high speed for 30 minutes, filter through a microporous membrane, fill with nitrogen, potting, and autoclave at 115°C to obtain the product.
Embodiment 3
[0055] The preparation of embodiment 3 Edaravone lipid microsphere preparation
[0056] Prescription (1000 bottles): Edaravone 30g
[0057] Soy Lecithin 100g
[0058] Poloxamer 188 60g
[0059] Oleic acid 35g
[0060] Sodium formaldehyde bisulfite 40g
[0061] Soybean oil for injection 600g
[0062] Water for injection 5000g
[0063] Preparation Process
[0064] (1) 30g of edaravone was dissolved in 100ml of ethanol, and added in 600g of soybean oil to obtain an oil phase;
[0065] (2) 100g of soybean lecithin, 60g of poloxamer 188, 35g of oleic acid, and 40g of sodium formaldehyde bisulfite were dissolved in water to obtain an aqueous phase;
[0066] (3) Mix the oil phase and the water phase, transfer to a tissue grinder, rotate at 8000r / min, stir at high speed for 20 minutes, filter through a microporous membrane, fill with nitrogen, potting, and autoclave at 115°C to obtain the produc...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com