Pantoprazole sodium liposomes freeze-dry preparations and method of preparing the same
A technology of pantoprazole sodium fat and pantoprazole sodium, which is applied in the field of pantoprazole sodium liposome freeze-dried preparations, can solve the quality requirements that injections are difficult to reach the expiration date, poor stability of pantoprazole sodium, etc. problem, to achieve the effect of solving the problem of quality stability, safety proof, and reduction of toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
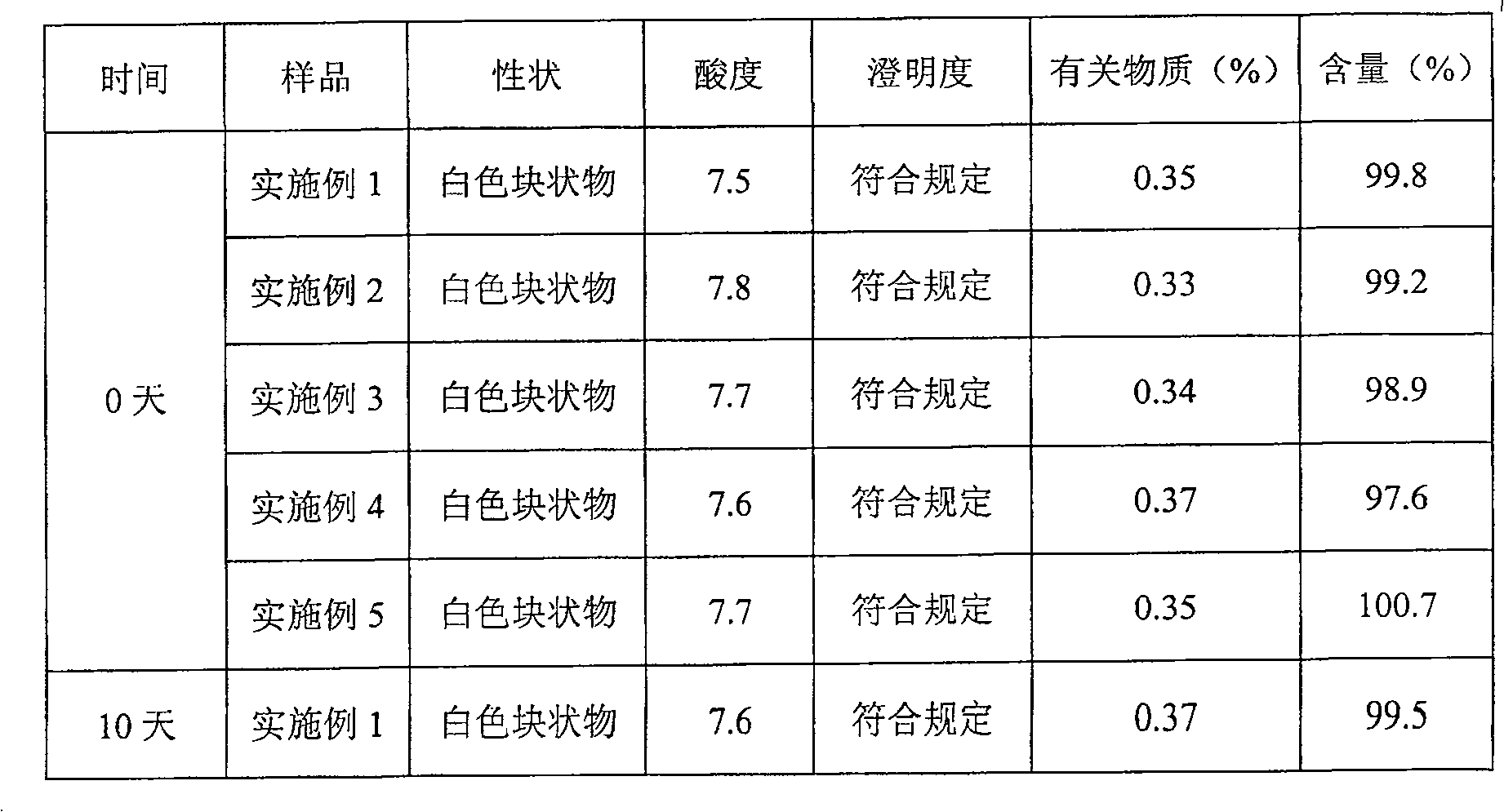
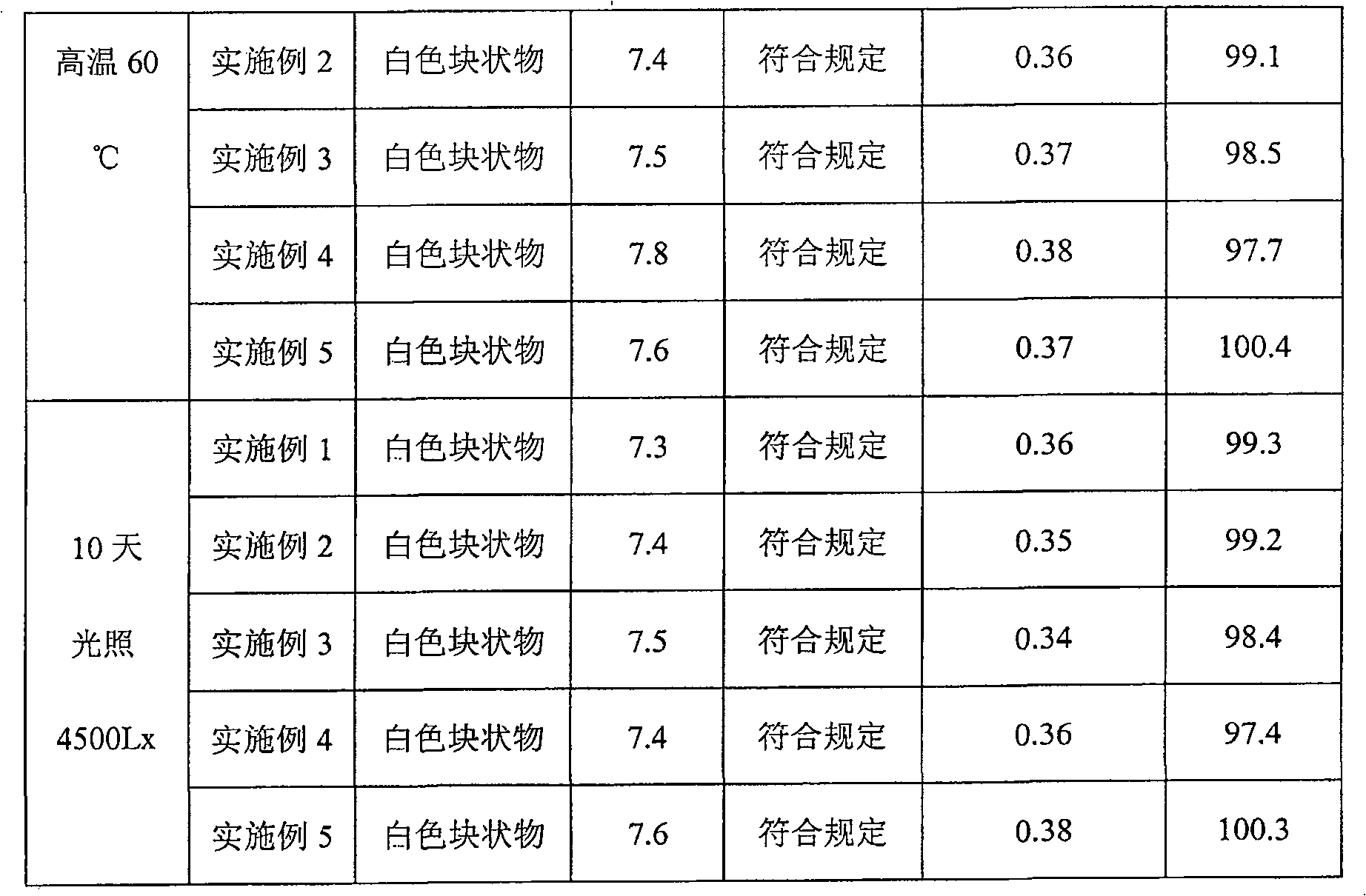
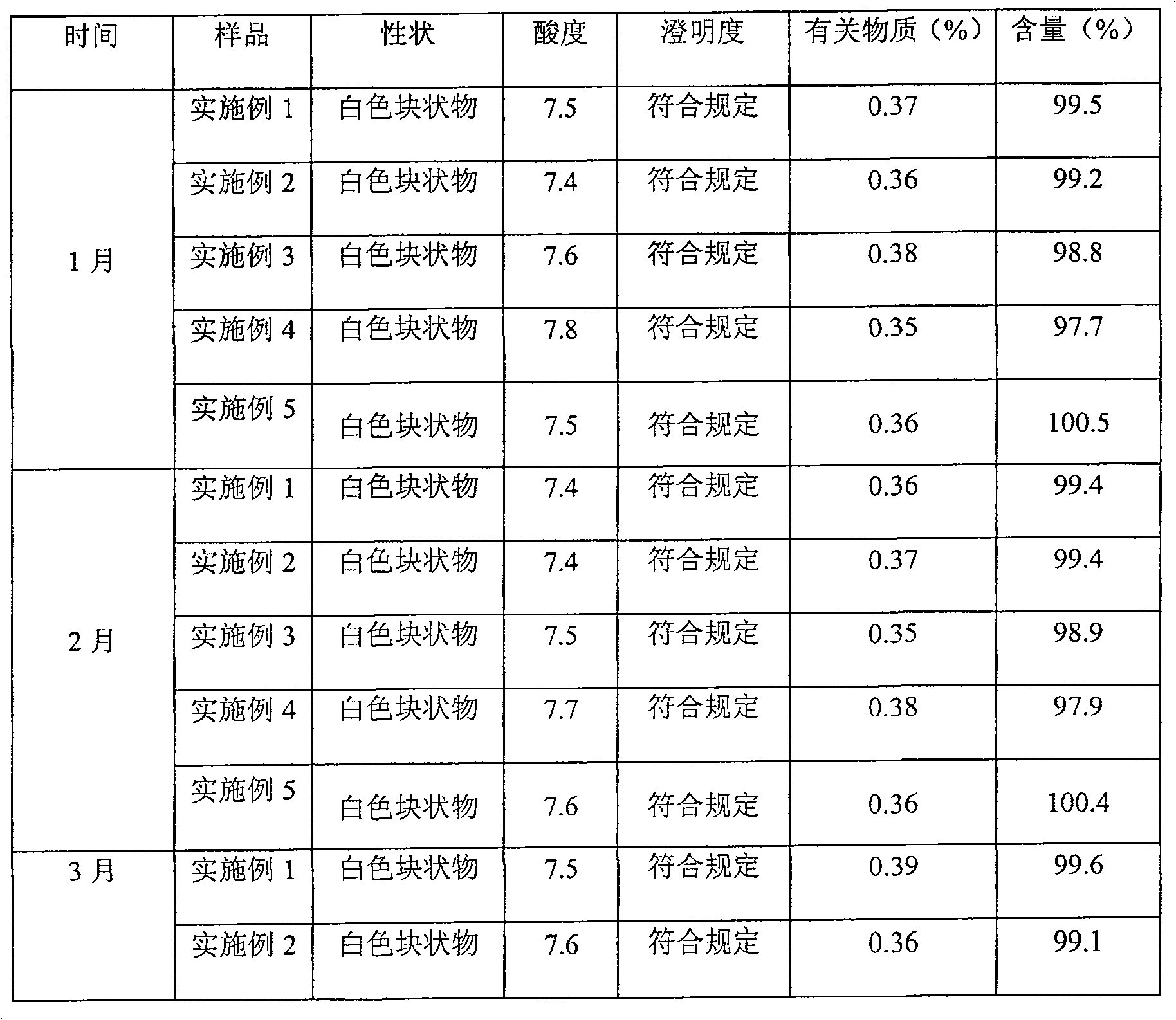
Examples
Embodiment 1
[0028] Pantoprazole Sodium 20g
[0029] Soy Lecithin 60g
[0030] Cholesterol 20g
[0031] Vitamin E 80mg
[0032] Disodium hydrogen phosphate 15g
[0033] Sodium dihydrogen phosphate 5g
[0034] Mannitol 200g
[0035] Preparation Process:
[0036] (1) Take by weighing 60 g of soybean lecithin, 20 g of cholesterol and 80 mg of vitamin E, dissolve them in chloroform, and mix them uniformly to form a liposome solution. The liposome solution was placed on a thin film evaporator, and the chloroform was released under reduced pressure to obtain a lipid film.
[0037] (2) Weigh 15 g of disodium hydrogen phosphate and 5 g of sodium dihydrogen phosphate according to the prescription, dissolve and prepare a phosphate buffer solution with a pH value of 6.0 to 8.0. Add phosphate buffer solution into the lipid film, and after the lipid is completely hydrated, prepare liposomes with a high-pressure homogenizer, check that the particle size of the liposomes is qualified, and set asid...
Embodiment 2
[0042] Pantoprazole Sodium 40g
[0043] Soy Lecithin 150g
[0044] Cholesterol 40g
[0045] Vitamin E 160mg
[0046] Citric acid 20g
[0047] Sodium citrate 50g
[0048] Glucose 800g
[0049] Preparation Process:
[0050] (1) Weigh 150 g of soybean lecithin, 40 g of cholesterol and 160 mg of vitamin E in the prescribed amount, dissolve them in chloroform, and mix them uniformly. The liposome solution was placed on a thin wax evaporator, and the chloroform was removed under reduced pressure to obtain a lipid film.
[0051] (2) Weigh 20 g of citric acid and 50 g of sodium citrate according to the prescription and dissolve to prepare a citrate buffer solution with a pH value of 6.0-8.0. Add the citrate buffer solution into the prepared lipid film, and after the lipid film is completely hydrated, prepare liposomes with a high-pressure homogenizer, check that the particle size of the liposomes is qualified, and set aside.
[0052] (3) Take by weighing 40g of pantoprazole so...
Embodiment 3
[0056] Pantoprazole Sodium 40g
[0057] Soy Lecithin 60g
[0058] Cholesterol 20g
[0059] Vitamin E 80mg
[0060] Citric acid 5g
[0061] Sodium citrate 12g
[0062] Mannitol 200g
[0063] Preparation Process:
[0064] (1) Weigh 60 g of soybean lecithin, 20 g of cholesterol and 80 mg of vitamin E in the prescribed amount, dissolve them in chloroform, and mix them evenly. The liposome solution was placed on a thin wax evaporator, and the chloroform was removed under reduced pressure to obtain a lipid film.
[0065] (2) Weigh 5 g of citric acid and 12 g of sodium citrate according to the prescription and dissolve to prepare a citrate buffer solution with a pH value of 6.0-8.0. Add the citrate buffer solution into the lipid film, and after the lipid film is completely hydrated, prepare liposomes with a high-pressure homogenizer, check that the particle size of the liposomes is qualified, and set aside.
[0066] (3) Take by weighing 40g of pantoprazole sodium, dissolve in...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com