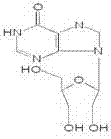
A kind of preparation method of inosine and composition injection thereof
A technology of inosine injection and composition, which is applied in the direction of drug combination, medical preparations containing active ingredients, and devices for making drugs into special physical or taking forms, etc., which can solve the safety of patients' medication and the loss of production enterprises , color yellowing and other problems, to achieve the effect of ensuring the quality of medicines
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
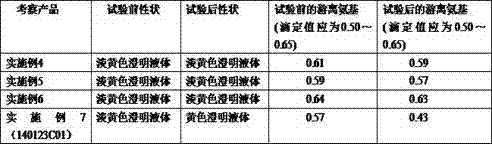
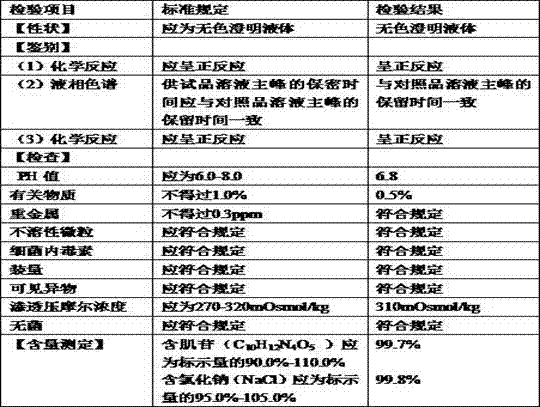
[0073] Accurately weigh the raw and auxiliary materials of each formula in units of 1 million ml of water for injection: inosine 6 kg, sodium chloride 9 kg, glycine 2 kg; medicinal activated carbon 0.1 kg.
[0074] The preparation process comprises the following steps:
[0075] (1) Take 0.1 kg of medicinal activated carbon and make a paste with water for injection.
[0076] (2) Inject 500,000 ml of water for injection into the concentrated preparation tank, add inosine, sodium chloride, and glycine raw materials to stir and dissolve, adjust the pH to 6.5-7.0 with 10% sodium hydroxide solution, and add the adjusted wet medicinal activated carbon Heat to 96°C for 15 minutes.
[0077] (3) After the preparation is completed, the liquid medicine is refluxed and filtered, and the concentrated medicine is filtered using a titanium sintered rod with a pore size not greater than 15 μm for decarburization.
[0078] (4) Use a small beaker to collect the medicinal liquid at the return p...
Embodiment 2
[0087] Accurately weigh the raw and auxiliary materials of each formula in units of 1 million ml of water for injection: inosine 6 kg, sodium chloride 9 kg, glycine 1.5 kg; medicinal activated carbon 0.1 kg.
[0088] The preparation process comprises the following steps:
[0089] (1) Take 0.1 kg of medicinal activated carbon and make a paste with water for injection.
[0090] (2) Inject 500,000 ml of water for injection into the concentrated preparation tank, add inosine, sodium chloride, and glycine raw materials to stir and dissolve, adjust the pH to 6.5-7.0 with 10% sodium hydroxide solution, and add the adjusted wet medicinal activated carbon Heat to 97°C for 15 minutes.
[0091] (3) After the preparation is completed, the liquid medicine is refluxed and filtered, and the concentrated medicine is filtered using a titanium sintered rod with a pore size not greater than 15 μm for decarburization.
[0092] (4) Use a small beaker to collect the medicinal liquid at the return...
Embodiment 3
[0101] Accurately weigh the raw and auxiliary materials of each formula in units of 1 million ml of water for injection: inosine 6 kg, sodium chloride 9 kg, glycine 2.5 kg; medicinal activated carbon 0.1 kg.
[0102] The preparation process comprises the following steps:
[0103] (1) Take 0.1 kg of medicinal activated carbon and make a paste with water for injection.
[0104] (2) Inject 500,000 ml of water for injection into the concentrated preparation tank, add inosine, sodium chloride, and glycine raw materials to stir and dissolve, adjust the pH to 6.5-7.0 with 10% sodium hydroxide solution, and add the adjusted wet medicinal activated carbon Heat to 95°C for 15 minutes.
[0105] (3) After the preparation is completed, the liquid medicine is refluxed and filtered, and the concentrated medicine is filtered using a titanium sintered rod with a pore size not greater than 15 μm for decarburization.
[0106] (4) Use a small beaker to collect the medicinal liquid at the return...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com