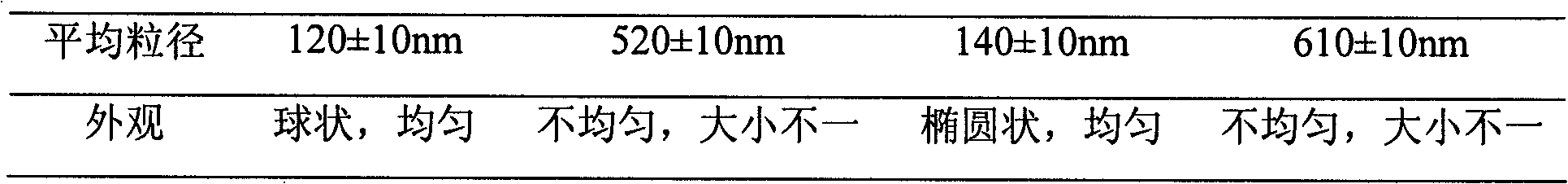
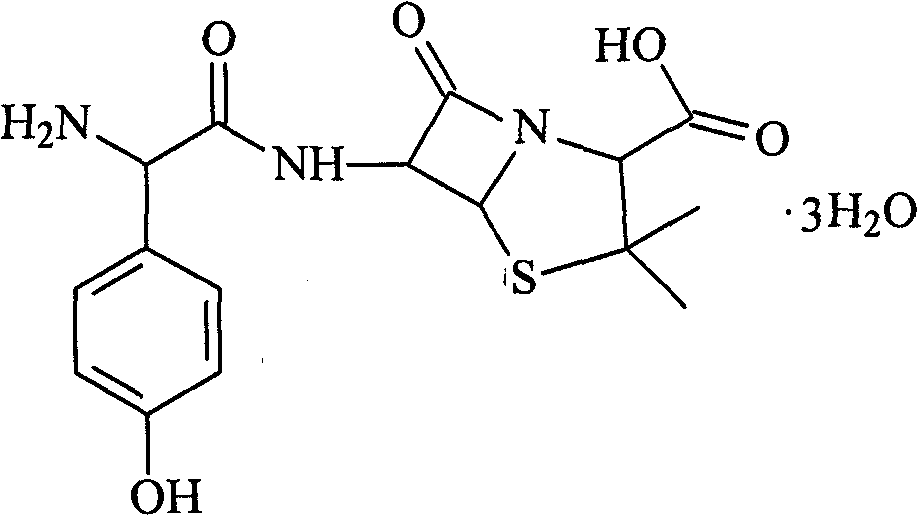
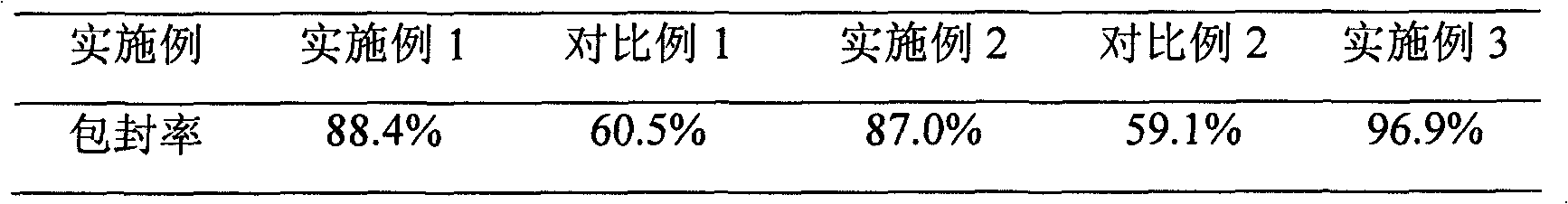
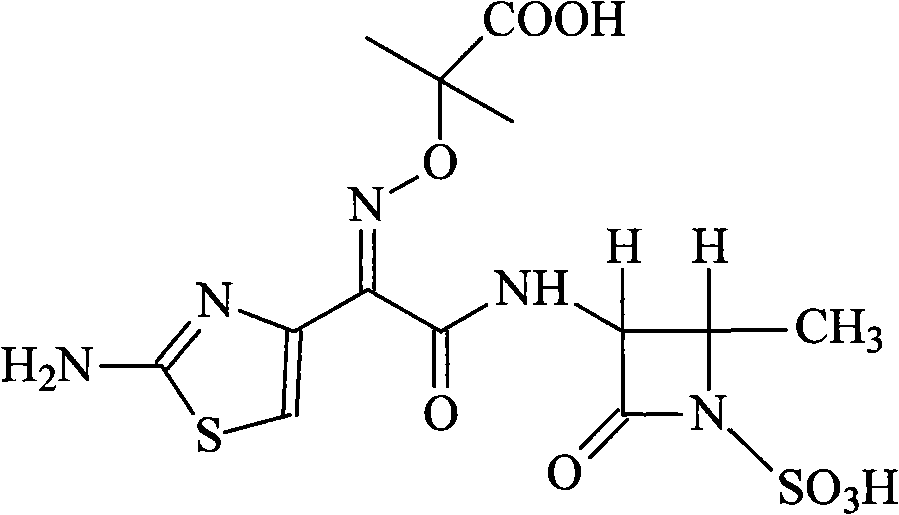
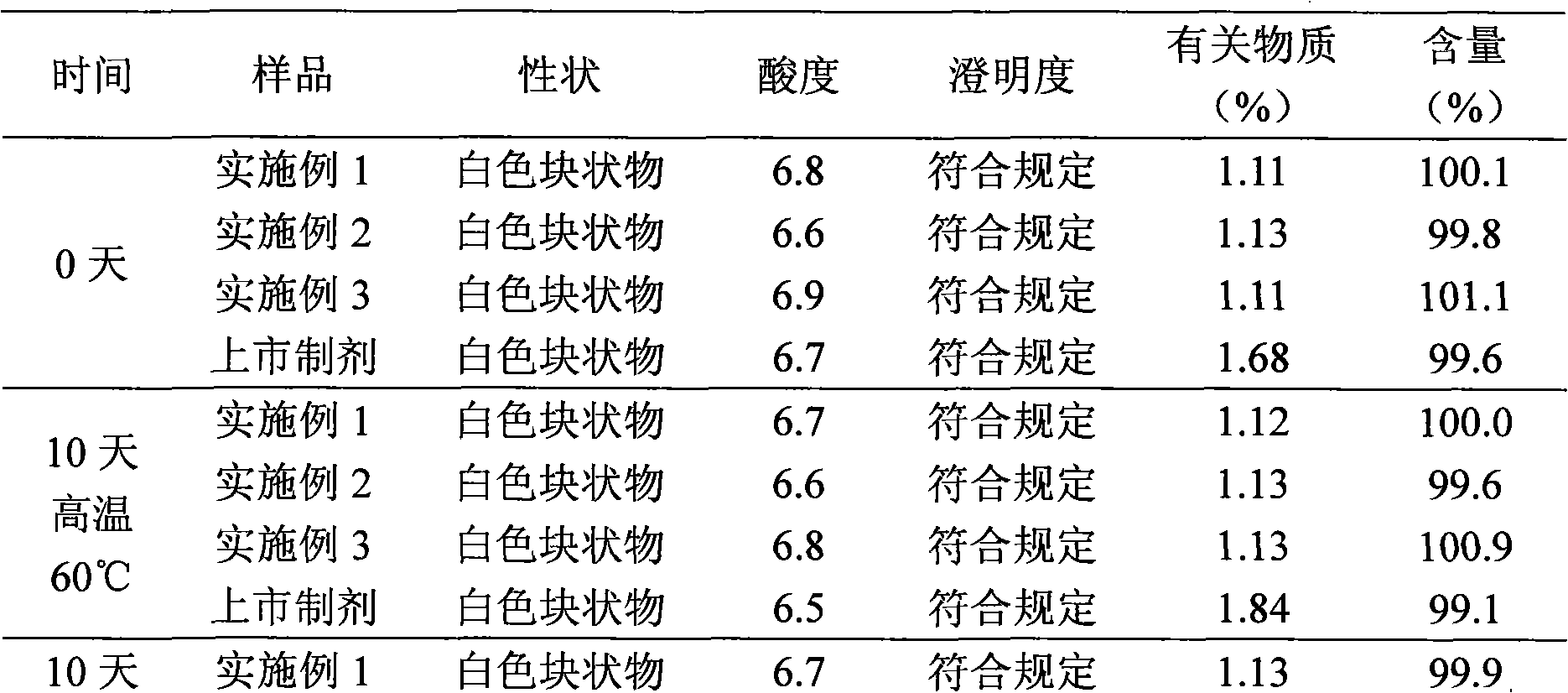
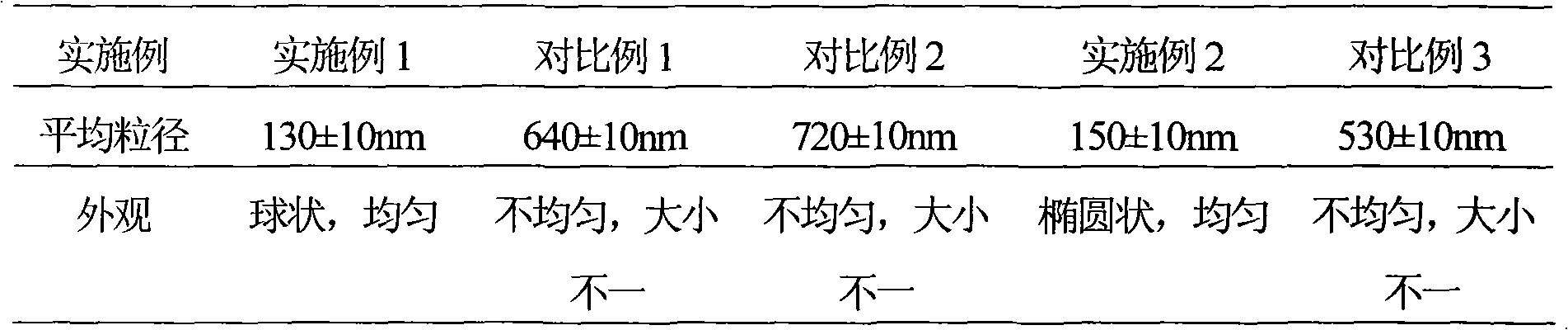
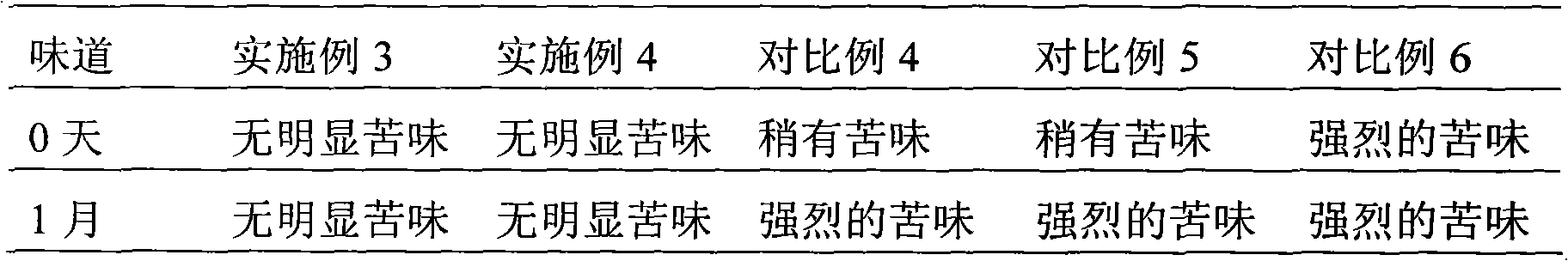
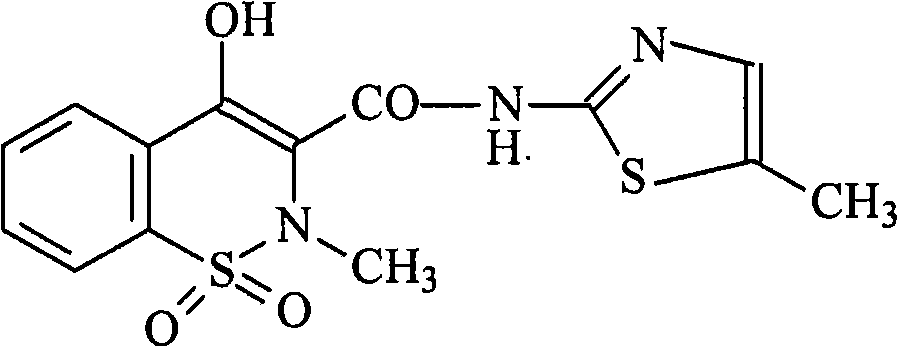
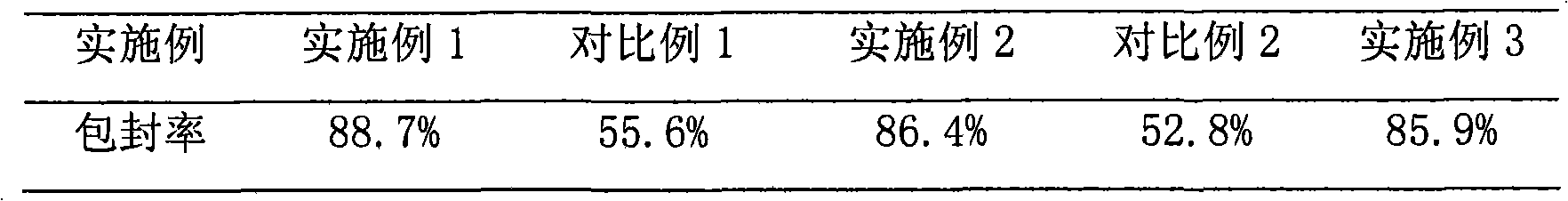
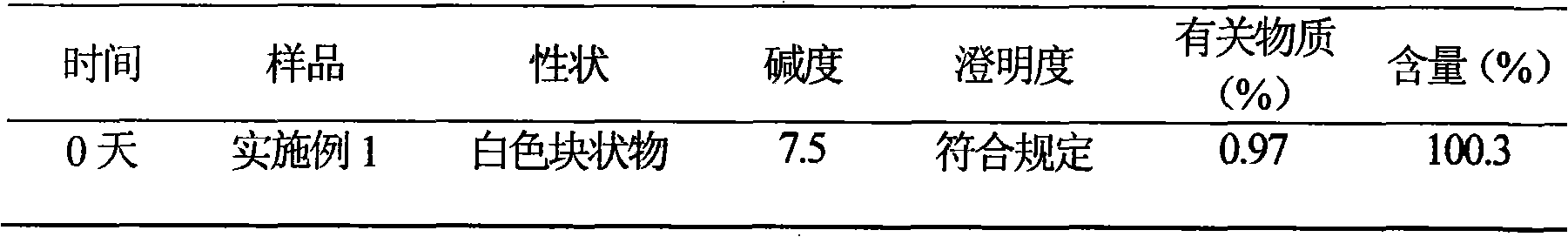
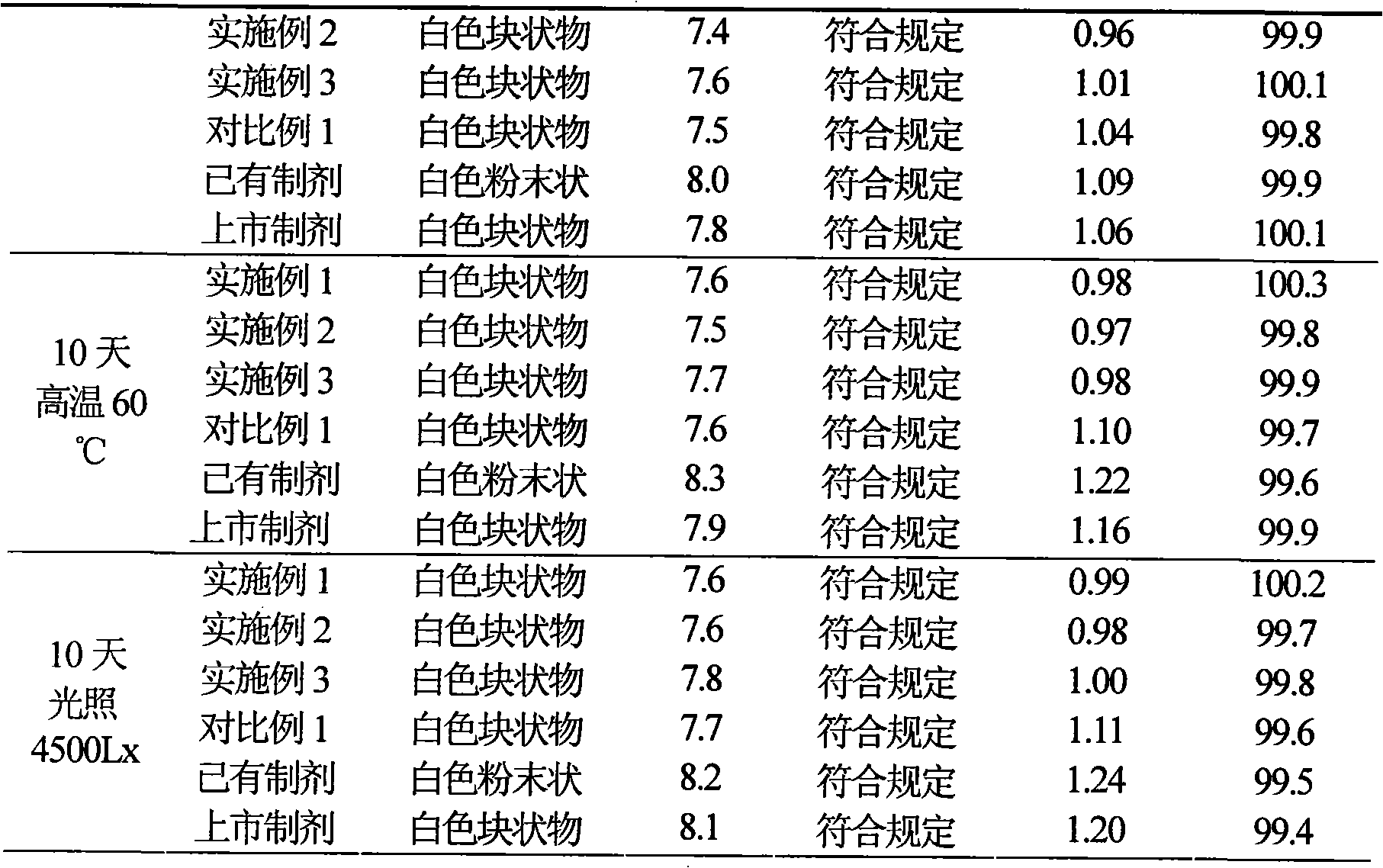
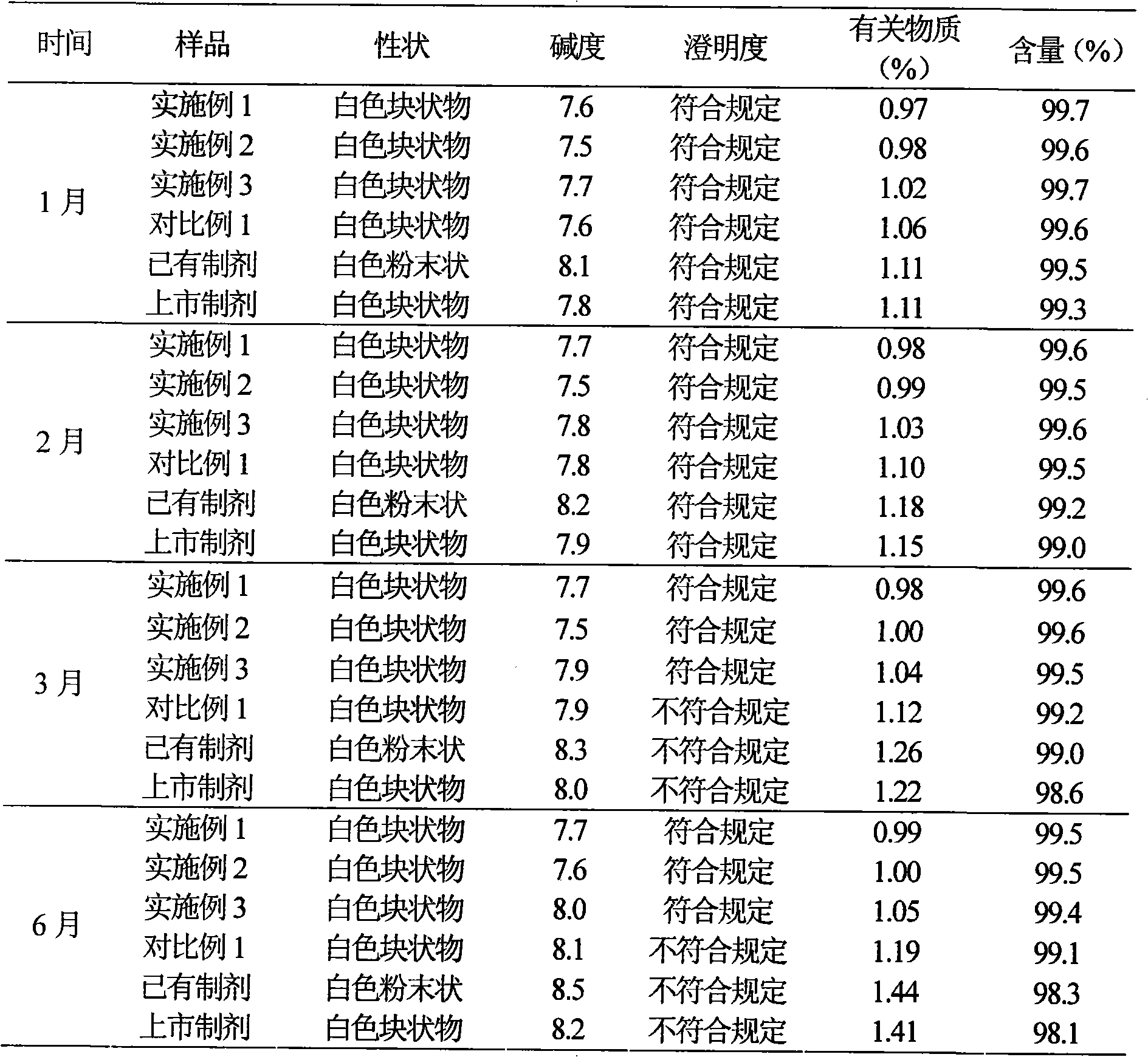
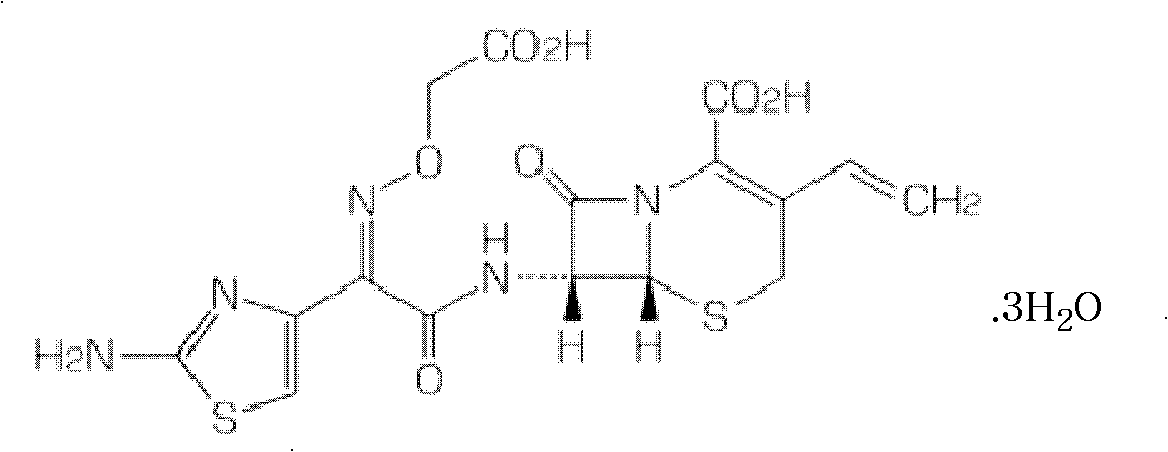
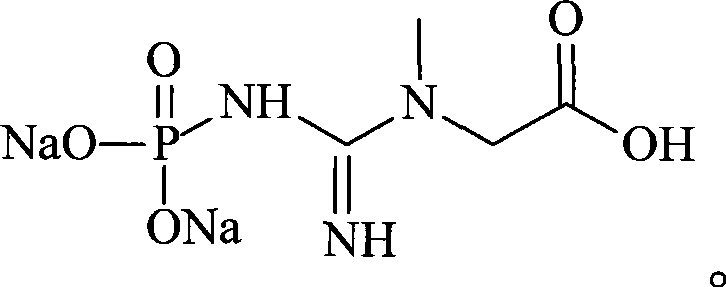
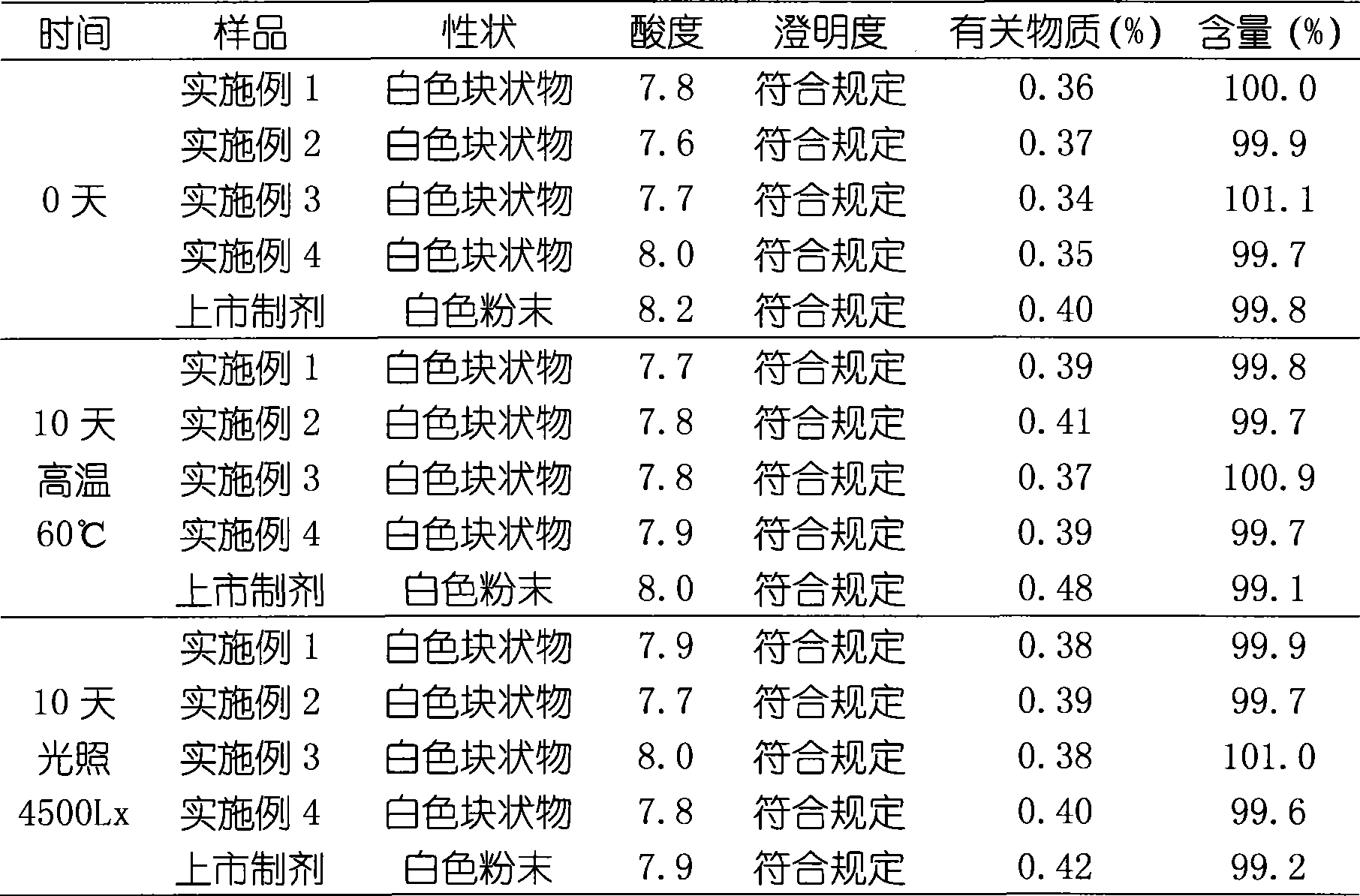
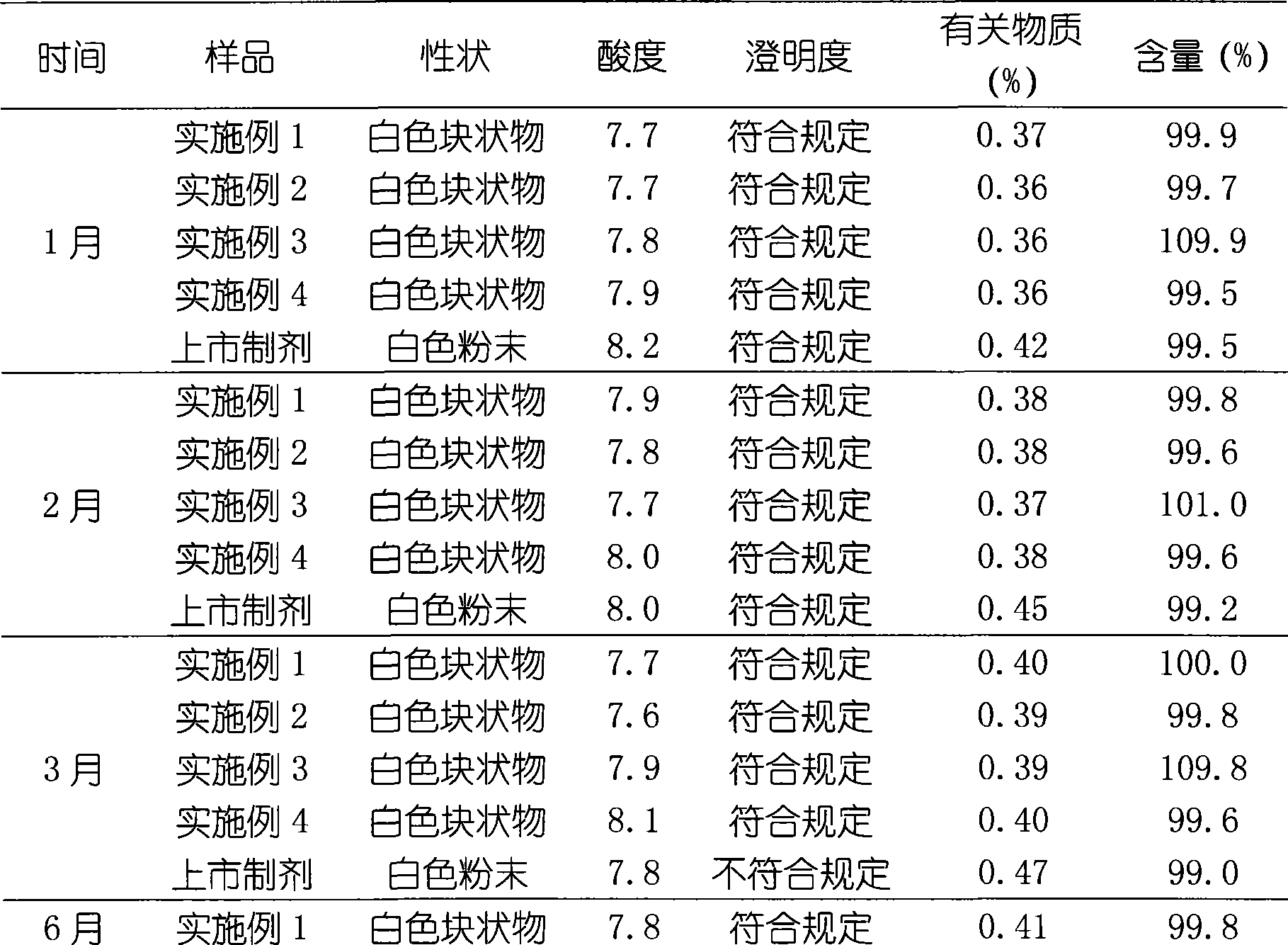
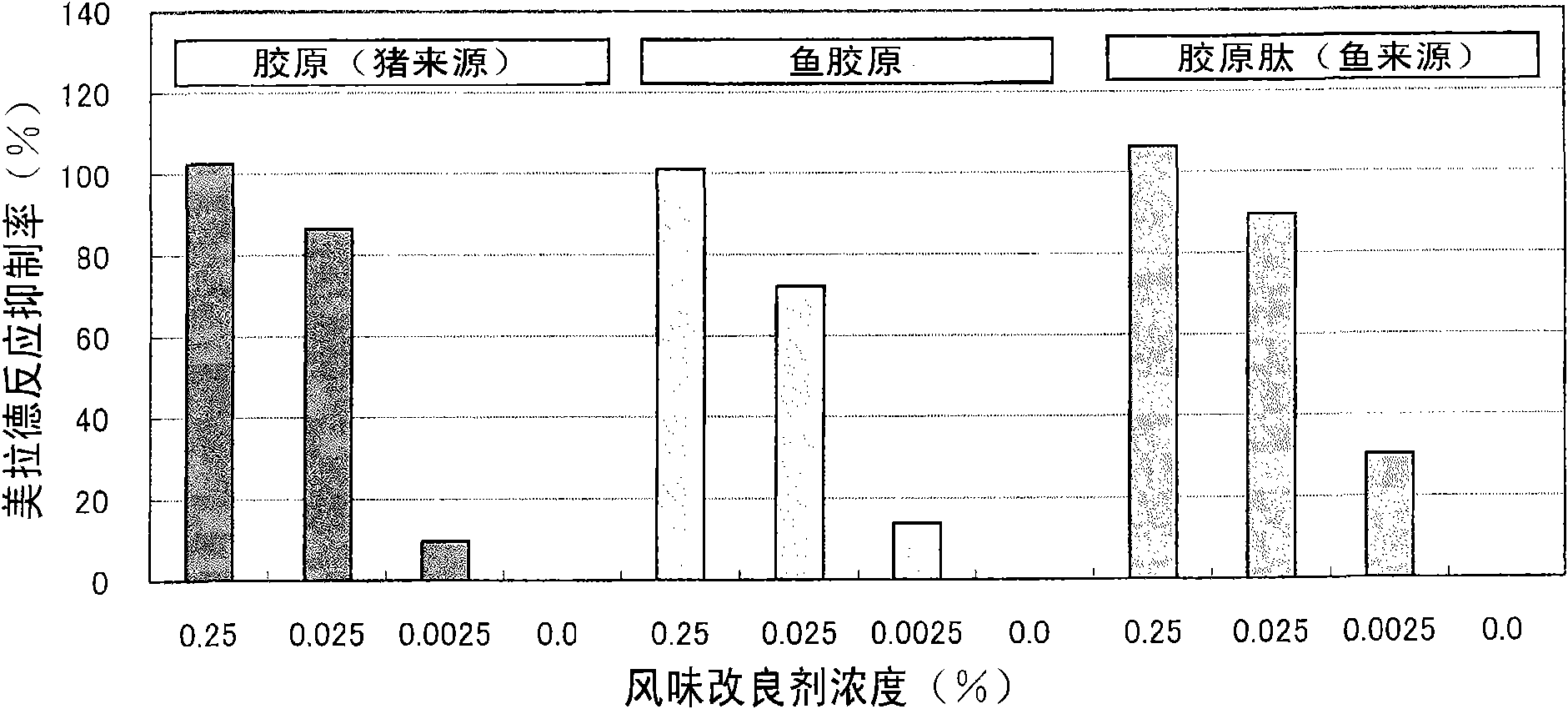
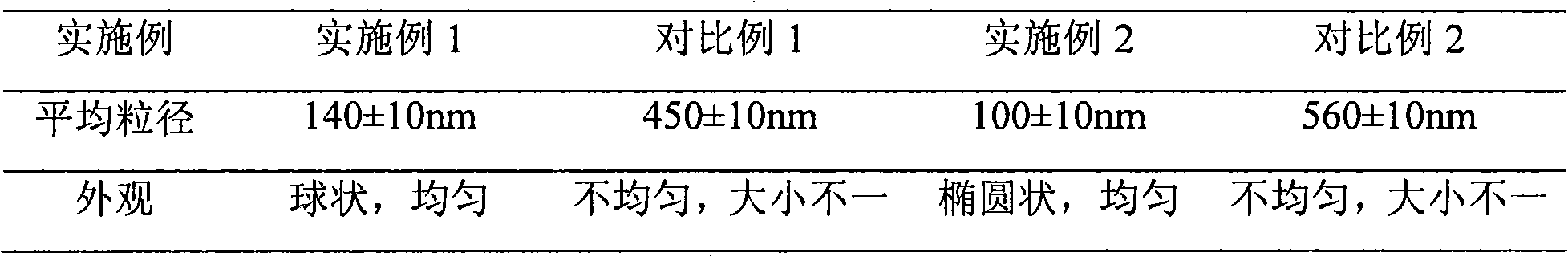
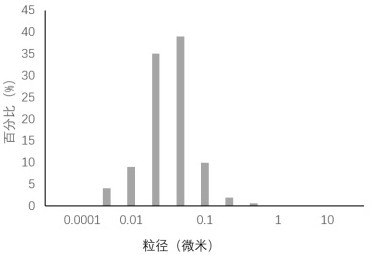
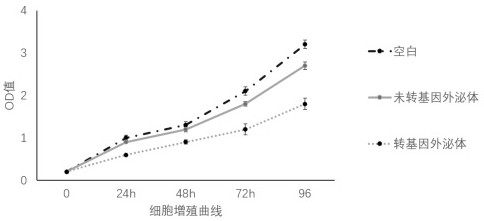
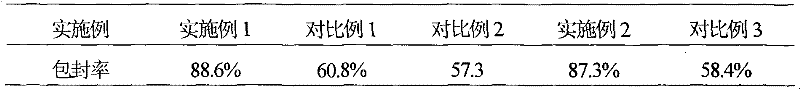
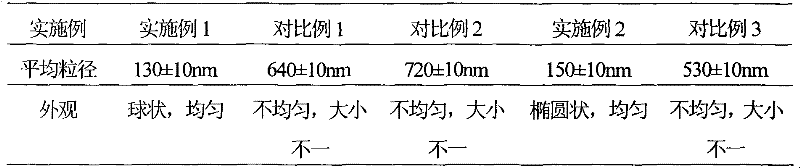
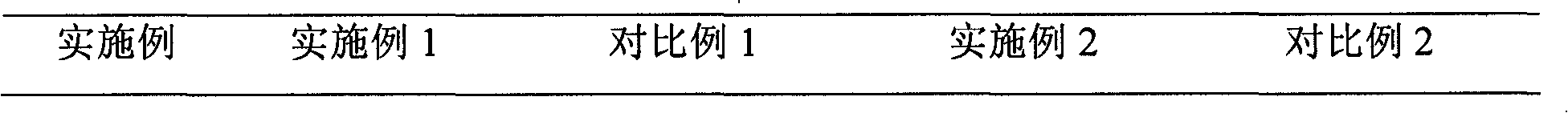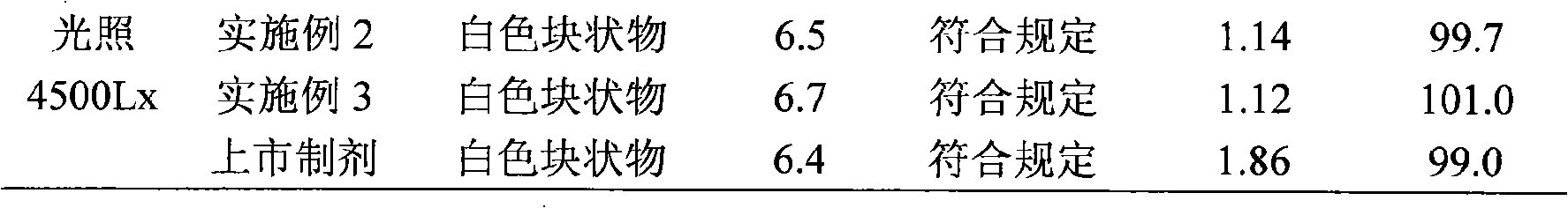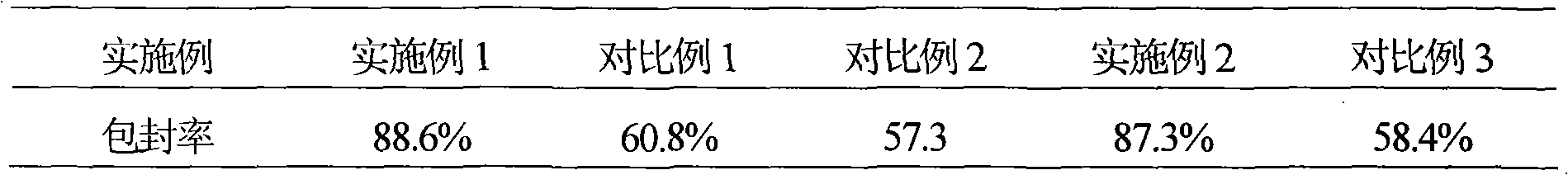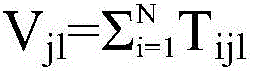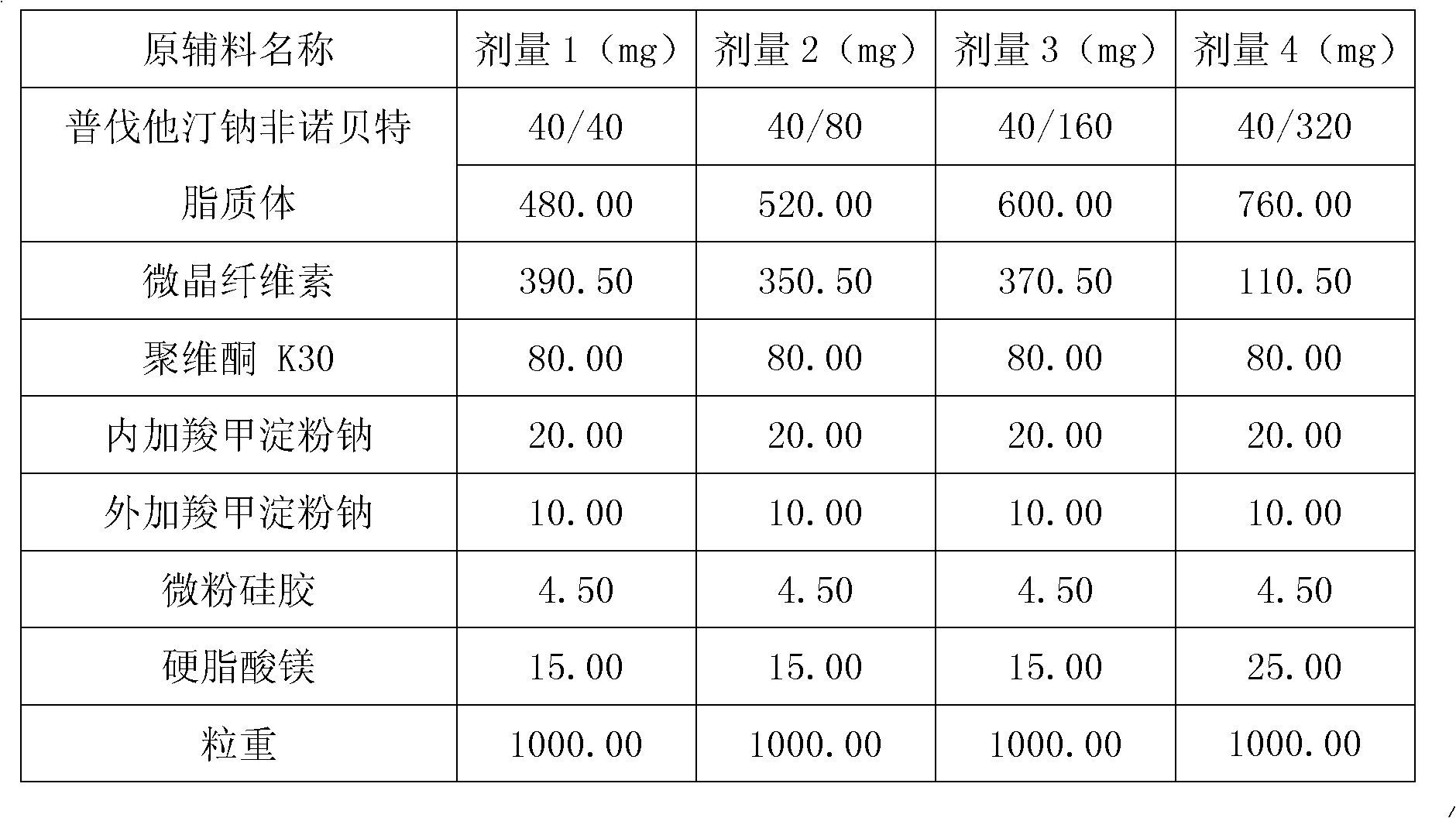Patents
Literature
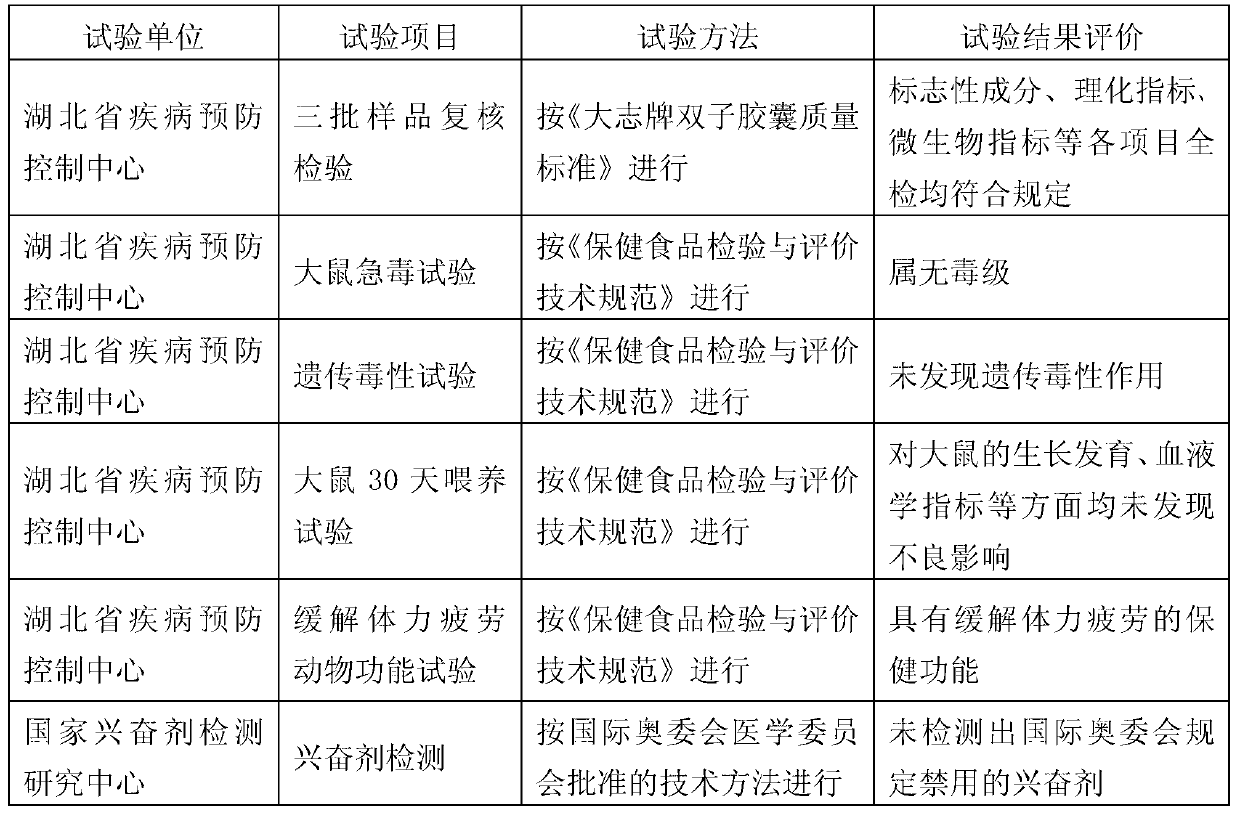
61results about How to "Safety proof" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Composite liposome for injection containing 12 vitamins and preparation method thereof
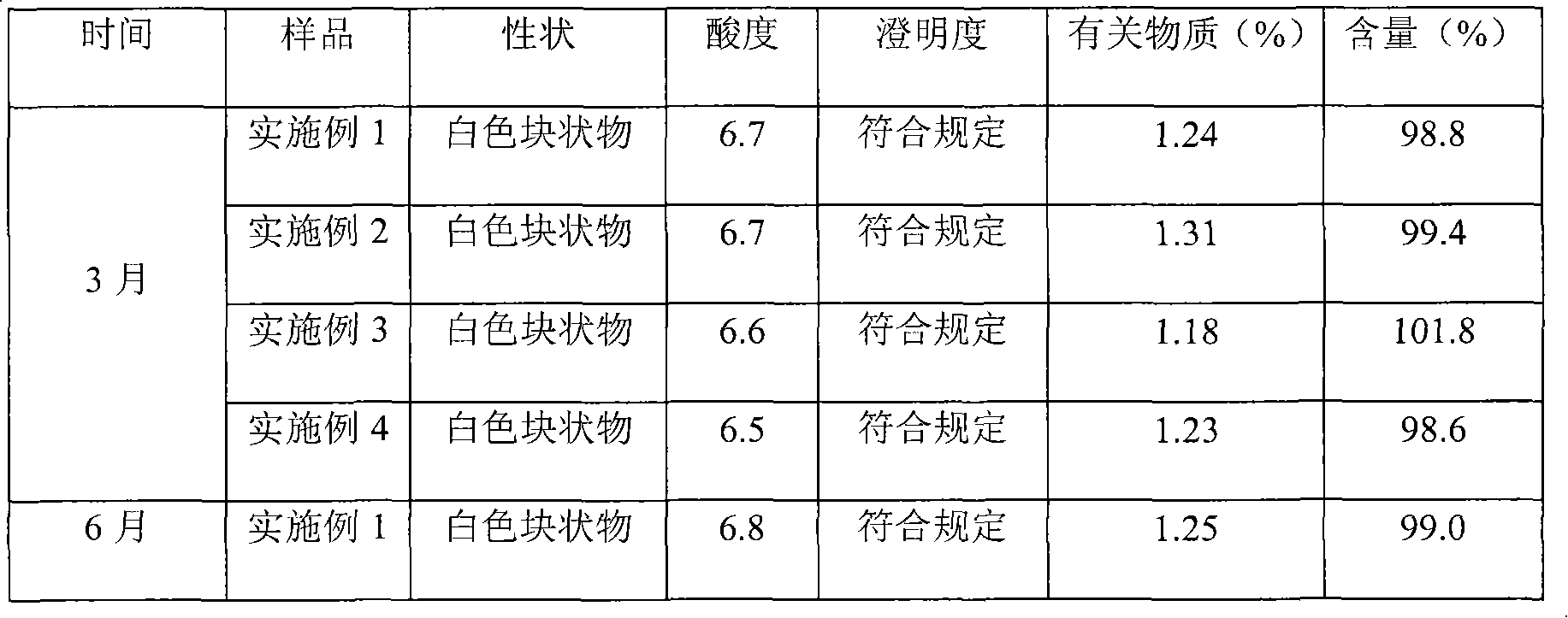
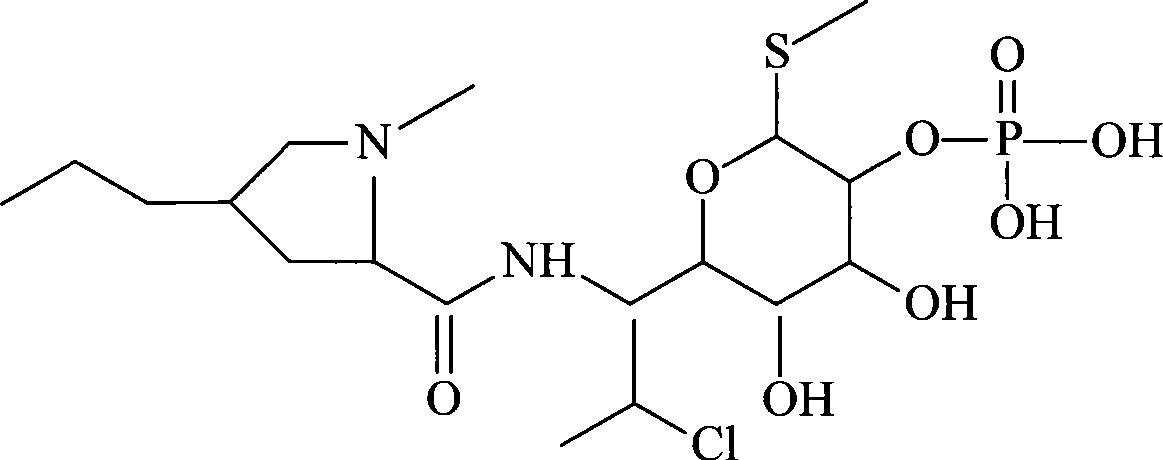
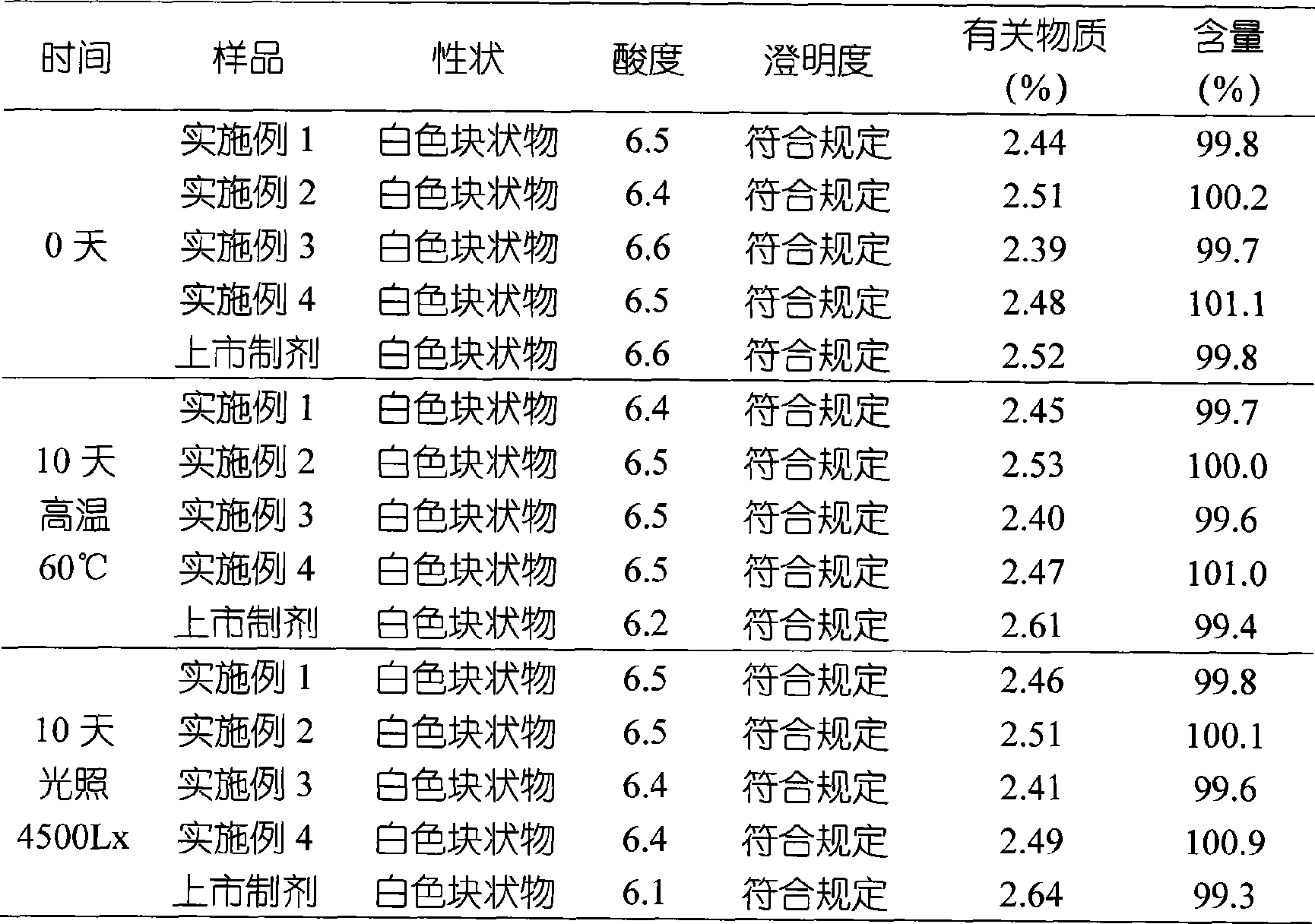
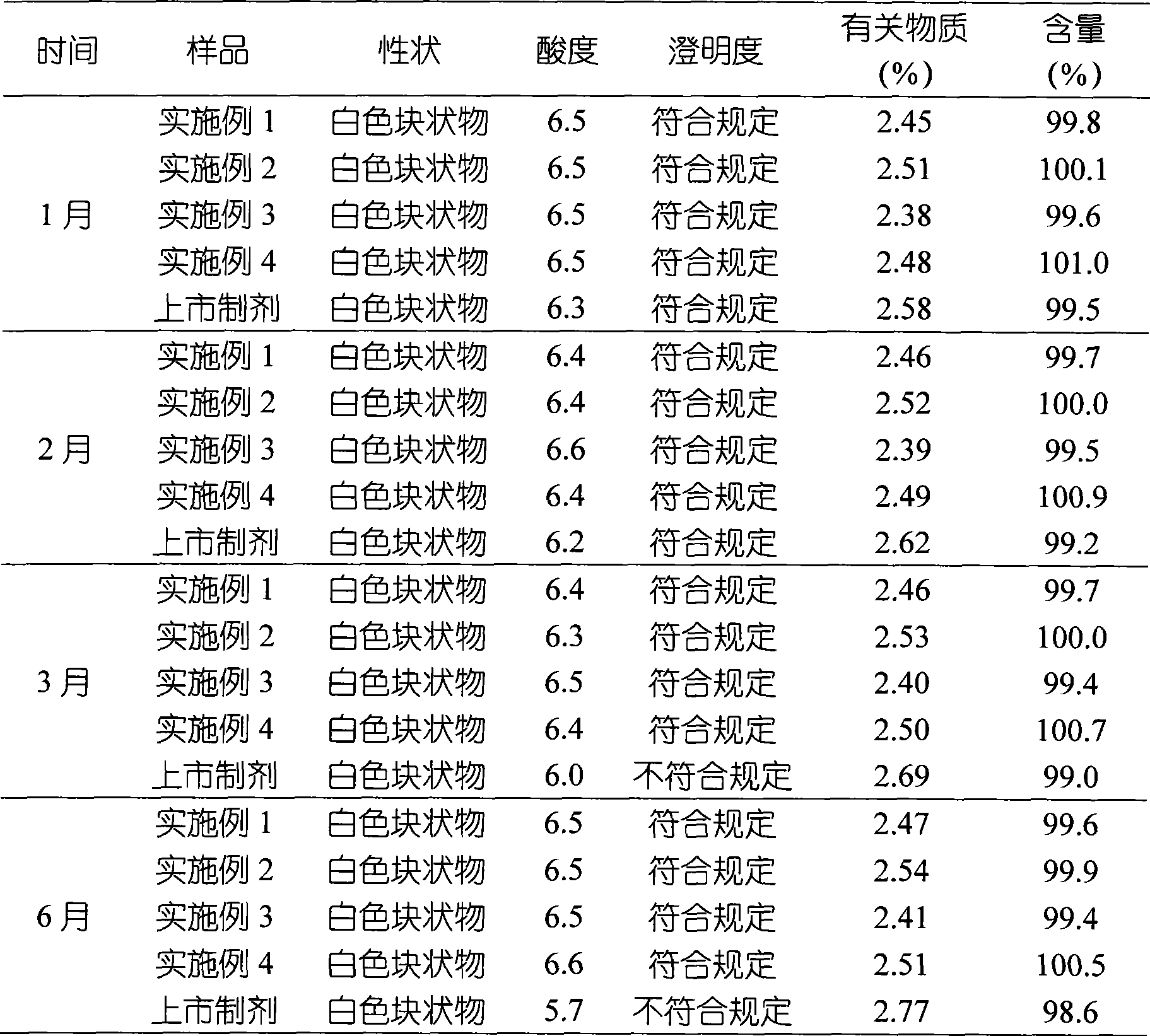
InactiveCN101491499ASafety proofMetabolism disorderAmide active ingredientsAlpha-TocopherolSodium phosphates
The invention relates to a liposome lyophilized preparation prepared from twelve compound vitamins. The lyophilized preparation is characterized in that the lyophilized preparation is prepared from a liposome which consists of soybean lecithin and glycocholic acid and is encapsulated with twelve vitamins of retinyl palmitate, cocarboxylase tetrahydrate, riboflavin sodium phosphate, pyridoxine hydrochloride, cyanocobalamin, cholecalciferol, ascorbic acid, racemization-alpha tocopherol, D-biotin, niacinamide, folacin and dexpanthenol.
Owner:灵康药业集团股份有限公司
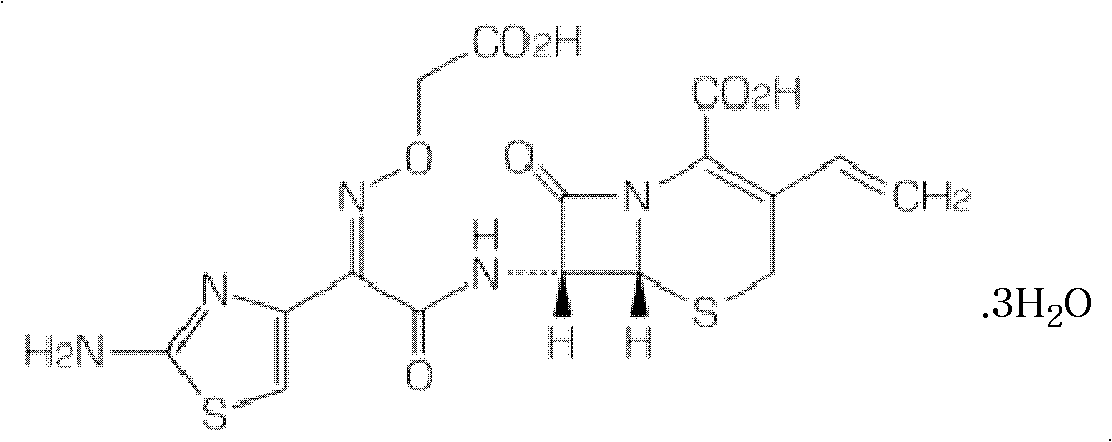
Aztreonam liposomes freeze-dry preparations and method of preparing the same
InactiveCN101249074AImprove stabilitySolve the problem of quality stabilityAntibacterial agentsOrganic active ingredientsFreeze-dryingAztreonam
The invention discloses a freeze-dried preparation of aztreonam liposomes, wherein aztreonam is encapsulated in antioxidant-containing liposomes made of neutral phospholipids, negative charge phospholipids and cholesterol. The freeze-dried preparation has stable quality. Additionally, the aztreonam is encapsulated to minimize toxicity and adverse effects without affecting drug effectiveness.
Owner:HAINAN LINGKANG PHARMA CO LTD
Clindamycin phosphate lipidosome freeze-dried preparation and preparation method thereof
InactiveCN101530393AAchieve vitrificationAvoid damageAntibacterial agentsOrganic active ingredientsFreeze-dryingCholesterol
The invention relates to a clindamycin phosphate lipidosome freeze-dried preparation and a preparation method thereof. The clindamycin phosphate lipidosome freeze-dried preparation is characterized by comprising the following components in portion by weight: 15 to 25 portions of clindamycin phosphate, 10 to 40 portions of dimyristyl acid lecithin, 1 to 10 portions of cholesterol, 1 to 5 portions of antioxidant, and 5 to 25 portions of cryoprotectant.
Owner:HAINAN LINGKANG PHARMA CO LTD
Edaravone lipid microsphere formulation and preparation method
InactiveCN101536979ASafety proofImprove stabilityOrganic active ingredientsNervous disorderLipid formationEdaravone Injection
The invention relates to an edaravone lipid microsphere formulation and a preparation method thereof. The edaravone lipid microsphere formulation is prepared by taking the mixed emulsifier of granulesten and poloxamer188 with specific rate as an emulsifier and injection soybean oil as oil phase solvent, and also contains pharmaceutically regular antioxidant. Not only does the prepared edaravone lipid microsphere injection have simple process, low cost and easy industrial production, but also obviously improved stability compared with the existing edaravone injection.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Ranitidine hydrochloride lipidosome capsule and new application thereof
InactiveCN101623258AImprove efficacyImprove bioavailabilityDigestive systemAntiviralsYolkHerpetic stomatitis
The invention provides a ranitidine hydrochloride lipidosome capsule and new application thereof. Particularly, certain amounts of yolk lecithin, cholesterin, natrium glycocholicum, tween 80 and an active ingredient ranitidine hydrochloride are combined and are prepared into ranitidine hydrochloride lipidosome by film dispersion technology, and the ranitidine hydrochloride lipidosome is mixed with the general accessories of medicine to prepare the capsule. The ranitidine hydrochloride lipidosome capsule better solves the problems of easy deliquescence, moisture absorption, color change and poor stability of the ranitidine hydrochloride, increases preparation medical effect and biological availability and can be used for treating herpetic stomatitis of childrem.
Owner:HAINAN MEIDA PHARMA
Amoxicillin lipidosome solid preparation and new application thereof
InactiveCN101623259AFix stability issuesPromote dissolutionAntibacterial agentsDigestive systemChemistryPoor quality
The invention provides an amoxicillin lipidosome solid preparation and a new application thereof. Certain amounts of yolk lecithin, cholesterin, natrium glycocholicum, tween 80 and an active ingredient amoxicillin are combined and are prepared into amoxicillin lipidosome by film dispersion technology, and the amoxicillin lipidosome is mixed with the certain accessories to prepare various solid preparations. The amoxicillin lipidosome solid preparation not only can solve the problems of poor dissolution rate and poor quality stability of the amoxicillin, increases the medical effect and the bioavailability of the preparation, can treat bacterial liver abscess and obtain good invention effect.
Owner:HAINAN MEIDA PHARMA
Sub-micro emulsion frozen preparation of aztreonam
InactiveCN101548956ASafety proofImprove stabilityAntibacterial agentsPowder deliveryEmulsionAztreonam
The invention provides a sub-micro emulsion frozen preparation of aztreonam and a preparation method thereof. The frozen preparation is prepared mainly with the following components according to parts by weight: 1-20 portions of aztreonam, 1-40 portions of biodegradable polymer, 1-20 portions of emulsifying agent, 10-50 portions of skeleton supporting agent and 0.5-20 portions of stabilizing agent.
Owner:HAINAN LINGKANG PHARMA CO LTD
Loratadine-ambroxol pharmaceutical composite and liposome solid preparation thereof
InactiveCN101627998AImprove solubilityImprove stabilityOrganic active ingredientsPharmaceutical product form changeYolkMedicine
The invention relates to a loratadine-ambroxol pharmaceutical composite and a liposome solid preparation thereof and a preparation method thereof; the liposome comprises the following components according to the parts by weight percent: 1 part of loratadine, 5 parts of ambroxol hydrochloride, 3-30 parts of yolk lecithin, 1-14 of cholesterol, 1.2-10 parts of sodium deoxycholate and 3-18 parts of poloxamer 188.
Owner:HAINAN YONGTIAN PHARMA INST
Meloxicam liposome and pharmaceutical composition thereof
InactiveCN101632640AUnexpected effectImprove stabilityOrganic active ingredientsAntipyreticSolubilityMeloxicam
The invention provides a meloxicam liposome and a pharmaceutical composition thereof. The meloxicam liposome consists of one part by weight of meloxicam, 1.5-10 parts by weight of phospholipid, 1-3 parts by weight of cholesterin and 1-4 parts by weight of poloxamer 188. The invention is made in a such way that the meloxicam is made into the liposome, and the liposome is made into solid pharmaceutical preparation. The preparation components and the preparation art are simple, compared with the prior meloxicam preparation, the invention can not only improve the stability of the meloxicam solid preparation, but also can improve the dissolvability and the bioavailability of the meloxicam.
Owner:HAINAN YONGTIAN PHARMA INST
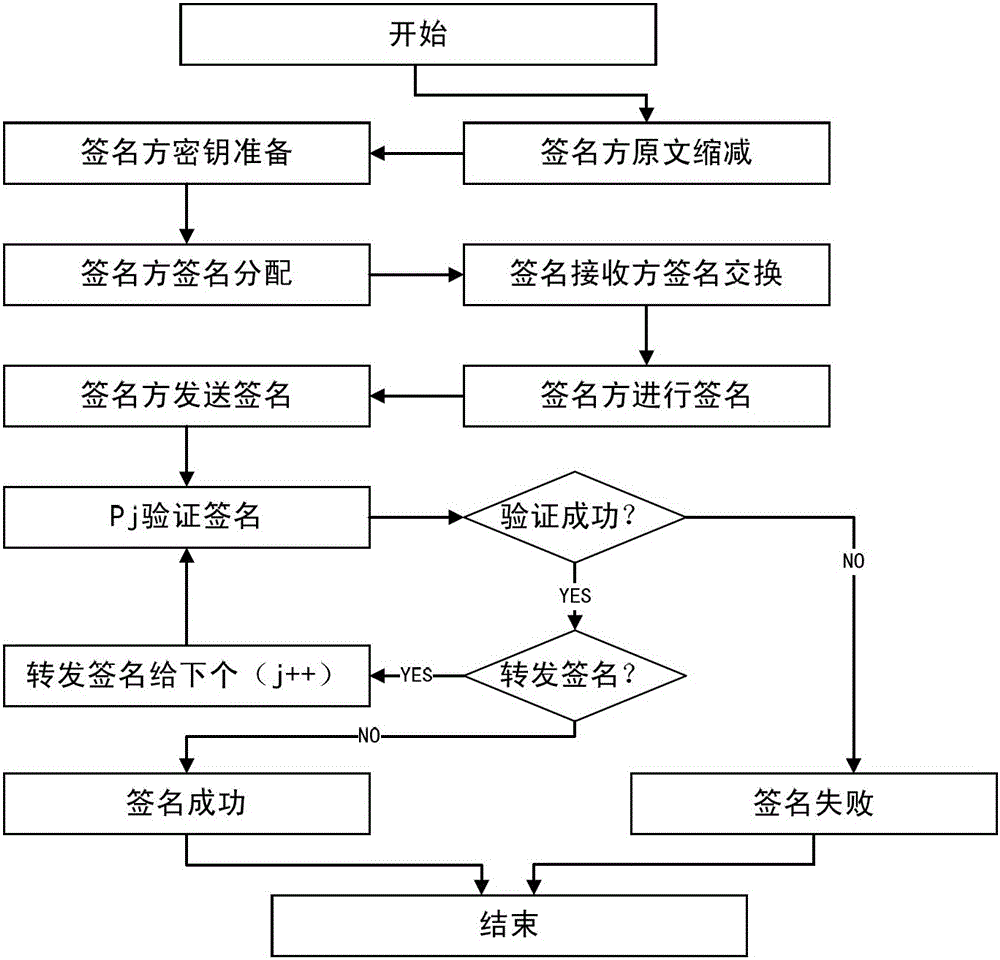
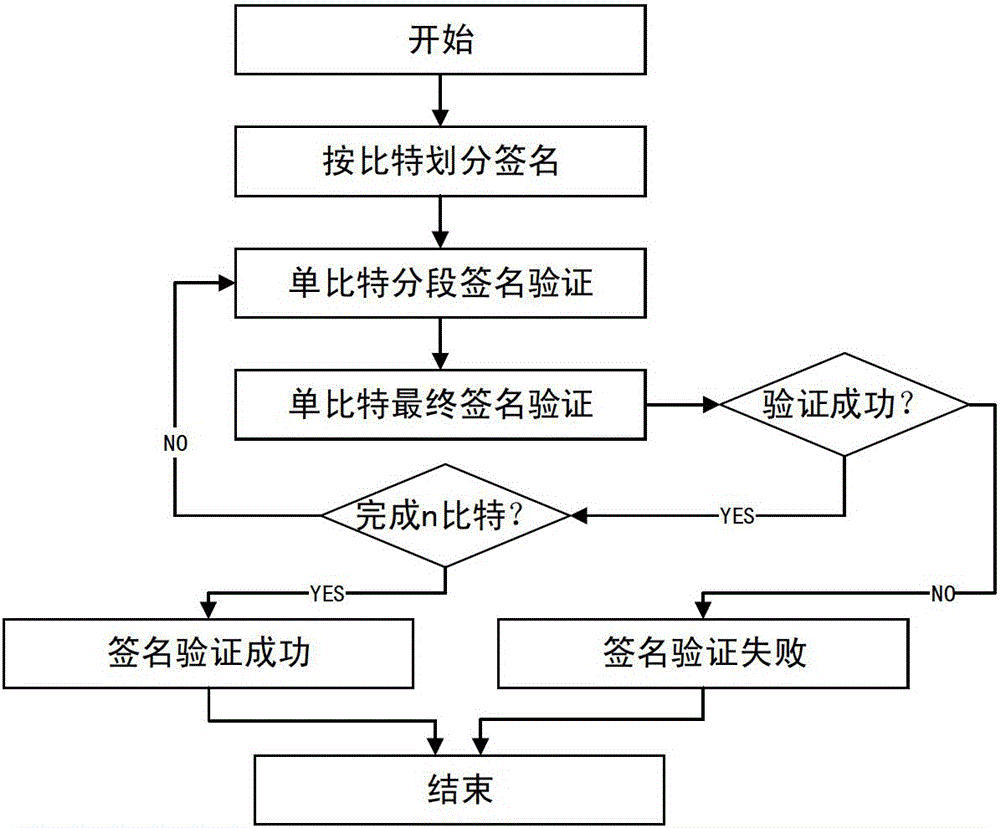
Multi-party quantum digital signature method without trusted center
ActiveCN106452790AOvercoming the Weakness of Computational ComplexitySafety proofKey distribution for secure communicationUser identity/authority verificationQuantumQuantum digital signature
The invention discloses a multi-party quantum digital signature method without a trusted center. The method comprises the following steps: step a, a sender performs operation on an original text to obtain a reduced original text; step b, the sender generates a signature and sends the signature to verification parties separately; step c, each two verification parties exchange the partially owned signature; step d, the sender sends the original text and the signature generated in step b to one verification party; step e, the current verification party performs operation on the received original text to obtain the reduced original text, and performs comparison verification on the signature received from the sender or the previous verification party and the owned signature after the exchange in step c according to each bit of the reduced original text; and after the verification is passed, the other verification parties perform verification in sequence until a preset number of verification parties pass the verification. By adoption of the multi-party quantum digital signature method disclosed by the invention, the problem that a large number of keys are needed in single bit signature is overcome, the multi-party signature demands can be satisfied, and the application scenes are greatly expanded.
Owner:ZHEJIANG SHENZHOU QUANTUM NETWORK TECH CO LTD
Valsartan liposome, preparation method thereof and medicinal composition containing same
InactiveCN101810580AThe drug works quicklyAchieve targeted deliveryPharmaceutical non-active ingredientsPill deliveryValsartanAdditive ingredient
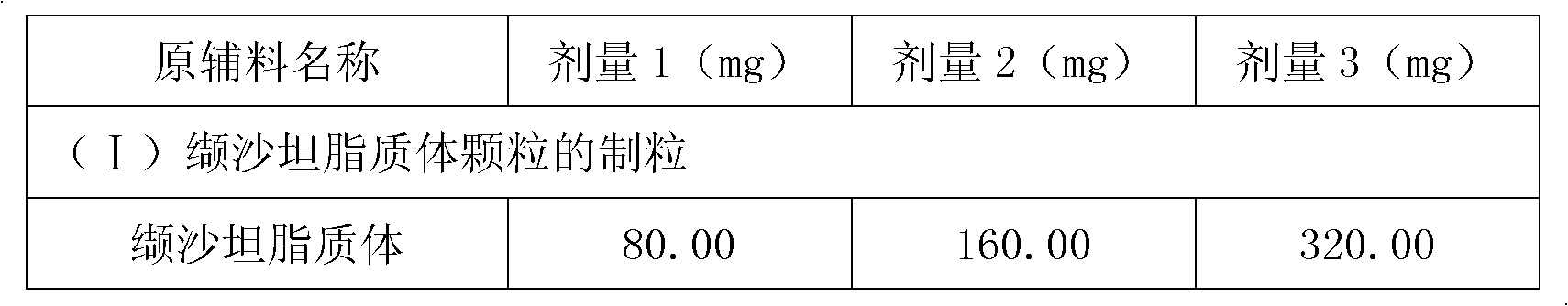
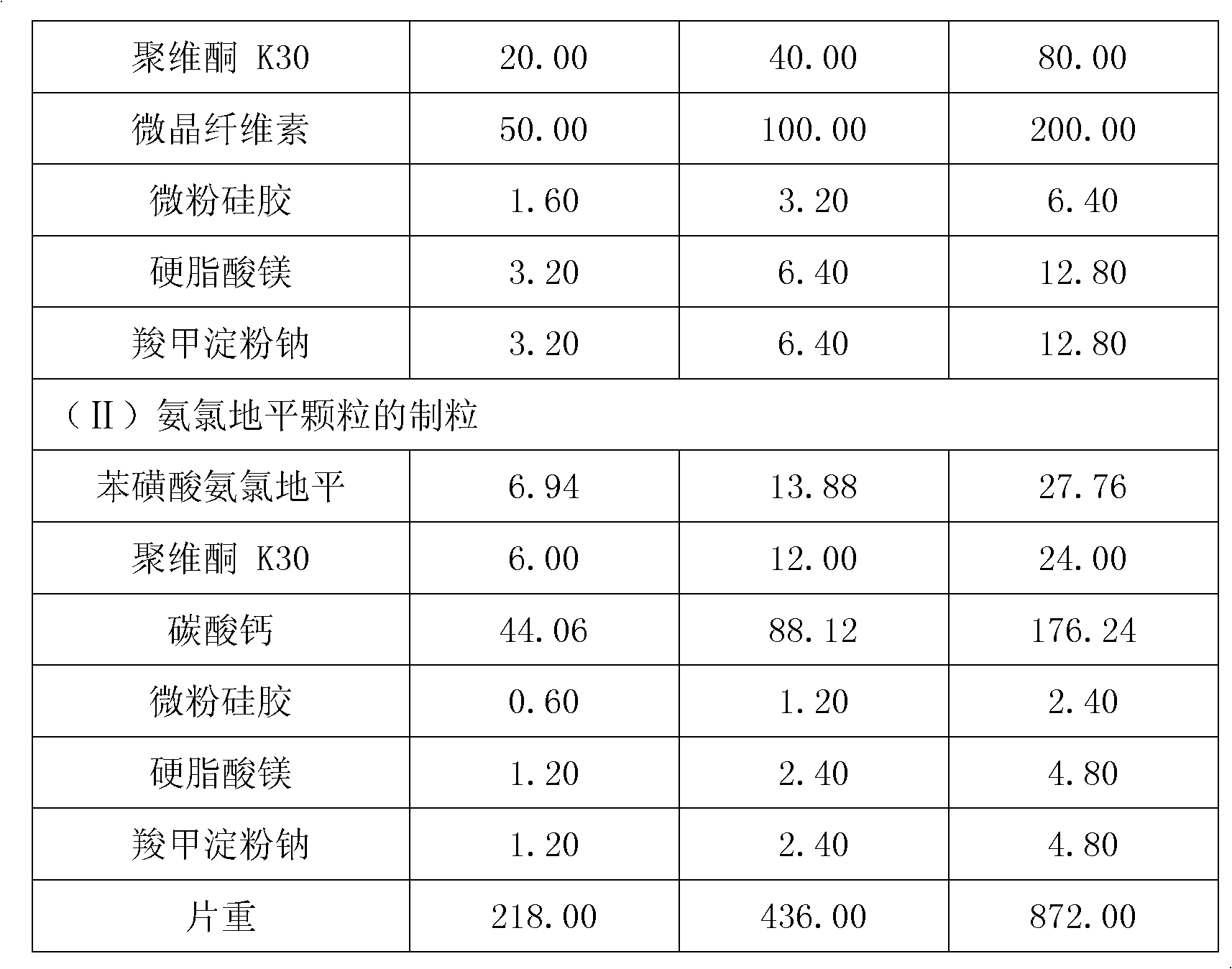
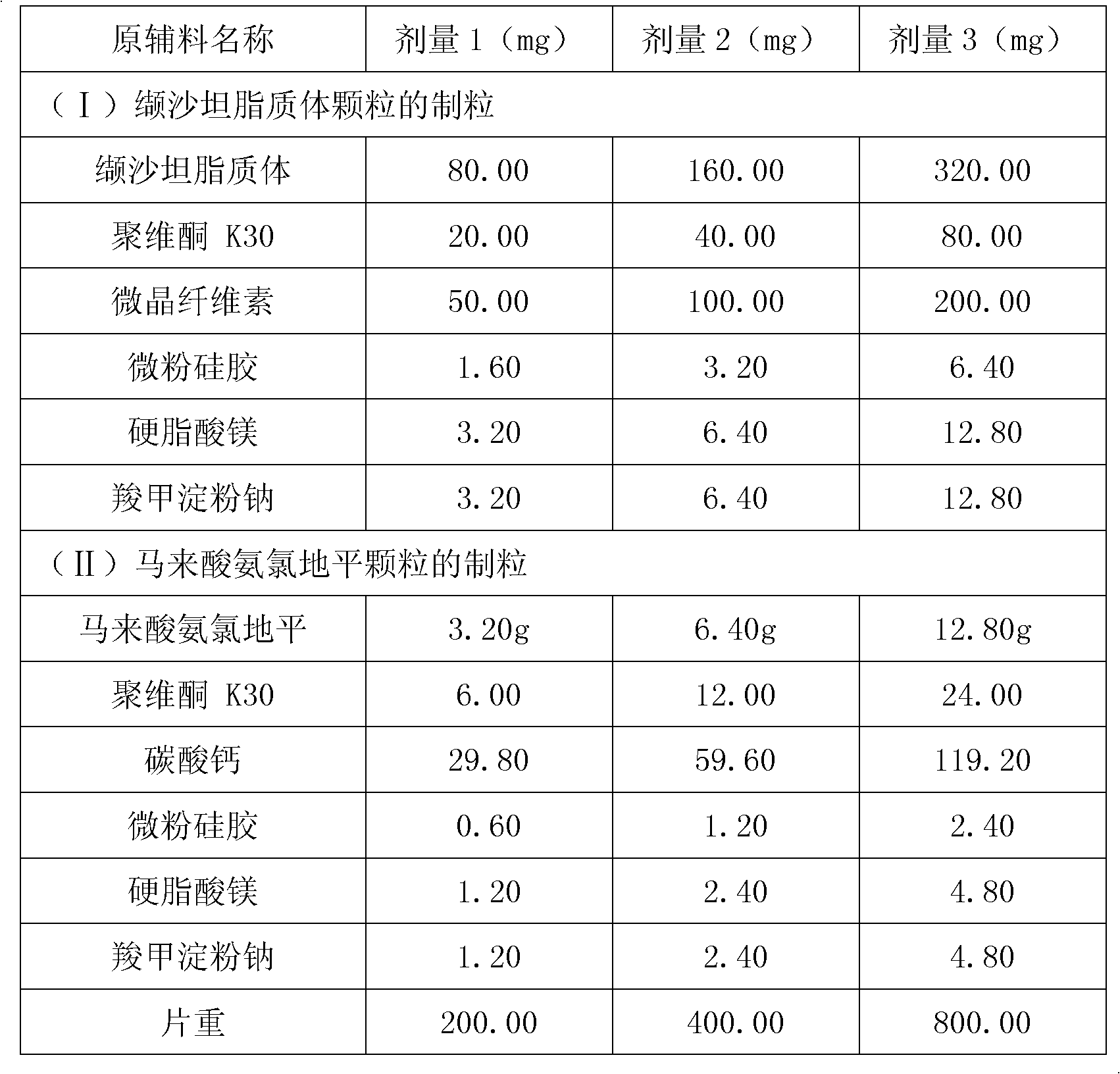
The invention relates to valsartan liposome, a preparation method thereof and a medicinal composition containing the same. Active components of the medicinal composition are valsartan and / or amlodipine. The valsartan liposome consists of the valsartan or pharmaceutically acceptable salt thereof and phospholipid, wherein the weight of the phospholipid is 1 to 10 times that of the valsartan or the pharmaceutically acceptable salt thereof; and the medicinal composition prepared by the valsartan liposome not only meets the requirement of Chinese Pharmacopoeia, but also has the advantages of quicker dissolution, quicker drug effect exertion, obviously improved biological utilization compared with conventional valsartan medicinal composition.
Owner:王丽燕
Traditional Chinese medicine health care food for relieving physical fatigue and preparation method thereof
ActiveCN103340405AReasonable compositionEfficacy balanceAntinoxious agentsFood preparationAlcoholWhole body
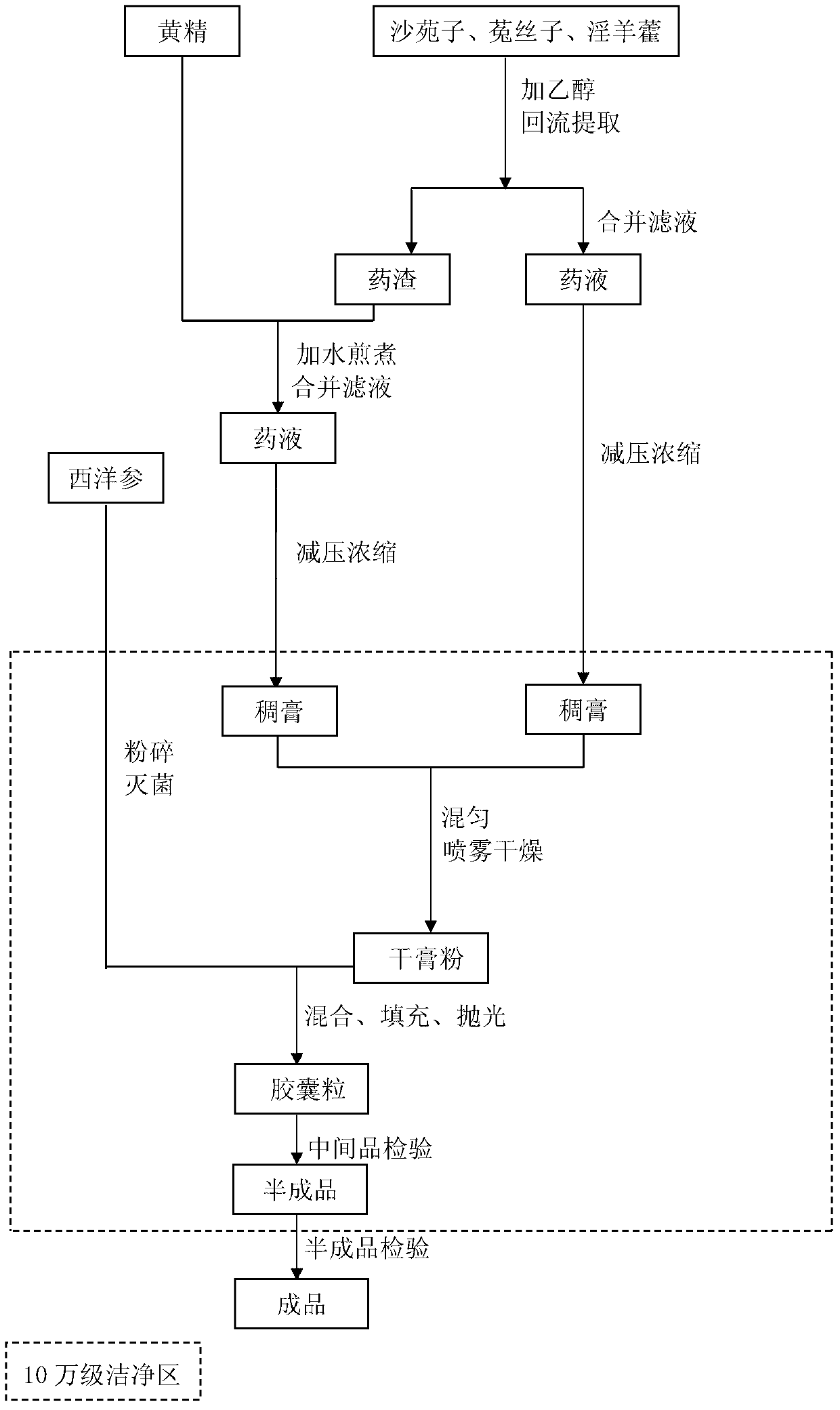
The invention discloses a traditional Chinese medicine health care food for relieving physical fatigue and a preparation method thereof. The health care food is prepared by five pure traditional Chinese medicine raw materials including flatstem milkvetch seed, semen cuscutae, herba epimedii, rhizoma polygonati and American ginseng. The raw materials are as follows in parts by weight: 20-50 parts of flatstem milkvetch seed, 20-40 parts of semen cuscutae, 5-20 parts of herba epimedii, 5-20 parts of rhizoma polygonati and 1-6 parts of American ginseng. The raw materials are orderly treated by preparation methods such as pretreatment, alcohol extraction, water extraction, thick paste mixing, spray-drying, crushing, mixing, filling, polishing, packing and the like so as to obtain an oral capsule preparation. The traditional Chinese medicine health care food disclosed by the invention has reasonable components and balanced effect, plays an overall regulation advantage and regulates the function of each visceral organ in the whole body, so the traditional Chinese medicine health care food is a good pure traditional Chinese medicine health care food that is helpful to relieve physical fatigue, also causes no untoward effects, has high safety even being taken for a long time, and causes no dependency. The preparation method related to the invention is simple, low in cost and capable of realizing industrial production.
Owner:SHAANXI DAZHI PHARMA
Granular formulation containing cefixime liposomes and preparation method thereof
InactiveCN101966154AHigh encapsulation efficiencyReduce leak rateOrganic active ingredientsAntiinfectivesYolkCholesterol
The invention relates to a cefixime liposome, a preparation method and a granular formulation containing the cefixime liposome. The granular formulation comprises the cefixime liposome and a pharmaceutically acceptable vector, wherein the cefixime liposome is prepared from the following components in parts by weight: 1 part of cefixime, 1.25-5 parts of hydrogenated soybean lecithin, 1.25-5 parts of hydrogenated egg yolk lecithin, 2.5-10 parts of cholesterol and 0.1-4.5 parts of polysorbate 80. The granular formulation not only accords with the requirement on Chinese pharmacopoeia, but also has the advantages of stabler storage and rapider drug effect exertion and remarkably improved bioavailability compared with the common pharmaceutical cefixime composition at a room temperature.
Owner:王丽燕
Cefotiam hydrochloride/anhydrous sodium carbonate medicinal composition suspension injection and new use thereof
InactiveCN101912361AHigh parcel rateCommon low priceOrganic active ingredientsInorganic non-active ingredientsCefotiam HydrochlorideSodium carbonate anhydrous
The invention provides cefotiam hydrochloride / anhydrous sodium carbonate medicinal composition suspension injection and new use thereof. The preparation is prepared from 166 to 282.2 weight parts of biodegrading agent, 6.64 to 26.56 weight parts of emulsifying agent, 9.96 to 26.56 weight parts of stabilizer, 13.28 to 49.8 weight parts of additive, 83 weight parts of cefotiam hydrochloride and 17 weight parts of anhydrous sodium carbonate. The invention further discloses new use of the cefotiam hydrochloride suspension injection for treating subacute thyroiditis in clinic.
Owner:HAINAN YONGTIAN PHARMA INST
Tablet containing cefixime liposome and preparation method thereof
InactiveCN101966159AStable storageImprove bioavailabilityAntibacterial agentsOrganic active ingredientsYolkCholesterol
The invention relates to a cefixime liposome and a preparation method thereof as well as a tablet containing the cefixime liposome. The tablet comprises a cefixime liposome and a pharmaceutically acceptable vector, wherein the cefixime liposome comprises the following components in parts by weight: 1 part of cefixime, 1.25-5 parts of hydrogenated soybean lecithin, 1.25-5 parts of hydrogenated yolk lecithin, 1.25-10 parts of cholesterol and 0.1-3 parts of polysorbate 80. The tablet not only meets the requirement on Chinese pharmacopoeia but also has the advantages of more stable storage at normal temperature, more obvious curative effect and obvious improvement of bioavailability compared with the traditional cefixime medicament composition.
Owner:王丽燕
Lansoprazole sodium submicron emulsion freeze-drying preparation
InactiveCN101543474AImprove stabilityGuarantee product qualityOrganic active ingredientsPowder deliveryFreeze-dryingEvaporation
The invention provides a method for preparing Lansoprazole sodium submicron emulsion freeze-drying preparation and the product thereof. The method includes the following steps: (1) Lansoprazole and sodium hydroxide are dissolved together in water, biodegradable polymer is dissolved in and mixed and stirred with organic solvent to form W / O type emulsion; (2) stabilizing agent and apodemal agent are dissolved in water, and the obtained solution is added to the W / O type emulsion to be stirred to form W / O / W type emulsion; and (3) the W / O type emulsion is added to the emulsifying agent aqueous solution and stirred into the same at room temperature, and the submicron emulsion freeze-drying preparation is obtained after the operations of pressure reduction for organic solvent evaporation, eccentric separation, water rinsing, freezing and drying are performed.
Owner:HAINAN LINGKANG PHARMA CO LTD
Capsule containing cefixime liposome and preparation method thereof
InactiveCN101966166AHigh dissolution rateThe drug works quicklyAntibacterial agentsOrganic active ingredientsYolkCholesterol
The invention relates to a cefixime liposome and a preparation method thereof as well as a capsule containing the cefixime liposome. The capsule comprises the cefixime liposome and a pharmaceutically acceptable vector, wherein the cefixime liposome is prepared from the following components in parts by weight: 1 part of cefixime, 1.25-5 parts of hydrogenated soybean lecithin, 1.25-5 parts of hydrogenated egg yolk lecithin, 1.25-10 parts of cholesterol and 0.1-2.5 parts of polysorbate 80. The capsule not only accords with the requirement on Chinese pharmacopoeia, but also has the advantages of stabler storage and rapider drug effect exertion and remarkably improved bioavailability compared with the common pharmaceutical cefixime pharmaceutical composition at a room temperature.
Owner:王丽燕
Dispersible tablet containing cefixime liposome and preparation method thereof
InactiveCN101966160AHigh encapsulation efficiencyReduce leak rateAntibacterial agentsOrganic active ingredientsCholesterolNiosome
The invention relates to a cefixime liposome, a preparation method thereof and a dispersible tablet containing the cefixime liposome. The dispersible tablet comprises the cefixime liposome and a pharmaceutically acceptable carrier, wherein the cefixime liposome comprises the following components in parts by weight: 1 part of cefixime, 1.25-5 parts of hydrogenated soybean lecithin, 1.25-5 parts of hydrogenated egg yolk lecithin, 1.25-10 parts of cholesterol and 0.2-3.5 parts of polysorbate 80. The dispersible tablet not only conforms to the requirements of a Chinese pharmacopoeia, but also has the advantages of more stable storage under normal temperature and remarkable improvement of bioavailability compared with the ordinary cefixime medicament composition, and can take effect more rapidly.
Owner:王丽燕
Ribavirin lipid microsphere effervescent granules
InactiveCN101703481AUnexpected effectNon-immunogenicOrganic active ingredientsAntiviralsYolkLipid formation
The invention relates to ribavirin lipid microspheres of a targeting drug delivery system and a method for preparing the same, in particular to ribavirin lipid microsphere effervescent granules and a method for preparing the same. The method comprises the steps of: adopting film dispersion technology to obtain the ribavirin lipid microspheres by combining a certain amount of yolk lecithin, cholesterol and deoxysodium cholate with an active component, namely ribavirin, and then mixing the ribavirin lipid microspheres with certain excipient to obtain the effervescent granules. The effervescent granules have the advantages of quick response, high bioavailability, and less toxic and side effects, so that the satisfactory technical effect is achieved.
Owner:HAINAN LINGKANG PHARMA CO LTD
Dry suspension containing cefixime liposome and preparation method thereof
InactiveCN101972231AStorage moreStorage stableOrganic active ingredientsAntiinfectivesPhysical ExertionsYolk
The invention relates to cefixime liposome and preparation method therefore as well as dry suspension containing the cefixime liposome. The dry suspension is composed of the cefixime liposome and pharmaceutically acceptable carriers, wherein the cefixime liposome comprises the following components in parts by weight: 1 part of cefixime, 1.25-5 parts of hydrogenated soybean lecithin, 1.25-5 parts of hydrogenated yolk lecithin, 2.5-10 parts of cholesterol and 0.1-5 parts of polysorbate 80. The dry suspension not only accords with the requirements of Chinese pharmacopoeia, but also has the advantages of better storage stability in normal temperature, faster effect exertion and obviously improved bioavailability in comparison with the common cefixime medicaments.
Owner:王丽燕
Creatine phosphate sodium lipidosome freeze-dried preparation and preparation method thereof
InactiveCN101530391ASafety proofUnexpected effectOrganic active ingredientsPharmaceutical non-active ingredientsAntioxidantPhosphate
The invention relates to a creatine phosphate sodium lipidosome freeze-dried preparation and a preparation method thereof. The creatine phosphate sodium lipidosome freeze-dried preparation is characterized by comprising the following components in portion by weight: 10 to 20 portions of creatine phosphate sodium, 10 to 40 portions of phospholipids, 0 to 10 portions of cholesterol, 1 to 10 portions of antioxidant, and 5 to 20 portions of cryoprotectant.
Owner:HAINAN LINGKANG PHARMA CO LTD
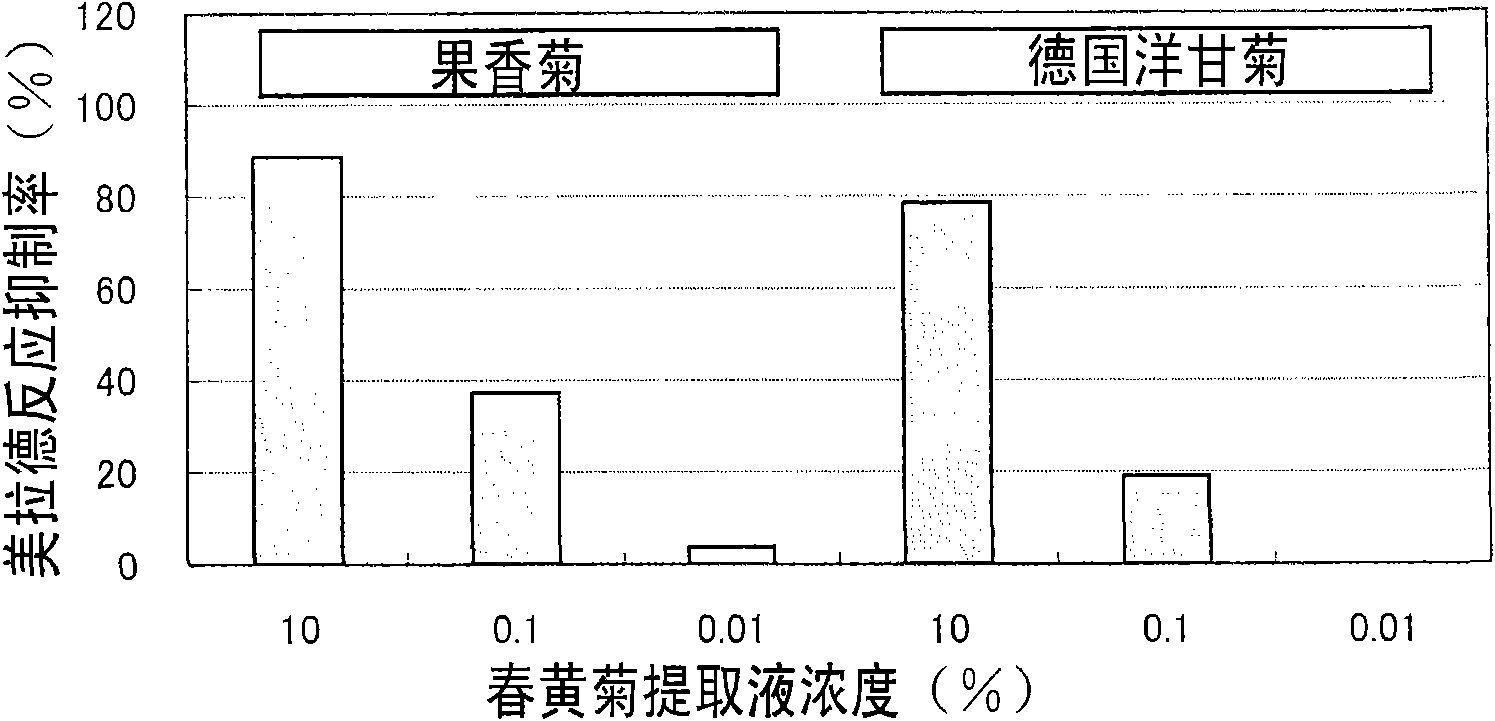
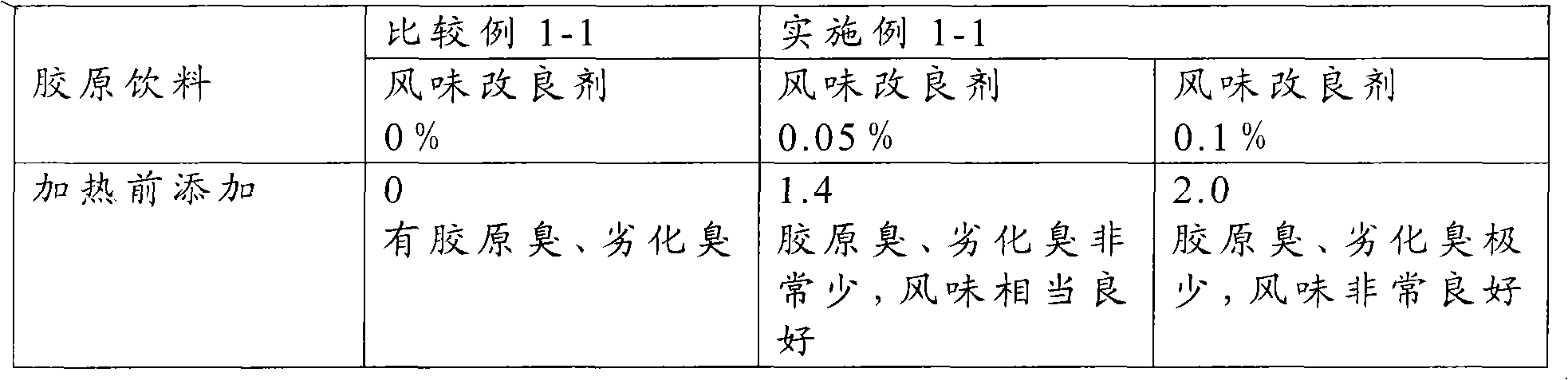
Flavor-improving agent for foods and drinks
ActiveCN101610686AOdor suppressionInhibitionFruits/vegetable preservation using acidsFood ingredient as flavour affecting agentSaururaceaeMatricaria
Owner:ARKRAY INC
Amoxicillin lipidosome solid preparation
InactiveCN101623259BFix stability issuesPromote dissolutionAntibacterial agentsDigestive systemYolkDissolution
The invention provides an amoxicillin lipidosome solid preparation and a new application thereof. Certain amounts of yolk lecithin, cholesterin, natrium glycocholicum, tween 80 and an active ingredient amoxicillin are combined and are prepared into amoxicillin lipidosome by film dispersion technology, and the amoxicillin lipidosome is mixed with the certain accessories to prepare various solid preparations. The amoxicillin lipidosome solid preparation not only can solve the problems of poor dissolution rate and poor quality stability of the amoxicillin, increases the medical effect and the bioavailability of the preparation, can treat bacterial liver abscess and obtain good invention effect.
Owner:HAINAN MEIDA PHARMA
Nimesulide liposome solid preparation and preparation method of drug composite thereof
InactiveCN101642433AImprove efficacyImprove bioavailabilityAntipyreticAnalgesicsAdditive ingredientCholesterol
The invention provides a Nimesulide liposome solid preparation and a preparation method of the drug composite thereof. For the Nimesulide liposome solid preparation of the invention, the active ingredient Nimesulide is prepared into liposome and then prepared into Nimesulide solid preparation with other ingredients, wherein the liposome is prepared by the following ingredients according to parts by weight: 1 part of Nimesulide, 3-10 parts of lauric acid glycerol monolaurate, 0.5-4 parts of cholesterol, and 0.1-2 parts of poloxamer188. Compared with the existing Nimesulide liposome solid preparation, the Nimesulide of the invention has high dissolution rate and improved bioavailability, and overcomes the detect that the bioavailability of the Nimesulide liposome solid preparation in the prior art is low.
Owner:HAINAN LINGKANG PHARMA CO LTD
Composite bone cement capable of forming pores and increasing the holding ability of bone, preparation method and applications thereof
PendingCN107899072AImprove connection strengthReduce long-term failure rateTissue regenerationProsthesisSolid phasesBone ingrowth
The invention discloses composite bone cement capable of forming pores and increasing the holding ability of bone, wherein the pre-preparation system of the bone cement comprises a solid phase component and a liquid phase component, the solid phase component comprises polymethacrylate and calcium phosphate as a pore forming agent, and the liquid phase component comprises hydroxyethyl methacrylate.According to the present invention, with the application of the bone cement in human body, the holding ability of bone can be increased at the early stage, and the pores can be formed along with thecalcium phosphate absorption by the human body at the late stage so as to promote the bone ingrowth, such that the binding strength of the bone cement-cancellous bone interface can be enhanced, and the late failure rate of the internal fixation can be reduced; and the composite bone cement has advantages f excellent injectability, excellent mechanical property, excellent curing rate and no toxicity, and can be used clinically as soon as possible.
Owner:PEKING UNIV THIRD HOSPITAL
Pantoprazole sodium liposomes freeze-dry preparations and method of preparing the same
InactiveCN100548298CSolve the problem of quality stabilitySmall toxicityOrganic active ingredientsDigestive systemFreeze-dryingCholesterol
The invention discloses a freeze-dried preparation of pantoprazole sodium liposomes, wherein pantoprazole sodium is encapsulated in antioxidant-containing liposomes made of soybean lecithin and cholesterol. The freeze-dried preparation is administered intravenously with stable quality, less toxicity and high effectiveness.
Owner:HAINAN LINGKANG PHARMA CO LTD
Application of genetic modification stem cell exosome to preparation of medicines or beautifying and whitening cosmetics
ActiveCN111643527ASafety proofPrevent proliferationCosmetic preparationsMicroencapsulation basedPharmaceutical drugExosome
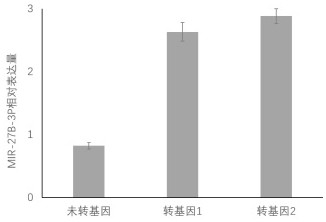
The invention relates to an application of a genetic modification stem cell exosome to preparation of medicines or beautifying and whitening cosmetics. miR-27b-3p is used for transfection of epidermalstem cells and an exosome from genetic modification stem cells is obtained. Experiments prove that the exosome can restrain expression of PIK3R3 proteins in melanocyte, can also restrain proliferation and migration of the melanocyte. Safety experiments also prove the safety of the exosome. Therefore, the exosome has good medical and cosmetic application prospects when being made into corresponding medicines or cosmetics.
Owner:GUANGDONG CELL BIOTECHNOLOGY CO LTD
Loratadine-ambroxol pharmaceutical composite and liposome solid preparation thereof
InactiveCN101627998BImprove solubilityImprove stabilityOrganic active ingredientsPharmaceutical product form changeYolkMedicine
Owner:HAINAN YONGTIAN PHARMA INST
Fasudil hydrochloride lipo-microballoons carrier preparation and preparation method thereof
InactiveCN101507707ASafety proofImprove stabilityOrganic active ingredientsSolution deliveryMicrosphereOil phase
The invention relates to a hydrochloric acid Fasudil fat microsphere preparation and a method for preparing the same. By taking soyabean lecithin as an emulsifying agent and corn oil for injection as an oil phase solvent and comprising an antioxidant commonly used in pharmaceutical products, the preparation is prepared. The preparation has the advantages of simple process, low cost and easy industrialized production; and compared with the prior hydrochloric acid Fasudil injection, the injection prepared by the method has obviously improved stability.
Owner:HAINAN LINGKANG PHARMA CO LTD
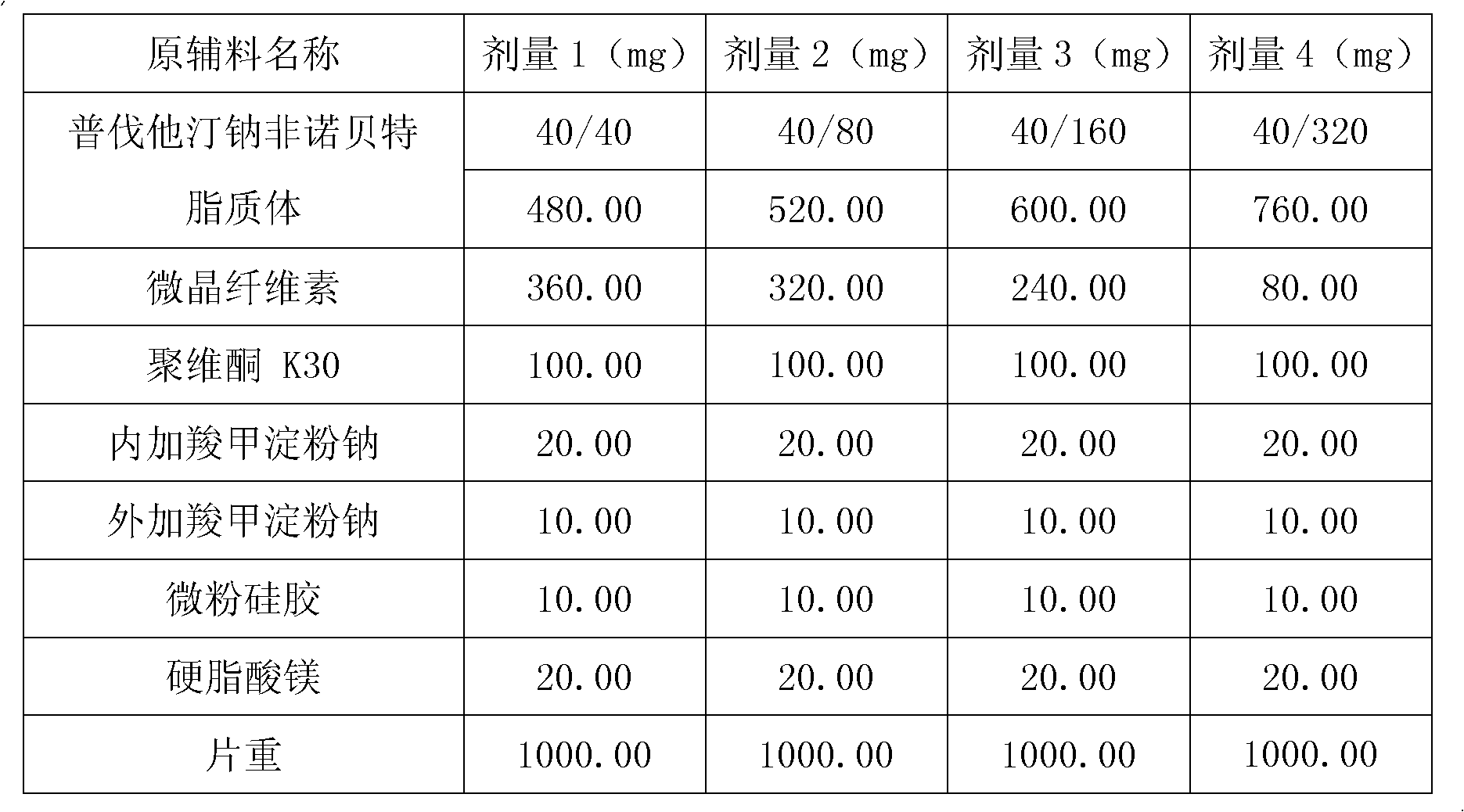
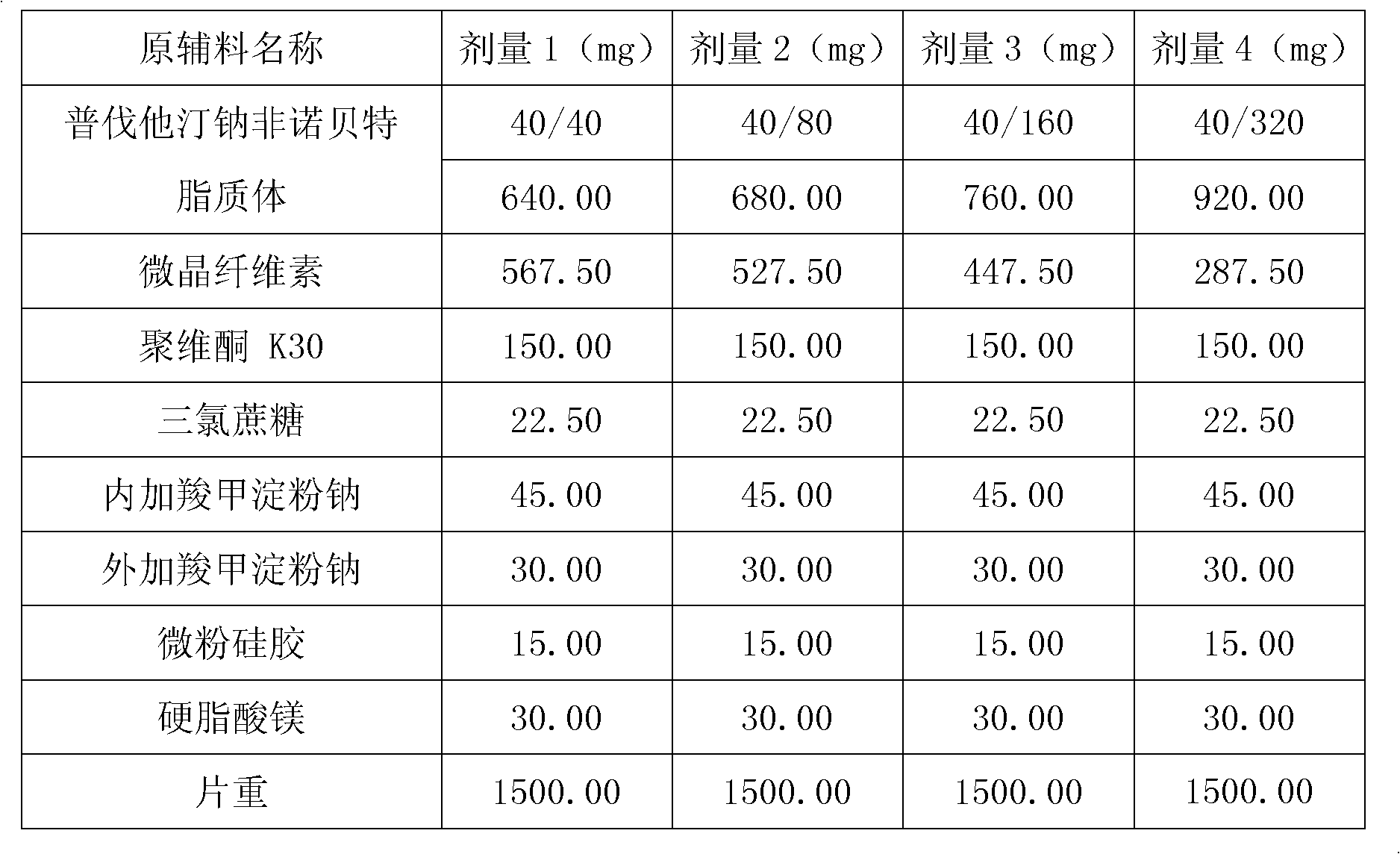
Medicine composition containing pravastatin sodium fenofibrate liposome and preparation method of medicine composition
InactiveCN102552143AHigh content of the main drugHigh dissolution rateMetabolism disorderPharmaceutical non-active ingredientsCholesterolDissolution
The invention relates to a medicine composition containing a pravastatin sodium fenofibrate liposome and a preparation method of the medicine composition. The medicine composition comprises a pravastatin sodium fenofibrate liposome and at least one pharmaceutically acceptable vector, wherein the pravastatin sodium fenofibrate liposome comprises pravastatin sodium, fenofibrate, phospholipid and cholesterol. The medicine composition prepared by the pravastatin sodium fenofibrate liposome not only satisfies the requirements of Chinese Pharmacopoeia and has the advantages of faster dissolution, faster medicine efficacy, high bioavailability, good stability as well as worth of being widely promoted and applied when being compared with the common pravastatin sodium fenofibrate medicine composition.
Owner:海南欣莱医药科技股份有限公司
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com