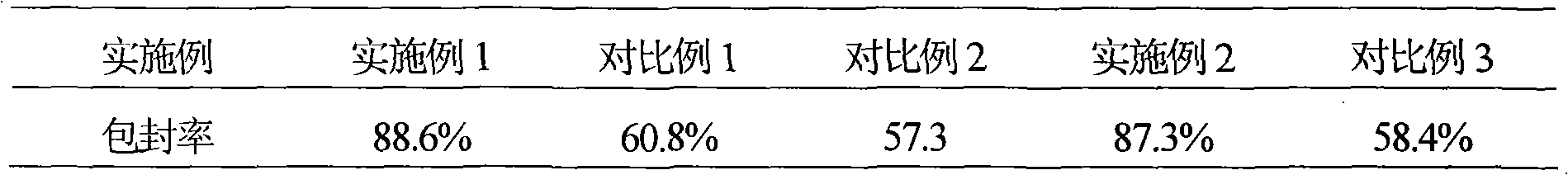Loratadine-ambroxol pharmaceutical composite and liposome solid preparation thereof
A technology of loratadine and solid preparation, which is applied in the field of medicine to achieve the effects of reducing drug toxicity, improving quality and reducing drug side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
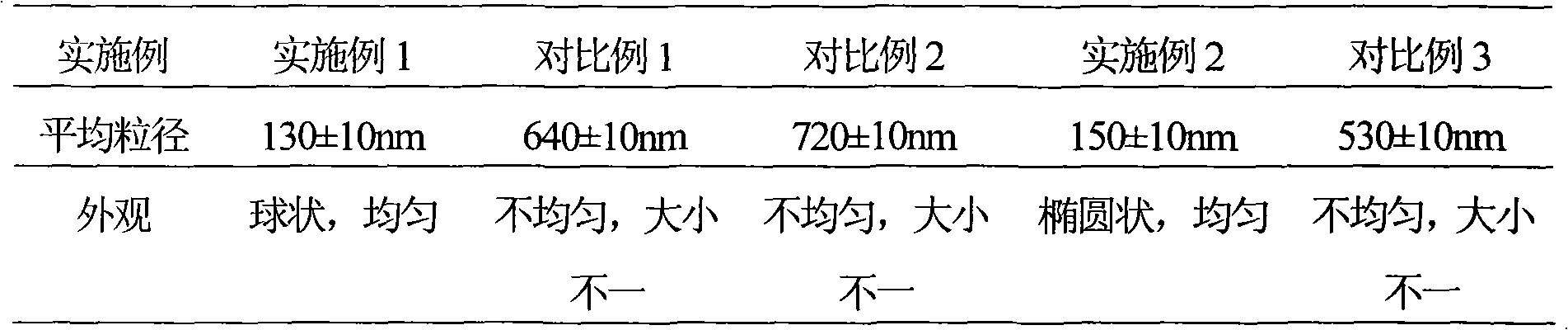
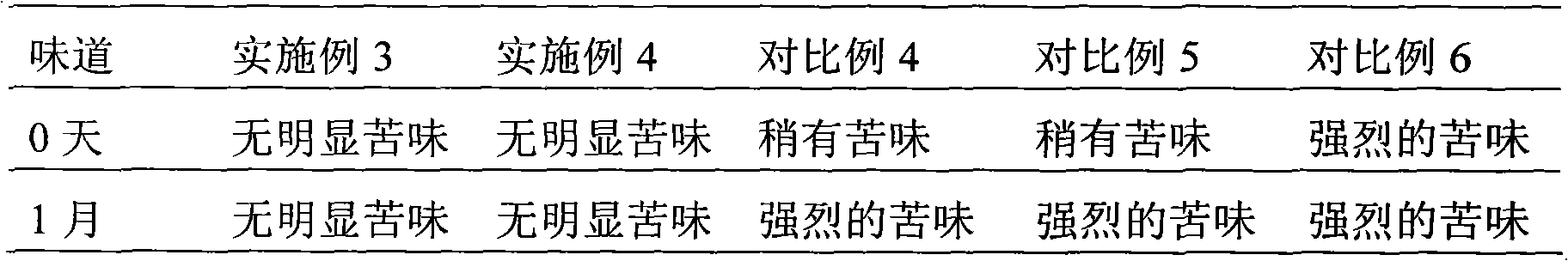
Embodiment 1
[0050] Example 1 The preparation of the liposome of loratadine ambroxol pharmaceutical composition
[0051] prescription:
[0052] Loratadine 5g
[0053] Ambroxol Hydrochloride 25g
[0055] Cholesterol 15g
[0056] Sodium deoxycholate 7.5g
[0057] Poloxamer 188 20g
[0058] Preparation Process
[0059] (1) Dissolve 30g of egg yolk lecithin, 15g of cholesterol, 7.5g of sodium deoxycholate and 20g of poloxamer 188 in 450ml of isopropanol and methanol at a volume ratio of 2:1, mix well, and place on a rotary thin film evaporator Isopropanol and methanol were removed under reduced pressure to obtain a phospholipid film;
[0060] (2) Add 200ml of acetic acid-sodium acetate buffer solution with pH=4.8, stir to completely hydrate the phospholipid film, then homogenize and emulsify with a tissue grinder for 20min at a speed of 14000r / min, to prepare a blank liposome suspension; (Loratadine 5g is 1 part, buffer solution 200ml is 200g is 40 par...
Embodiment 2
[0079] Example 2 The preparation of the liposome of loratadine ambroxol pharmaceutical composition
[0080] prescription:
[0081] Loratadine 5g
[0082] Ambroxol Hydrochloride 25g
[0084] Cholesterol 40g
[0085] Sodium deoxycholate 20g
[0086] Poloxamer 188 45g
[0087] Preparation Process
[0088] (1) 90g egg yolk lecithin, 40g cholesterol, 20g sodium deoxycholate and 45g poloxamer 188 were dissolved in 1900ml of isopropanol and methanol with a volume ratio of 2:1, mixed evenly, and reduced on a rotary thin film evaporator. Remove isopropanol and methanol under pressure to obtain a phospholipid film;
[0089] (2) Add 1000ml of citric acid-sodium citrate buffer solution with pH = 6.2, stir to completely hydrate the phospholipid membrane, then homogenize and emulsify for 15min with a tissue masher at a speed of 12000r / min to prepare blank liposomes suspension;
[0090] (3) Evenly disperse 5g of loratadine and 25g of ambroxol hydro...
Embodiment 3
[0103] Example 3 The preparation of the tablet of loratadine ambroxol pharmaceutical composition
[0104] Select the loratadine ambroxol medicine 30g of embodiment 1 (containing loratadine 5g, ambroxol hydrochloride 25g)
[0105] Composition of liposomes
[0106] Microcrystalline Cellulose 56g
[0107] Mannitol 35g
[0108] Croscarmellose Sodium 6.5g
[0109] Hypromellose 0.6g
[0110] Magnesium Stearate 1.3g
[0111] Preparation Process
[0112] (1) the liposome of the 30g loratadine ambroxol pharmaceutical composition prepared by embodiment 1 is pulverized, crosses 80 mesh sieves, and is subsequent use;
[0113] (2) Take by weighing 56g microcrystalline cellulose, 35g mannitol, 6.5g croscarmellose sodium, cross 80 mesh sieves, mix, and set aside;
[0114] (3) Mix the above-mentioned raw and auxiliary materials evenly, add 30 ml of 2% hypromellose 50% ethanol solution to make soft materials, pass through a 20-mesh sieve to granulate, dry at 65 ° C, and granulate th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com