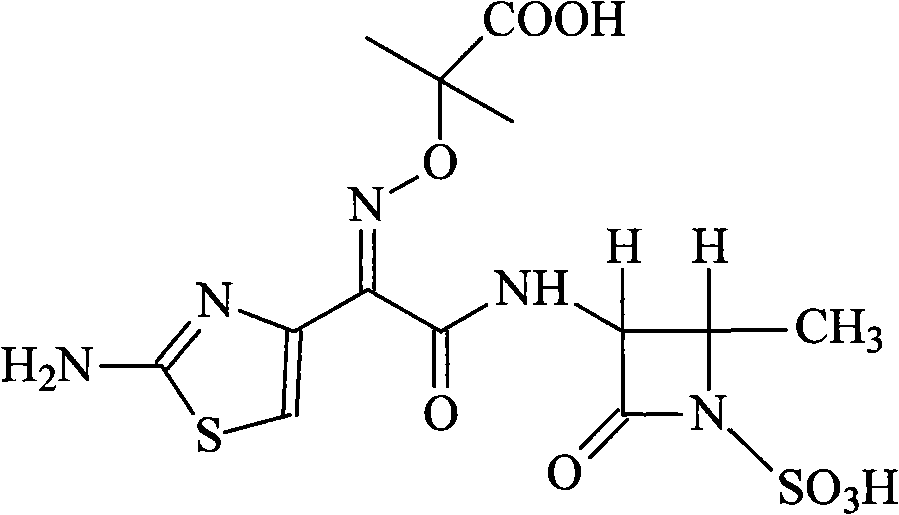
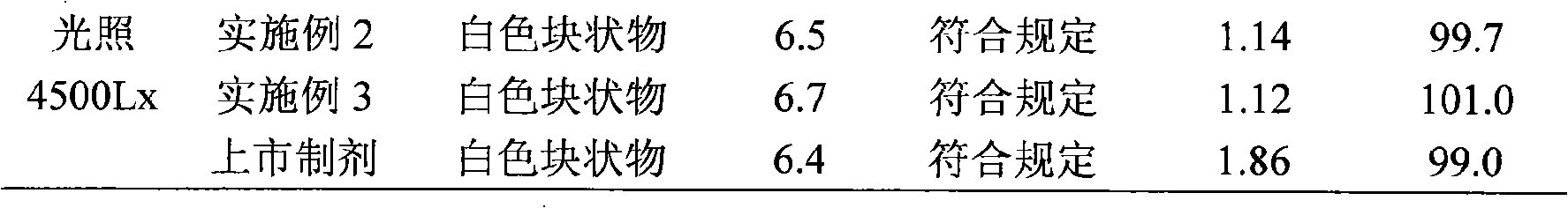Sub-micro emulsion frozen preparation of aztreonam
A freeze-dried preparation, aztreonam technology, applied in the field of freeze-dried preparations of aztreonam microemulsion, can solve the problems of high raw material and environmental requirements, thermal instability of double emulsion, poor stability of aqueous solution, etc., and achieve stable product quality, Reduce drug side effects and improve stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 2
[0069] Embodiment 2 prepares aztreonam microemulsion freeze-dried preparation
[0071] Polyethylene glycol / polylactic acid copolymer 400g
[0072] Polyvinyl alcohol 100g
[0073] Mannitol 260g
[0074] Povidone K15 15g
[0075] Preparation Process
[0076] (1) Dissolve 100g of aztreonam in 1500ml of water, 400g of polyethylene glycol / polylactic acid copolymer in 500ml of isopropanol, mix and stir for 15min at a speed of 600r / min to make a W / O emulsion ;
[0077] (2) Dissolve 260g of mannitol and 15g of povidone K15 in 1000ml of water, add the solution to the above-mentioned W / O emulsion, stir at room temperature for 30min, and rotate at 100r / min to make a W / O / W type double emulsion;
[0078] (3) Add the above-mentioned W / O / W type double emulsion into the aqueous solution of 100g polyvinyl alcohol, stir at room temperature for 60min, and rotate at 600r / min, evaporate isopropanol under reduced pressure, centrifuge at ...
Embodiment 3
[0081] The preparation of embodiment 3 aztreonam microemulsion freeze-dried preparation
[0082] Recipe: Aztreonam 200g
[0083] Polylactic acid / glycolic acid copolymer 300g
[0084] Lecithin 80g
[0085] Tween 80 50g
[0086] Lactose 500g
[0087] Dextran 40 58g
[0088] Preparation Process
[0089] (1) 200g of aztreonam was dissolved in 2000ml of water, 300g of polylactic acid / glycolic acid copolymer was dissolved in 1000ml of dichloromethane, the two were mixed and stirred for 20min, and the rotating speed was 300r / min to make a W / O emulsion;
[0090] (2) Dissolve 58g of dextran 40 and 500g of lactose in 1000ml of water, add the solution to the above-mentioned W / O type emulsion, stir at room temperature for 30min, and rotate at 200r / min to make W / O / W type double emulsion;
[0091] (3) Add the above-mentioned W / O / W type double emulsion into an aqueous solution of 80g lecithin and 50g Tween 80, stir at room temperature for 50min, and rotate...
Embodiment 4
[0094] Example 4 Quality Research
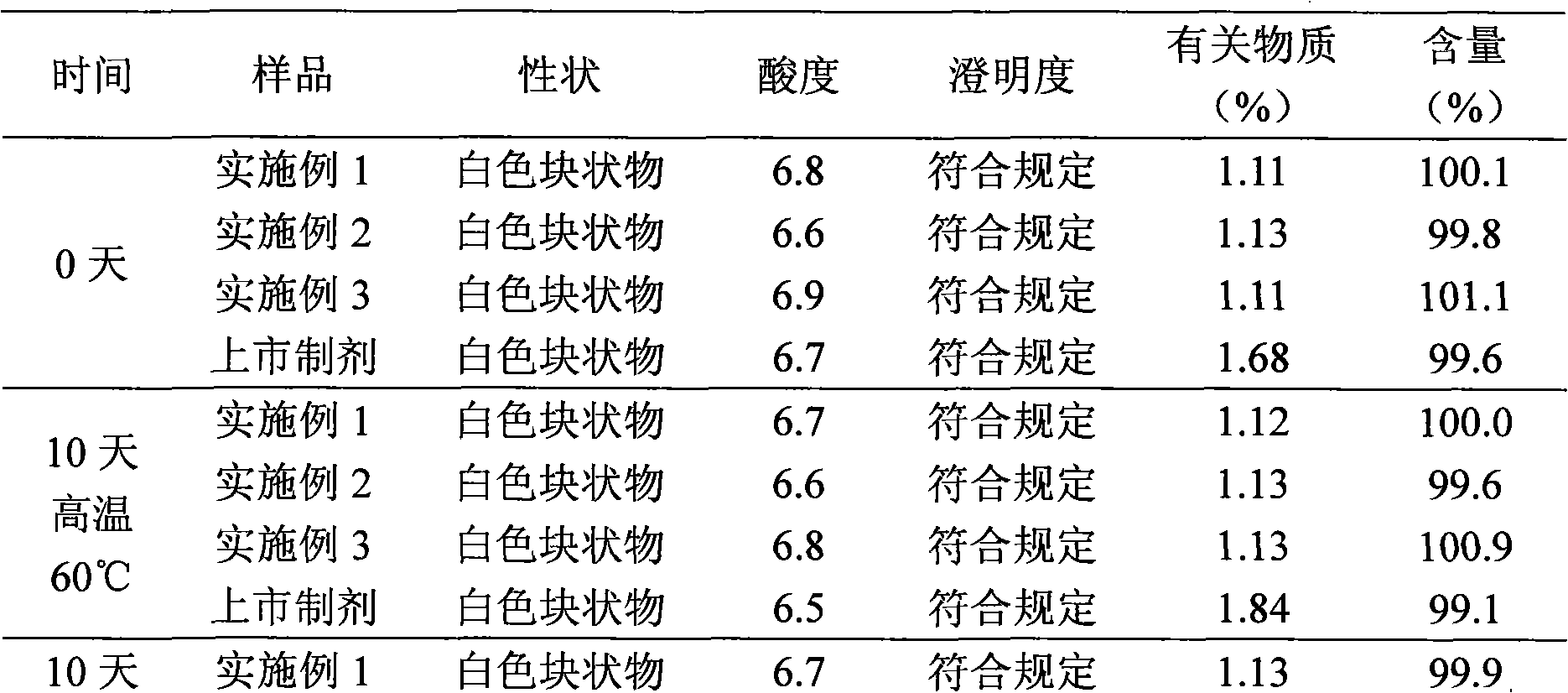
[0095] The samples prepared in the above examples and the marketed aztreonam powder injection (Shanxi Pude Pharmaceutical Co., Ltd., batch number 20080916) were placed at a high temperature of 60°C and a light of 4500Lx for 10 days to conduct an experimental investigation of the influencing factors. The results are shown in Table 1 ;At a high temperature of 40°C and a relative humidity of 75%±5% for 6 months, an accelerated test was carried out, the results are shown in Table 2; at a high temperature of 25°C and a relative humidity of 60%±10% for 18 months, a long-term test was carried out Investigate and detect the changes of various quality indicators, the results are shown in Table 3.
[0096] Table 1 Results of influencing factors
[0097]
[0098]
[0099] Table 2 Accelerated test results
[0100]
[0101] Table 3 Long-term test results
[0102]
[0103]
[0104] From the above results, it was found that the clarity of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com