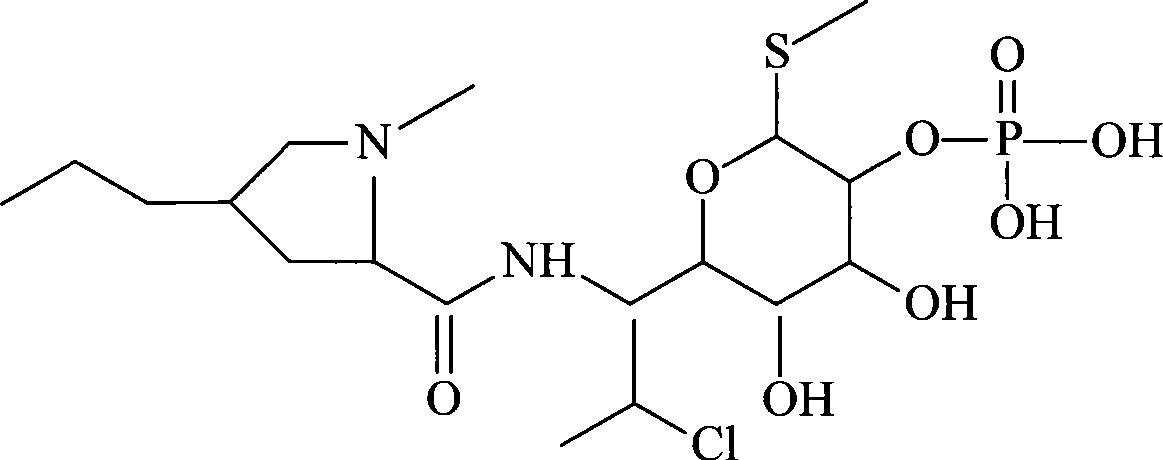
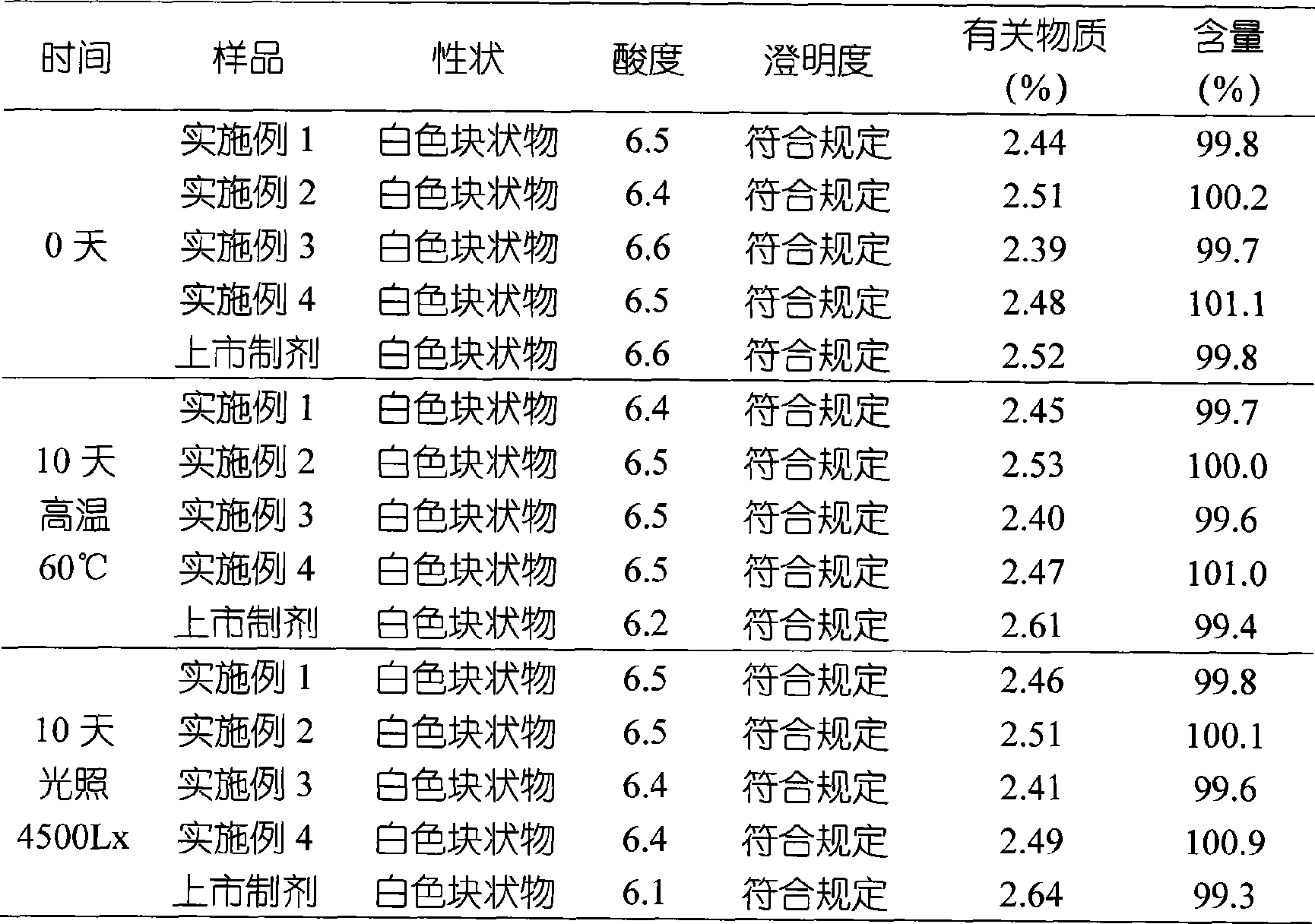
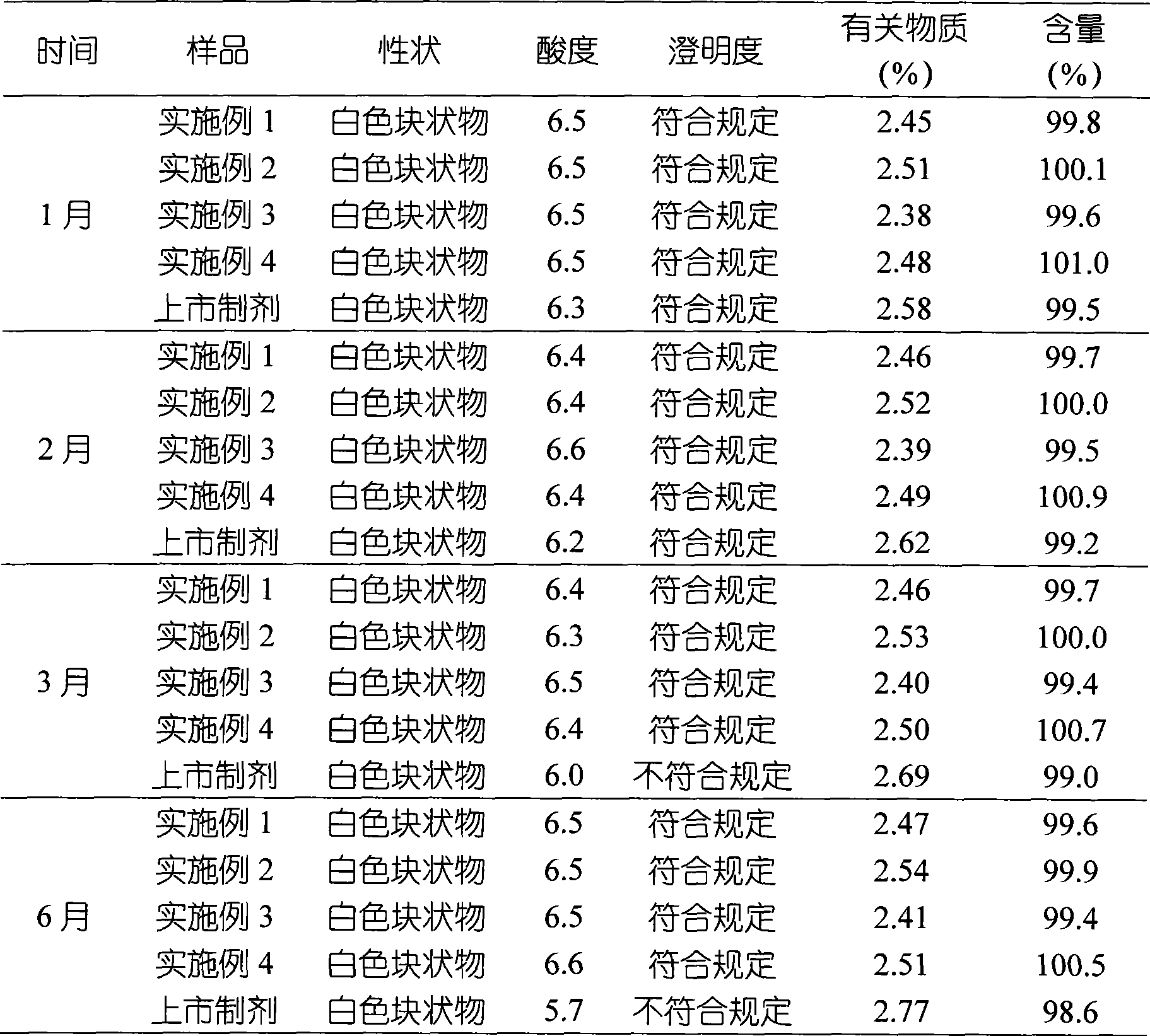
Clindamycin phosphate lipidosome freeze-dried preparation and preparation method thereof
A technology of clindamycin phosphate and phospholipid, which is applied in the field of liposome pharmaceutical preparations, can solve problems such as difficult to meet the quality requirements of the validity period and poor stability, and achieve the improvement of drug therapeutic index, stability, and reduction of The effect of drug side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Preparation of clindamycin phosphate liposome freeze-dried preparation
[0037] Prescription (100 bottles): Clindamycin Phosphate 30g
[0038] Dimyristic acid lecithin 80g
[0039] Cholesterol 20g
[0040] Vitamin E 10g
[0041] Mannitol 40g
[0042] Preparation Process
[0043] (1) Weigh 75g of myristic acid lecithin, 20g of cholesterol and 10g of vitamin E, dissolve them in 800ml of tert-butanol, heat and melt, add a phosphate buffer solution with a pH value of 6.8, heat and evaporate to remove tert-butanol, transfer to tissue and pound In a grinder, stir at high speed for 20 minutes, filter, sterilize, and sonicate for 10 minutes; (2) In a sterile room, under 100-grade conditions, add 30 g of sterilized clindamycin phosphate and 40 g of mannitol and dissolve, filter , filled in vials, freeze-dried to obtain clindamycin phosphate liposome freeze-dried agent.
Embodiment 2
[0044] Example 2 Preparation of clindamycin phosphate liposome lyophilized formulation
[0045] Prescription (100 bottles): Clindamycin Phosphate 60g
[0046] Dimyristic acid lecithin 30g
[0047] Cholesterol 6g
[0048] Propyl gallate 15g
[0049] Glycine 20g
[0050] Sorbitol 20g
[0051] Glucose 20g
[0052] Preparation Process
[0053] (1) Weigh 30g of lecithin myristicate, 6g of cholesterol and 15g of propyl gallate, dissolve in 500ml of a mixture of tert-butanol and isopropanol with a volume ratio of 1:1, heat and melt, add pH 6.8 phosphate Buffer solution, heated to remove tert-butanol and isopropanol, transferred to a tissue masher, stirred at high speed for 10 minutes, filtered, sterilized, and ultrasonicated for 20 minutes;
[0054] (2) In a sterile room, under the condition of grade 100, add 60 g of sterilized clindamycin phosphate and 20 g of ternary freeze-dried support agent glycine, 20 g of sorbitol, and 20 g of glucose...
Embodiment 3
[0055] Example 3 Preparation of clindamycin phosphate liposome lyophilized formulation
[0056] Prescription (100 bottles): Clindamycin Phosphate 90g
[0057] Dimyristic acid lecithin 120g
[0058] Cholesterol 25g
[0059] tert-Butyl-p-Hydroxyanisole 12g
[0060] Glycine 40g
[0061] Sorbitol 40g
[0062] Glucose 40g
[0063] Preparation Process
[0064] (1) Weigh 120g of lecithin myristicate, 25g of cholesterol and 12g of tert-butyl-p-hydroxyanisole and dissolve in 1000ml of a mixture of tert-butanol and isopropanol with a volume ratio of 1:1, heat and melt, and add pH 6.8 Phosphate buffer solution, heated to remove acetone, transferred to a tissue masher, stirred at high speed for 10 minutes, filtered, sterilized, and ultrasonicated for 10 minutes;
[0065] (2) In a sterile room, under 100-grade conditions, add 90 g of sterilized clindamycin phosphate, 40 g of ternary freeze-dried support agent glyc...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com