Aztreonam liposomes freeze-dry preparations and method of preparing the same
A technology of liposome freeze-drying and aztreonam, which is applied in the field of medicine, can solve the problems of poor stability of aztreonam, and achieve the effects of high encapsulation rate, reduced toxic and side effects, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
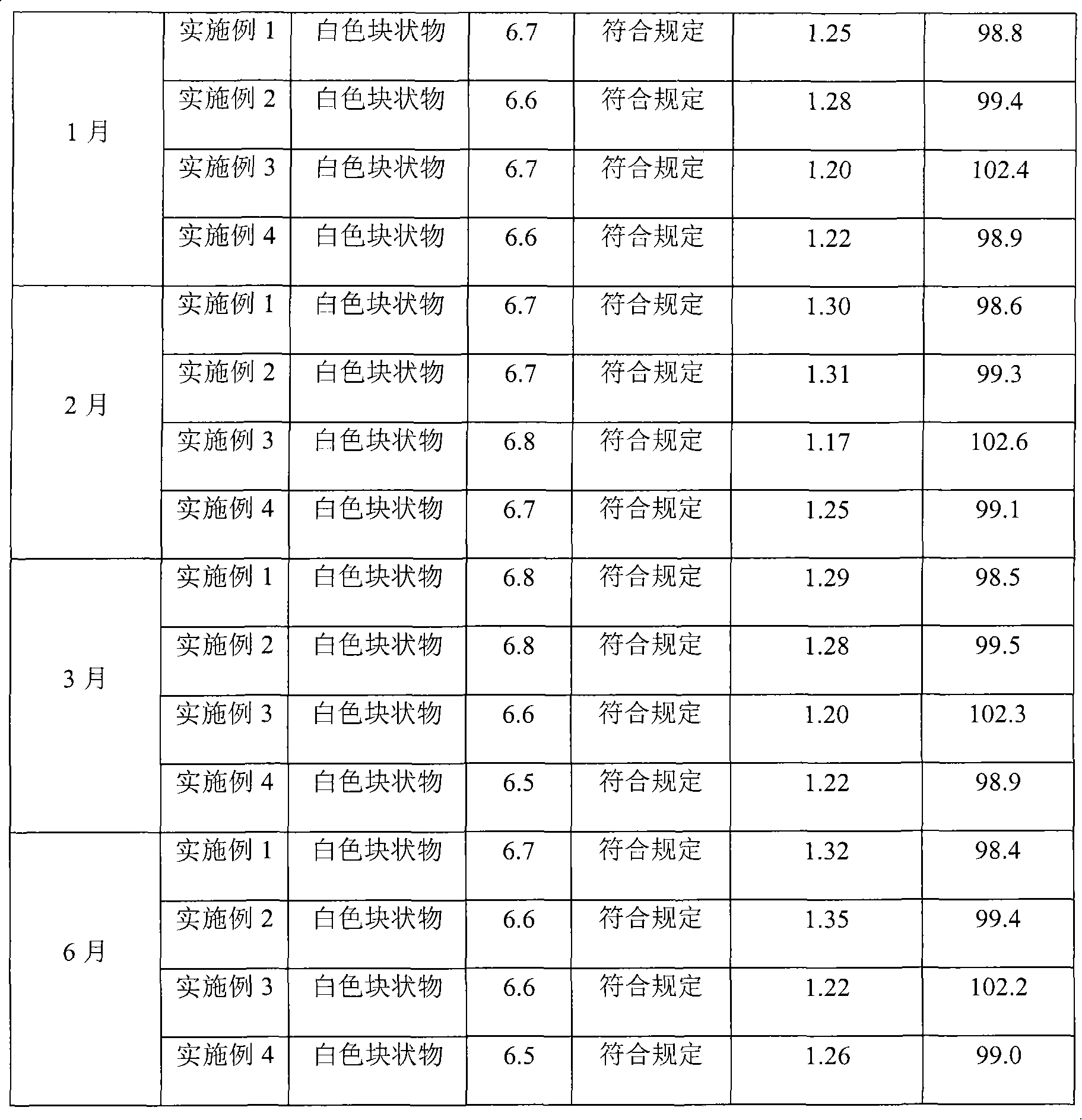
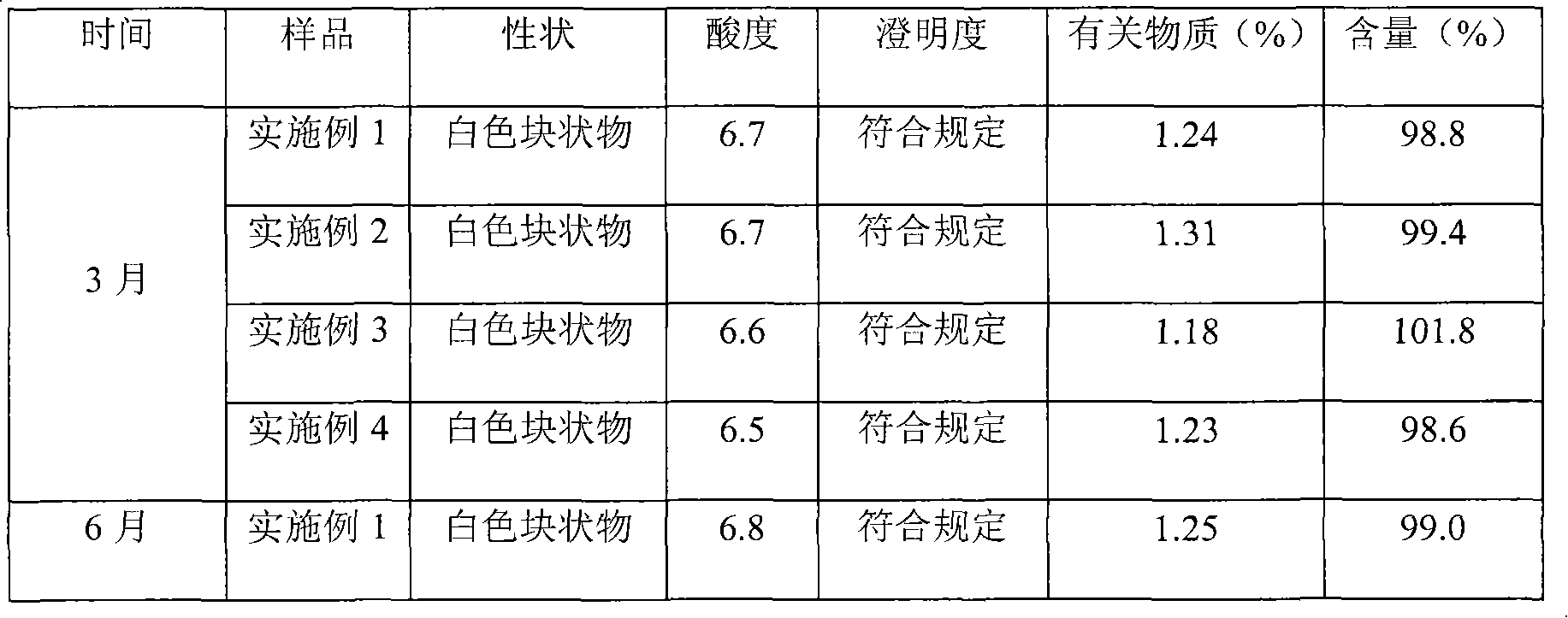
Embodiment 1
[0030] Aztreonam 100g
[0031] Lecithin Distearate 50g
[0032] Phosphatidylglycerol Distearate 0.5g
[0033] Cholesterol 2g
[0034] Vitamin E 50mg
[0035] Citric acid 5g
[0036] Anhydrous sodium carbonate 5g
[0037] Sucrose 200g
[0038] Glycine 200g
[0039] Water for injection 5000ml
[0040] Preparation Process
[0041] (1) According to the formula, dissolve lecithin distearate, phosphatidylglycerol distearate, cholesterol and vitamin E in chloroform and mix evenly, remove the solvent in the solution under reduced pressure with a rotary evaporator to form a lipid film, and prepare 0.25mol / L citric acid solution, use citric acid solution to hydrate the lipid film, and the hydration temperature is generally between 50°C and 60°C to obtain a blank liposome suspension.
[0042] (2) After hydration is complete, liposomes are prepared with a high-pressure particle homogenizer until the average particle size is controlled at 100-120 nm, and the particle size and unif...
Embodiment 2
[0047] Aztreonam 3000g
[0048] Hydrogenated Soy Lecithin 18000g
[0049] Dimyristate Phosphatidylglycerol 12000g
[0050] Cholesterol 9000g
[0051] Vitamin E 100g
[0052] Succinic acid 15000g
[0053] Sodium hydroxide 21000g
[0054] Lactose 2000g
[0055] Glycine 4000g
[0056] Water for injection 50000ml
[0057]Preparation process: operate with reference to the steps of Example 1, (1) use 0.3mol / L succinic acid solution to hydrate the lipid film; (3) use 0.5mol / L aqueous sodium hydroxide solution to adjust the blank lipid liposome suspension to pH 7.2; (4) prepare 4.5% lactose aqueous solution and add 1.0% glycine as liposome dispersion.
Embodiment 3
[0059] Aztreonam 500g
[0060] Lecithin Distearate 3200g
[0061] Phosphatidylglycerol Distearate 680g
[0062] Cholesterol 950g
[0063] Vitamin E 12g
[0064] Citric acid 5600g
[0065] Anhydrous sodium carbonate 8000g
[0066] Sucrose 1200g
[0067] Glycine 1250g
[0068] Water for injection 10000ml
[0069] Preparation process: operate with reference to the steps of Example 1, (1) use 0.25mol / L citric acid to hydrate the lipid film; (3) use 0.8mol / L anhydrous sodium carbonate aqueous solution to adjust the blank lipid Plastid suspension to 7.6; (4) prepare 9% sucrose aqueous solution and add 1.0% glycine as liposome dispersion.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com