Composite liposome for injection containing 12 vitamins and preparation method thereof
A technology of complex vitamins and liposomes, which is applied in liposome delivery, medical preparations containing active ingredients, drug combinations, etc., can solve problems such as poor stability, singleness, and quality problems, and avoid decomposition, toxic and side effects Effect of reduction, improvement of medicinal effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
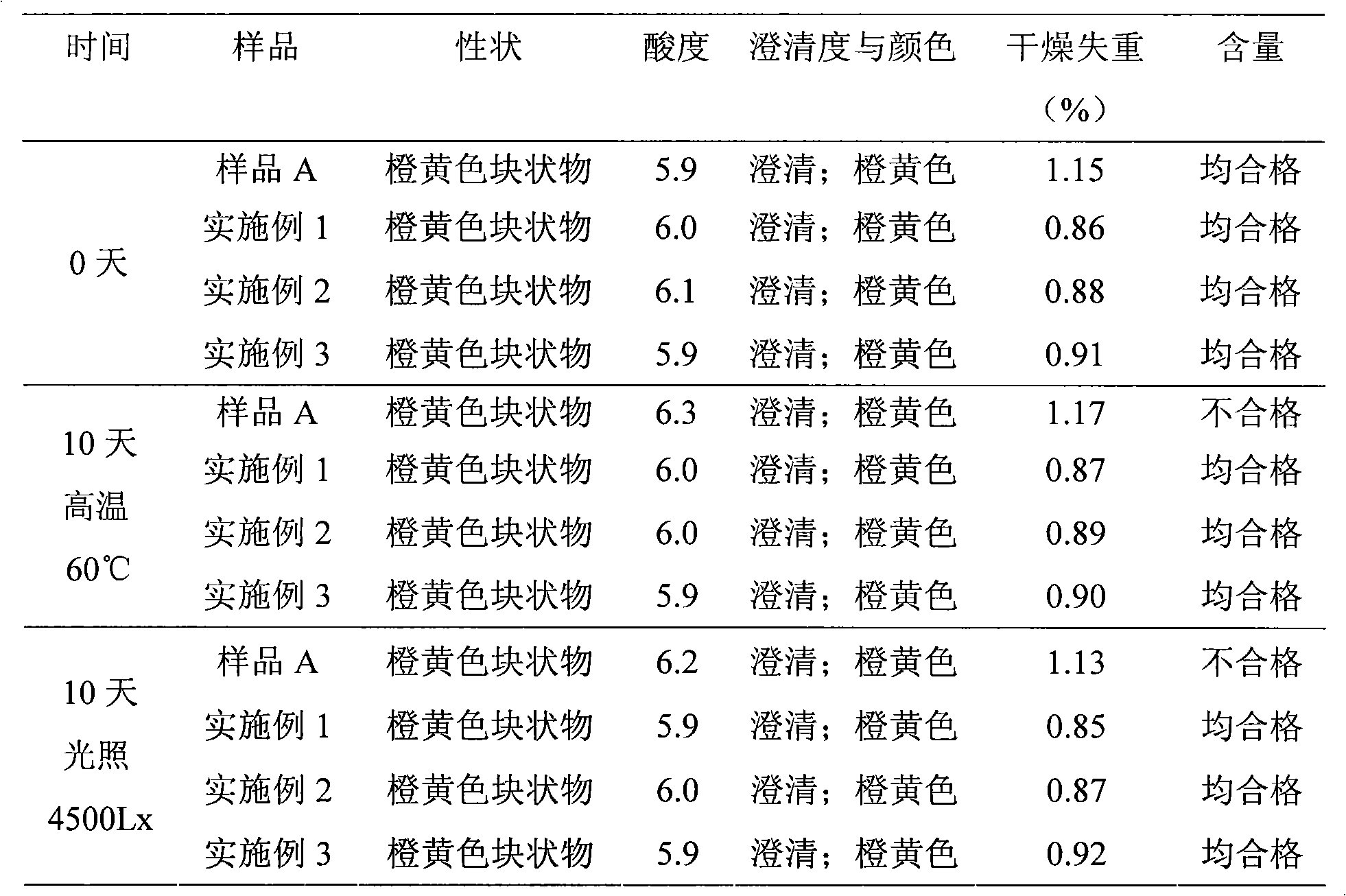
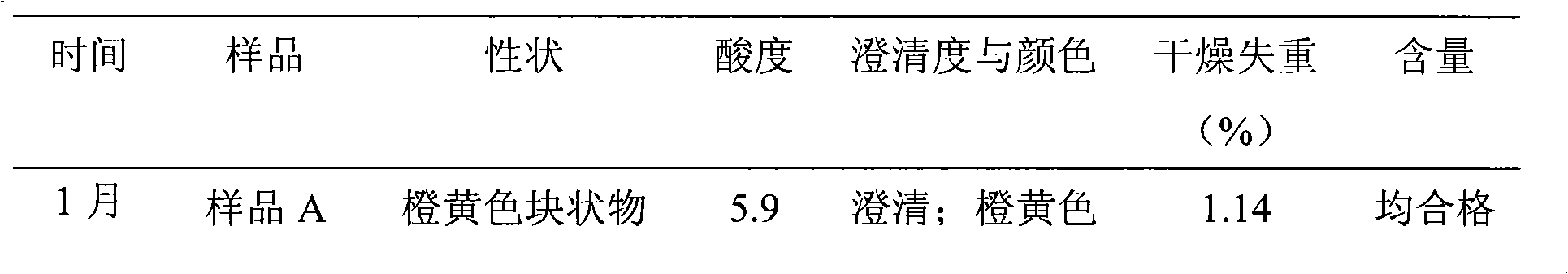
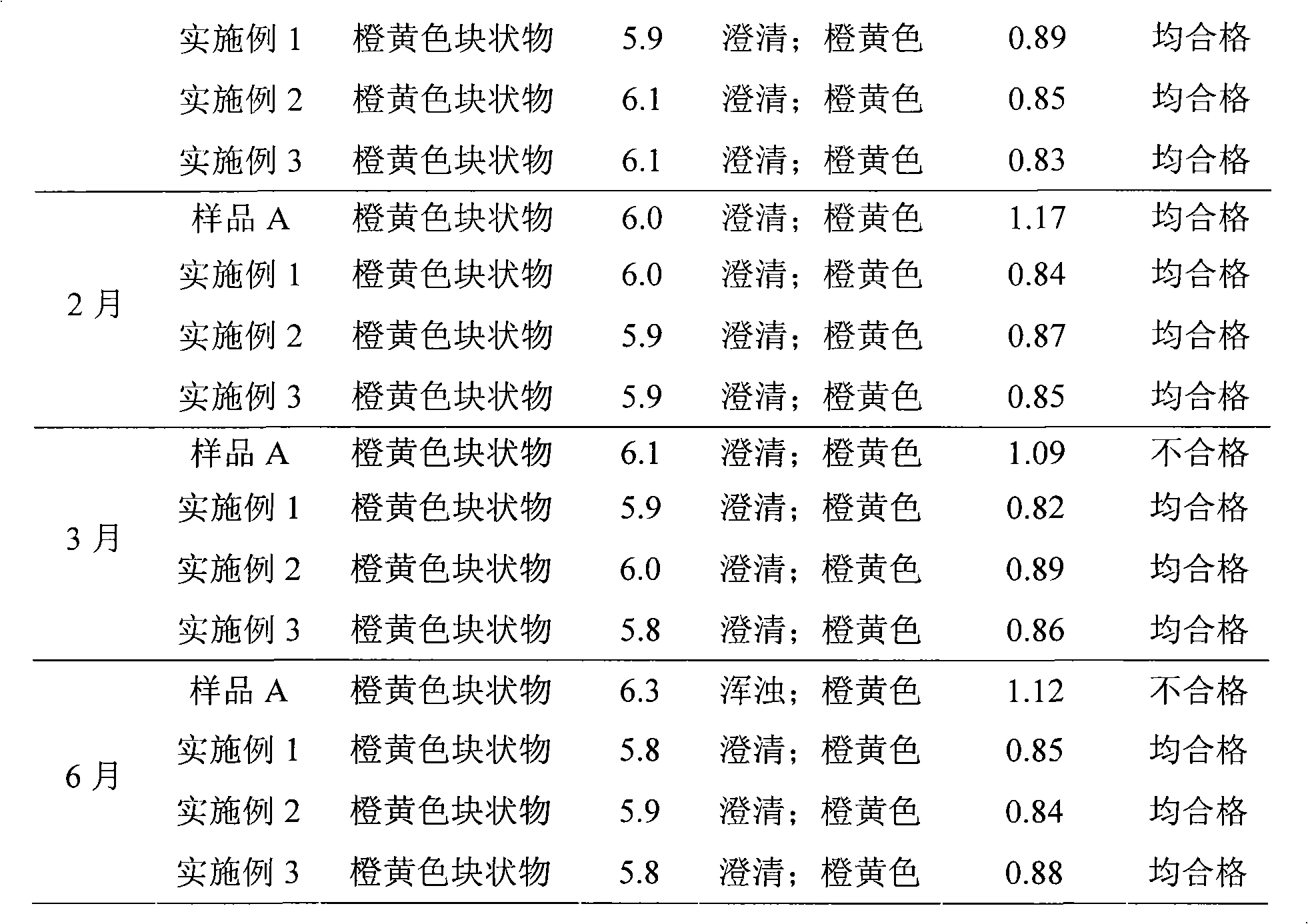
[0033] Example 1 12 kinds of multivitamin liposome freeze-dried preparations
[0034] Formulation: 1000 vials Quantities of components required in unit dosage form:
[0035] Retinyl Palmitate 4.2 million units Niacinamide 50.6g
[0036] Thiamine Phosphate Tetrahydrate 6.38g Folic Acid 496mg
[0037] Riboflavin Sodium Phosphate 6.24g Dexpanthenol 17.77mg
[0038] Pyridoxine Hydrochloride 6.05g Soy Lecithin 150g
[0039] Cyanocobalamin 7.2mg Glycocholic Acid 170g
[0040] Cholecalciferol 264,000 units Citric acid 35g
[0041] Ascorbic acid 137.5g Potassium citrate 60g
[0042] rac Alpha-Tocopherol 11.22g Glycine 300g
[0043] D-Biotin 82.5mg
[0044] Preparation Process:
[0045] (1) Weigh soybean lecithin and glycocholic acid, dissolve them with an appropriate amount of chloroform, mix uniformly, and then remove the chloroform from the formed solution under reduced pressure to prepare a lipid film;
[0046] (2) Prepare a buffer solution of citric acid and potassium cit...
example 2
[0050] Example 2 12 kinds of multivitamin liposome freeze-dried preparations
[0051] Formulation: 1000 vials Quantities of components required in unit dosage form:
[0052] Retinyl Palmitate 2.8 million units Niacinamide 41.4g
[0053] Thiamine Phosphate Tetrahydrate 5.22g Folic Acid 332mg
[0054] Riboflavin Sodium Phosphate 5.10g Dexpanthenol 14.54mg
[0055] Pyridoxine Hydrochloride 4.95g Soy Lecithin 100g
[0056] Cyanocobalamin 4.8mg Glycocholic Acid 110g
[0057] Cholecalciferol 176,000 units Citric acid 7.5g
[0058] Ascorbic Acid 112.5g Potassium Citrate 14g
[0059] rac Alpha-Tocopherol 9.18g Glycine 200g
[0060] D-Biotin 55.2mg
[0061] Preparation Process:
[0062] Adopt the production method as embodiment 1 to make the product of embodiment 2.
example 3
[0063] Example 3 12 kinds of multivitamin liposome freeze-dried preparations
[0064] Formulation: 1000 vials Quantities of components required in unit dosage form:
[0065] Retinyl Palmitate 3.5 million units Niacinamide 46.0g
[0066] Thiamine Phosphate Tetrahydrate 5.80g Folic Acid 414mg
[0067] Riboflavin Sodium Phosphate 5.67g Dexpanthenol 16.15mg
[0068] Pyridoxine Hydrochloride 5.50g Soy Lecithin 112.5g
[0069] Cyanocobalamin 6.0mg Glycocholic Acid 140g
[0070] Cholecalciferol 220,000 units Citric acid 30.8g
[0071] Ascorbic acid 125.0g Potassium citrate 41.6g
[0072] rac Alpha-Tocopherol 10.20g Glycine 250g
[0073] D-Biotin 69.0mg
[0074] Preparation Process
[0075] Adopt the production method as embodiment 1 to make the product of embodiment 3.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com