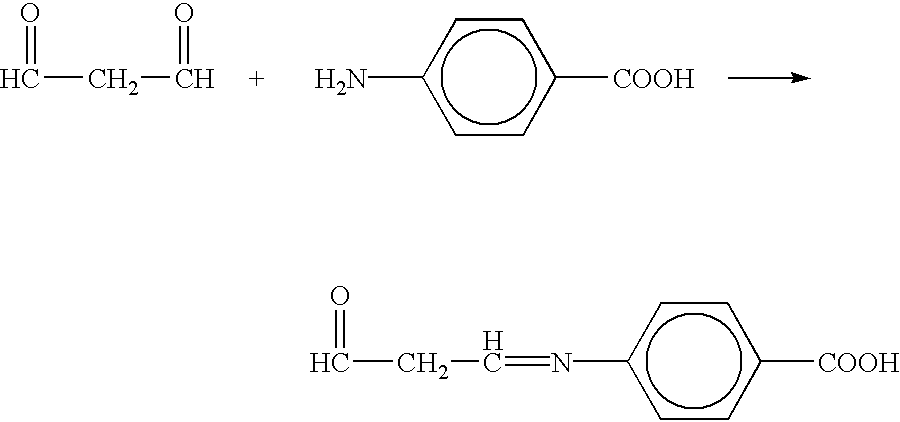
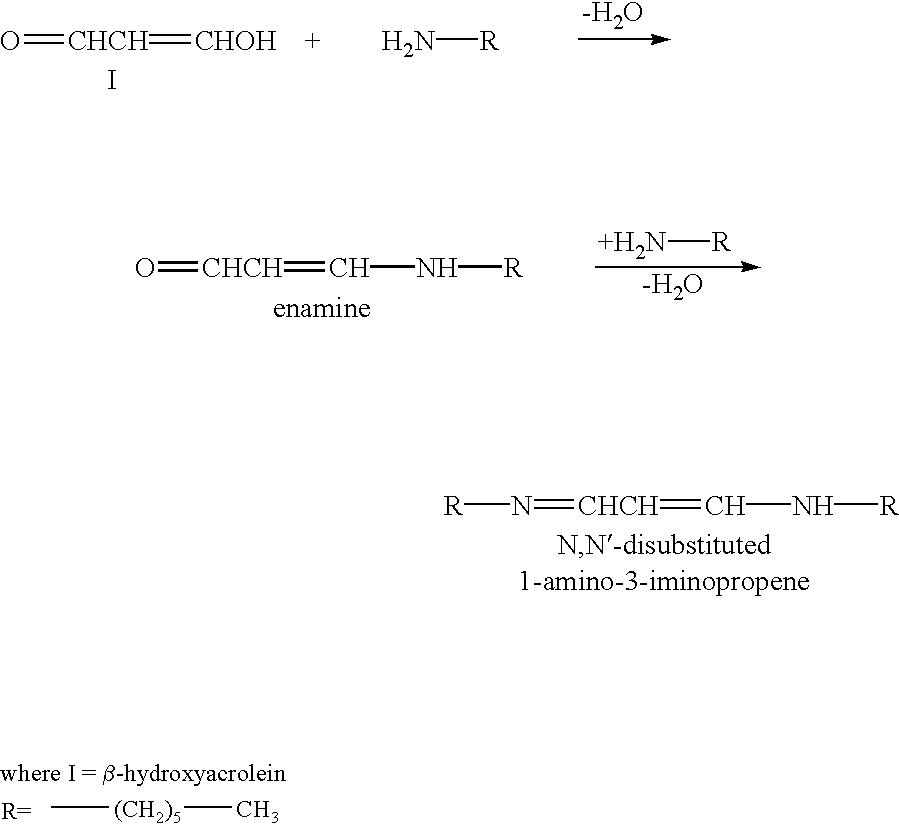
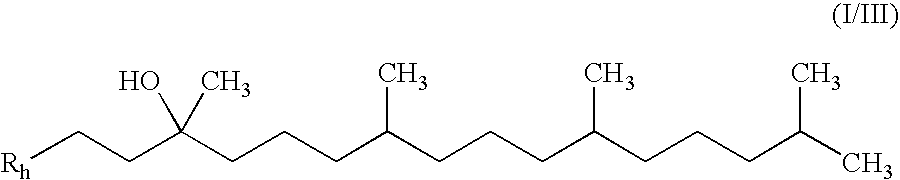
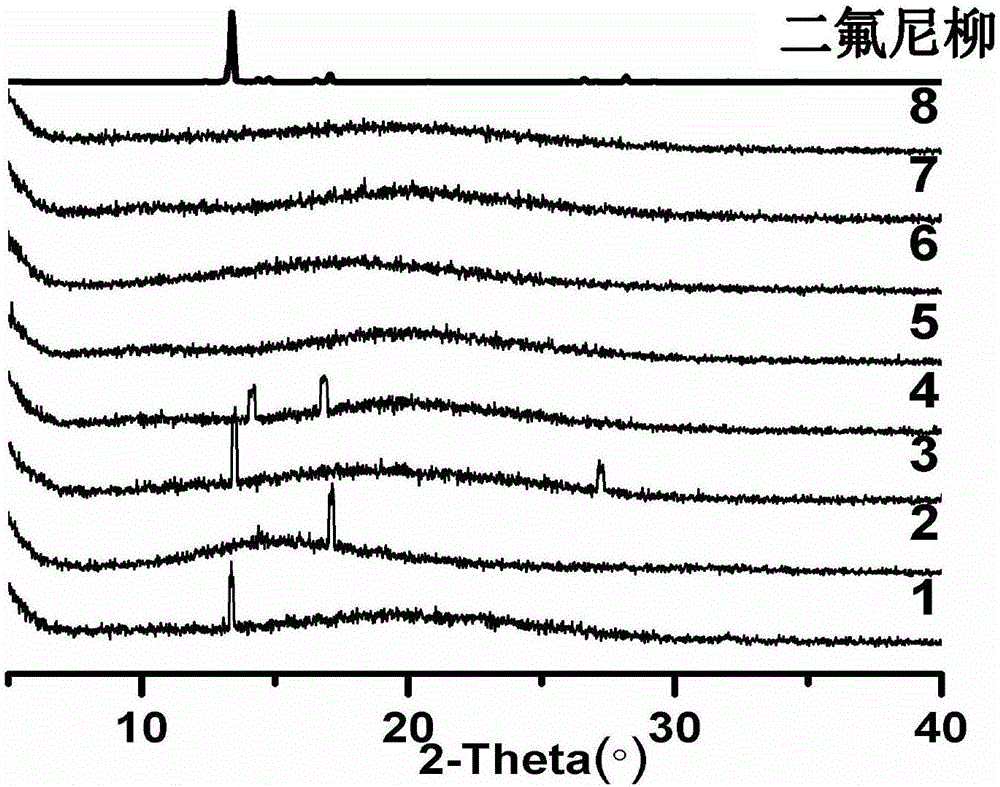
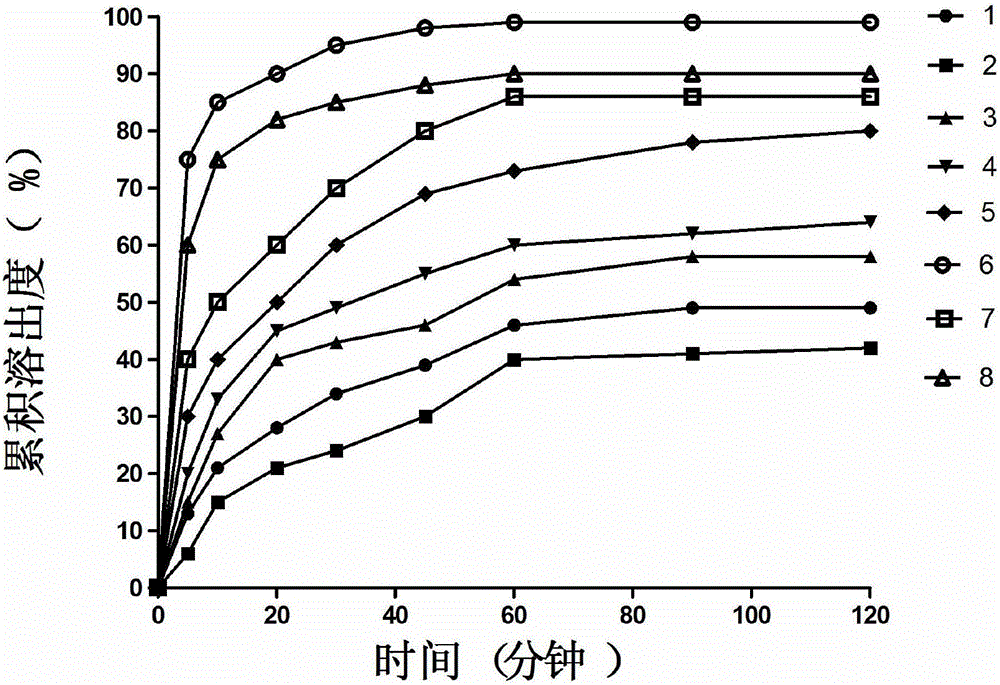
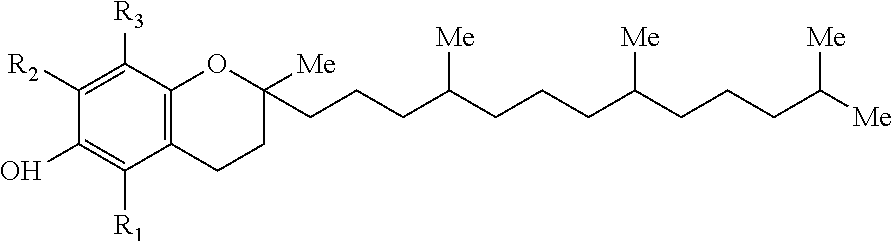
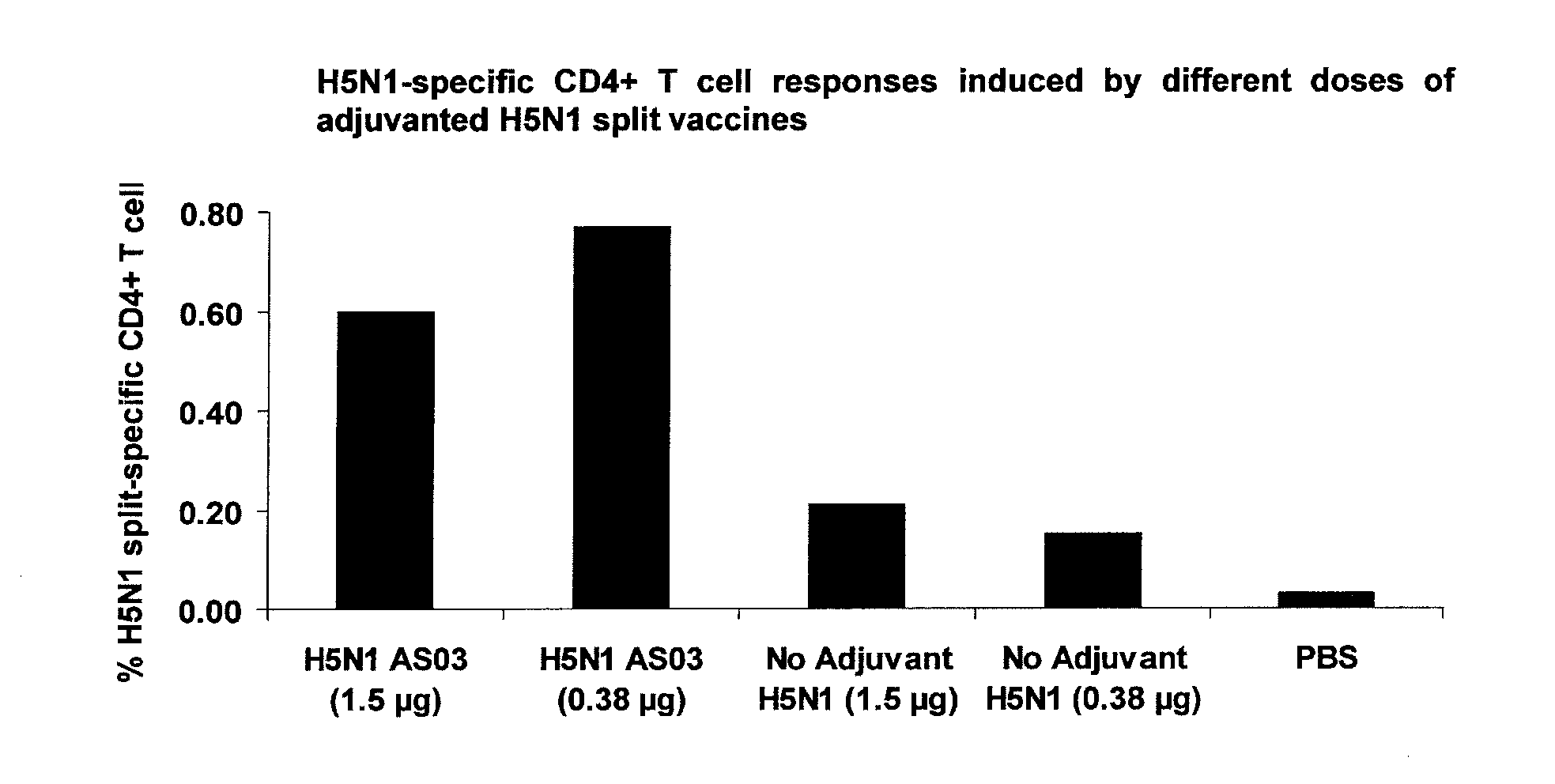
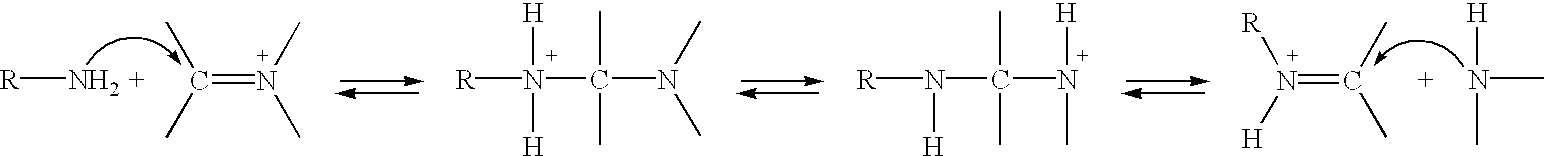Patents
Literature
155 results about "Alpha-Tocopherol" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
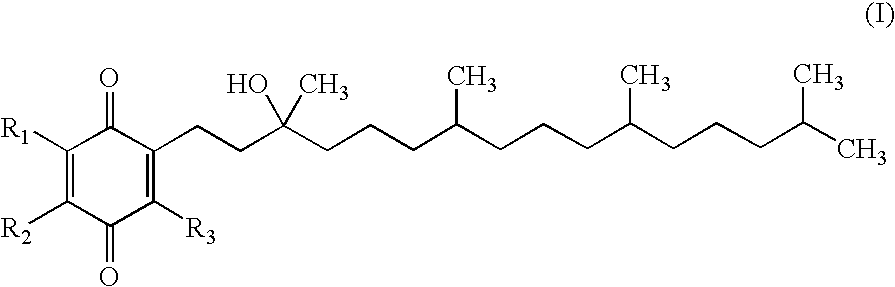
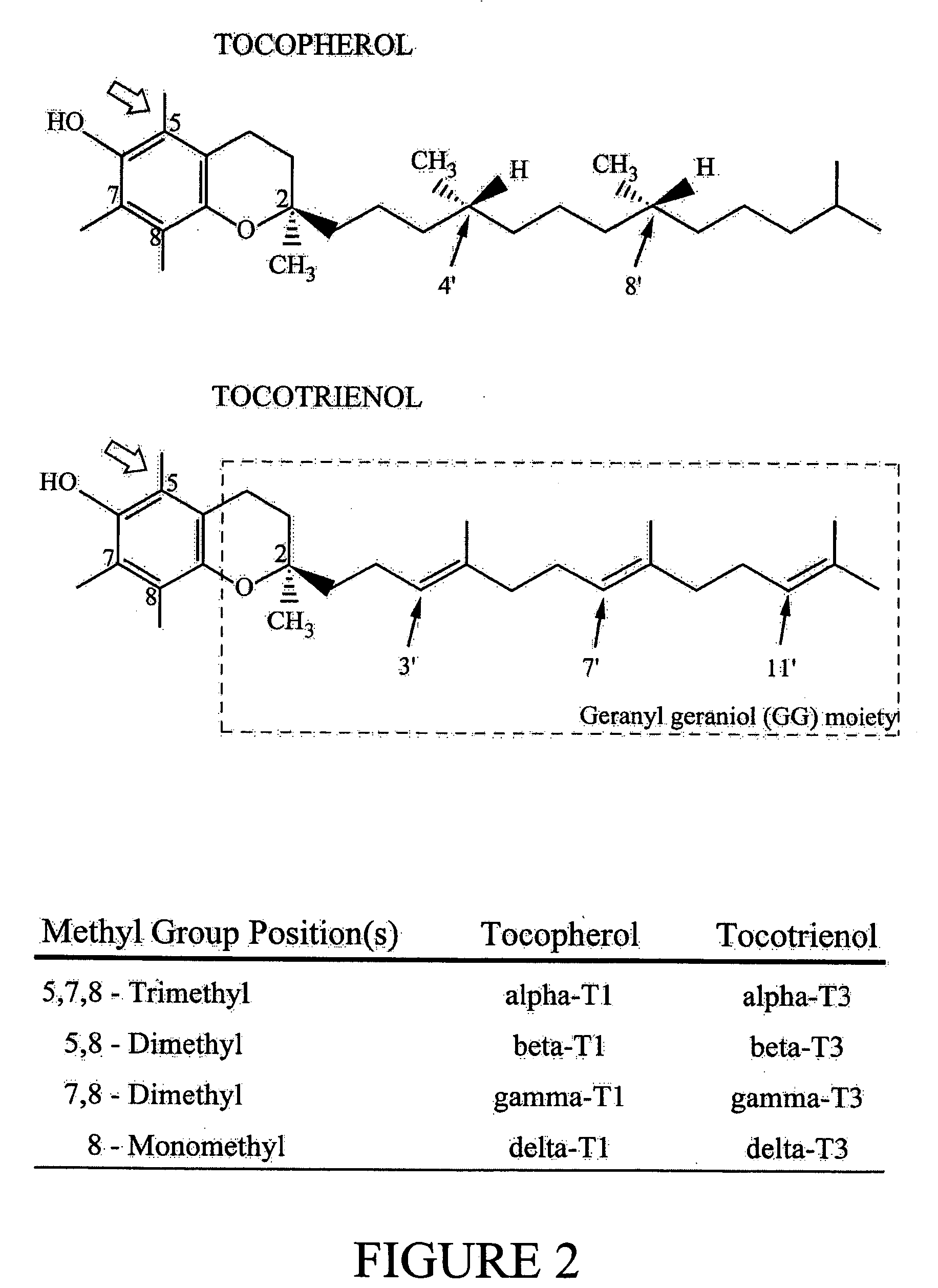
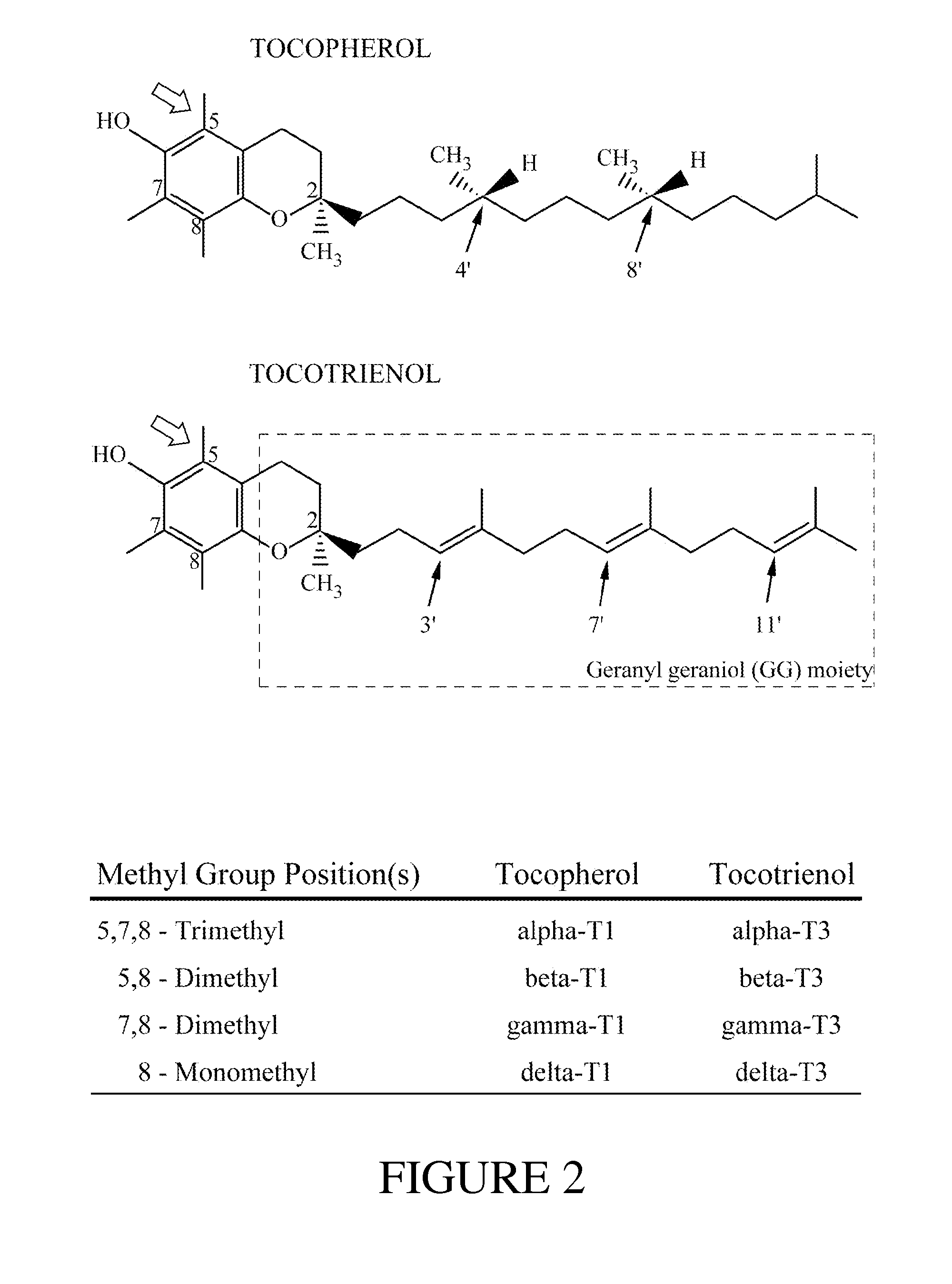
Α-Tocopherol is a type of vitamin E. It has E number "E307". Vitamin E exists in eight different forms, four tocopherols and four tocotrienols. All feature a chromane ring, with a hydroxyl group that can donate a hydrogen atom to reduce free radicals and a hydrophobic side chain which allows for penetration into biological membranes. Compared to the others, α-tocopherol is preferentially absorbed and accumulated in humans.
Methods of treating chronic inflammatory diseases using carbonyl trapping agents
InactiveUS6444221B1Improved therapeutic propertyImprove propertiesBiocidePeptide/protein ingredientsEtiologyBenzoic acid
Owner:SECANT PHARMA
Redox-active therapeutics for treatment of mitochondrial diseases and other conditions and modulation of energy biomarkers
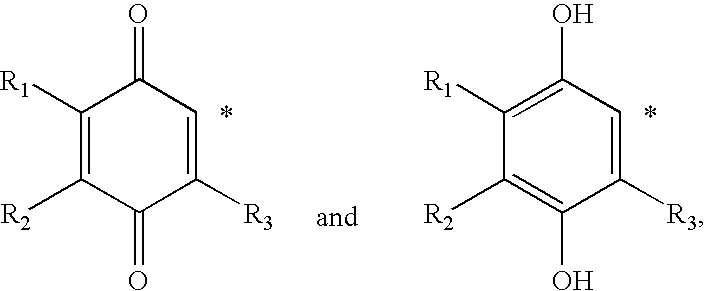
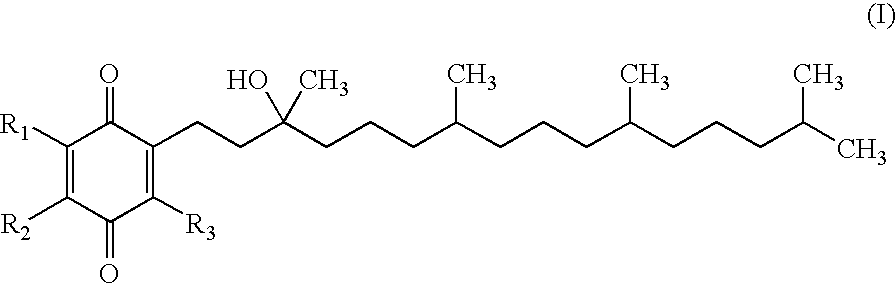
Methods of treating or suppressing mitochondrial diseases, such as Friedreich's ataxia (FRDA), Leber's Hereditary Optic Neuropathy (LHON), mitochondrial myopathy, encephalopathy, lactacidosis, stroke (MELAS), or Kearns-Sayre Syndrome (KSS) are disclosed, as well as compounds useful in the methods of the invention, such as alpha-tocopherol quinone. Methods and compounds useful in treating other disorders are also disclosed. Energy biomarkers useful in assessing the metabolic state of a subject and the efficacy of treatment are also disclosed. Methods of modulating, normalizing, or enhancing energy biomarkers, as well as compounds useful for such methods, are also disclosed.
Owner:PTC THERAPEUTICS INC
A combination of mitochondrial nutrients for relieving stress, preventing and improving stress-related disorders
InactiveUS20060257502A1Accelerated agingIncreasing oxidative metabolismBiocideCosmetic preparationsAlpha-TocopherolL-Carnosine
A dietary supplement of mitochondrial nutrients is designed for relieving stress, preventing and improving stress-related disorders, such as chronic fatigue syndrome, diabetes, age-associated cognitive dysfunction and diseases (Parkinson's and Alzheimer's disease). The supplement composition has the following nutrients: B vitamins (cyanocobalamin 2-1,000 ug, thiamin 1-1,000 mg, niacin 15-2,000 mg, pyridoxine 1-1,000 mg, Pantothenate 5-150 mg, folic acid 400-40,000 ug), alpha-tocopherol 10-800 mg, ascorbic acid 50-10,000 mg, calcium 20-2,000 mg, vitamin A 200-10,000 ug, alpha-lipoic acid 100-1,000 mg, N-acetyl cysteine 100-3,000 mg, L-carnosine 100-9,000 mg, tyrosine 100-9,000 mg, vanillin 10-100 mg, phosphatidylserine 10-800 mg, resveratrol 10-50 mg, dehydroepiandrosterone 1-50 mg, and melatonin 0.1-3 mg, all of which have been individually used experimentally or clinically for relieving stress, preventing and treating age- and stress-related disorders and diseases but no combination of these compounds has been used. Many embodiments also contain at least one adjunct ingredient such as coenzyme Q 10-200 mg, acetyl-L-carnitine 100-2,000 mg, choline 50-1,000 mg, and creatine 100-2,000 mg.
Owner:LIU JIANKANG
Method for preparing blend oil and cold-pressing and extracting peony seed oil
InactiveCN101653174AReduce lossesImprove nutrition and health valueEdible oils/fatsAlpha-TocopherolFiltration
The invention relates to a method for preparing blend oil and cold-pressing and extracting peony seed oil. The method comprises the following steps: collecting and dehulling seeds, cold-pressing and secondary-filtering, producing and filling the blend oil. The invention has a simple oil-extracting process and reduces the loss of active components in oil-extracting process. Cold pressing temperature is low so that phospholipid, pigment, and the like hardly enter oil, and the cold pressing oil has lighter color and lower phospholipid content and can achieve a new international four class oil standard only by mechanical filtration instead of any chemical refining. Cold pressing rap oil has high nutritive and healthcare values because thermosensitive alpha-tocopherol and phytosterols are fullyremained, active protein and other active components in peony seeds are remained so as to creates advantages for further developing new purposes of the seeds; no refining sewage is discharged in thecold pressing production process, therefore, the pollution controlling cost is lowered and the environmental protection is favorable.
Owner:兰州牡丹园艺开发公司
Redox-active therapeutics for treatment of mitochondrial diseases and other conditions and modulation of energy biomarkers
ActiveUS20100222436A1Reduce severityReduce in quantityBiocideSenses disorderDiseaseKearn sayre syndrome
Methods of treating or suppressing mitochondrial diseases, such as Friedreich's ataxia (FRDA), Leber's Hereditary Optic Neuropathy (LHON), mitochondrial myopathy, encephalopathy, lactacidosis, stroke (MELAS), or Kearns-Sayre Syndrome (KSS) are disclosed, as well as compounds useful in the methods of the invention, such as alpha-tocopherol quinone. Methods and compounds useful in treating other disorders are also disclosed. Energy biomarkers useful in assessing the metabolic state of a subject and the efficacy of treatment are also disclosed. Methods of modulating, normalizing, or enhancing energy biomarkers, as well as compounds useful for such methods, are also disclosed.
Owner:PTC THERAPEUTICS INC
Influenza vaccine
InactiveUS20080014217A1Stimulate immune responseSsRNA viruses negative-senseViral antigen ingredientsDiseaseSterol
The present invention relates to monovalent influenza vaccine formulations and vaccination regimes for immunising against influenza disease, their use in medicine, in particular their use in augmenting immune responses to various antigens, and to methods of preparation. In particular, the invention relates to monovalent influenza immunogenic compositions comprising an influenza antigen or antigenic preparation thereof from an influenza virus strain being associated with a pandemic outbreak or having the potential to be associated with a pandemic outbreak, in combination with an oil-in-water emulsion adjuvant comprising a metabolisable oil, a sterol and / or a tocopherol such as alpha tocopherol, and an emulsifying agent.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
Plant-derived protein extract compositions and methods
InactiveUS20050112078A1Reduce stimulationOrganic active ingredientsCosmetic preparationsAlpha-TocopherolColloidal oatmeal
Disclosed are skin care compositions comprising a plant-derived protein extract, such as a soy protein extract or a colloidal oatmeal preparation, supplemented by an enrichment composition, such as a non-alpha tocopherol, and methods of making and using such compositions.
Owner:JOHNSON & JOHNSON CONSUMER COPANIES
Less harmful nontoxic rubber automobile seal strip
The invention provides a less-harmful nontoxic rubber vehicle sealing strip which has excellent processability and physical properties and belongs to an environment friendly sulfidizing system. Main raw materials are as follows: 200 plus or minus 10 portions of ethylene propylene diene methylene, and 150 plus or minus 10portions of carbon black; 20 plus or minus 2 portions of light super fine calcium carbonate and 30 plus or minus 3 portions of talc as fillers; 20 plus or minus 2 portions of high-flash paraffin oil or styrene / Alpha-methyl styrene / vinyl toluene terpolymer as a softener; 10 plus or minus 1 portions of aluminum hydroxide as a flame retardant; 5 plus or minus 0.5 portions of zinc oxide and 2 plus or minus 0.2 portions of stearic acid as active agents together; 4 plus or minus 0.4 portions of polyethylene glycol of a hydroxyl polar group as an additive; 5 plus or minus 0.5 portions of calcium oxide as a neutralizer; 4 plus or minus 0.4 portions of nitrogen-free sulfidation material which is mainly sulphur; and 0.3 plus or minus 0.03 portion of Alpha-tocopherol as an inhibitor. The less harmful nontoxic rubber vehicle sealing strip can not only reduce environmental pollution, but also maintain reasonable cost.
Owner:ANHUI CHENYANG RUBBER & PLASTRIC CO LTD
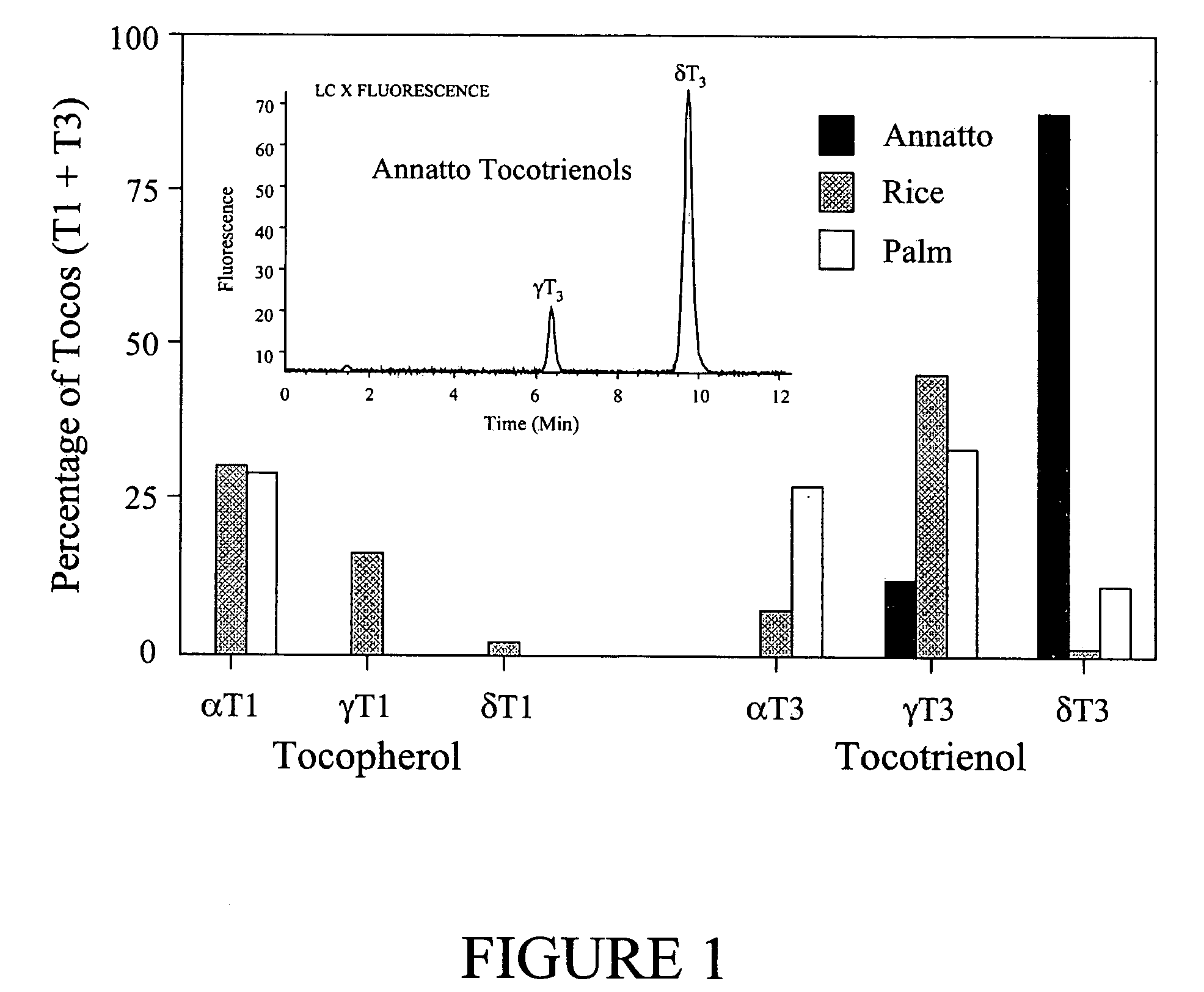
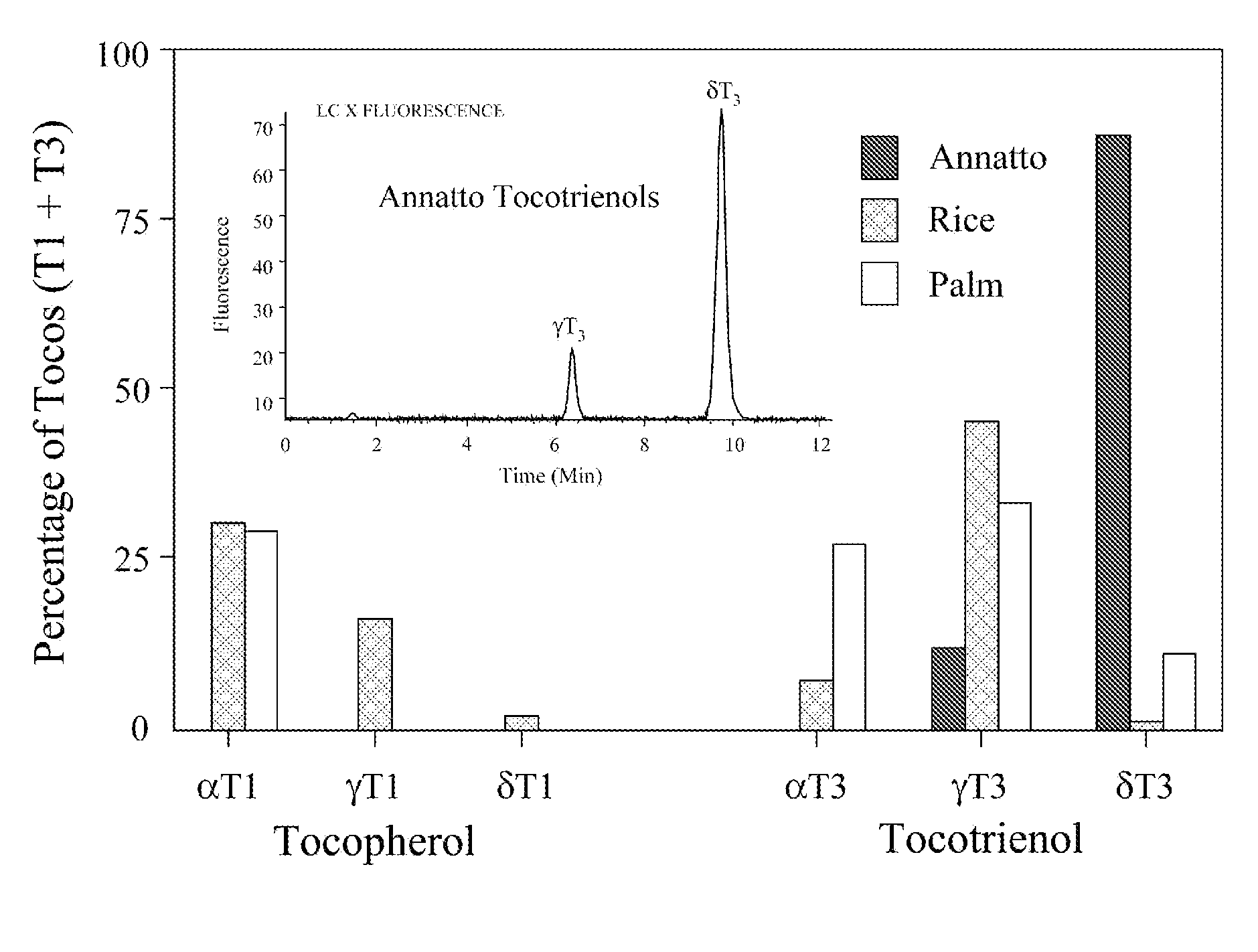
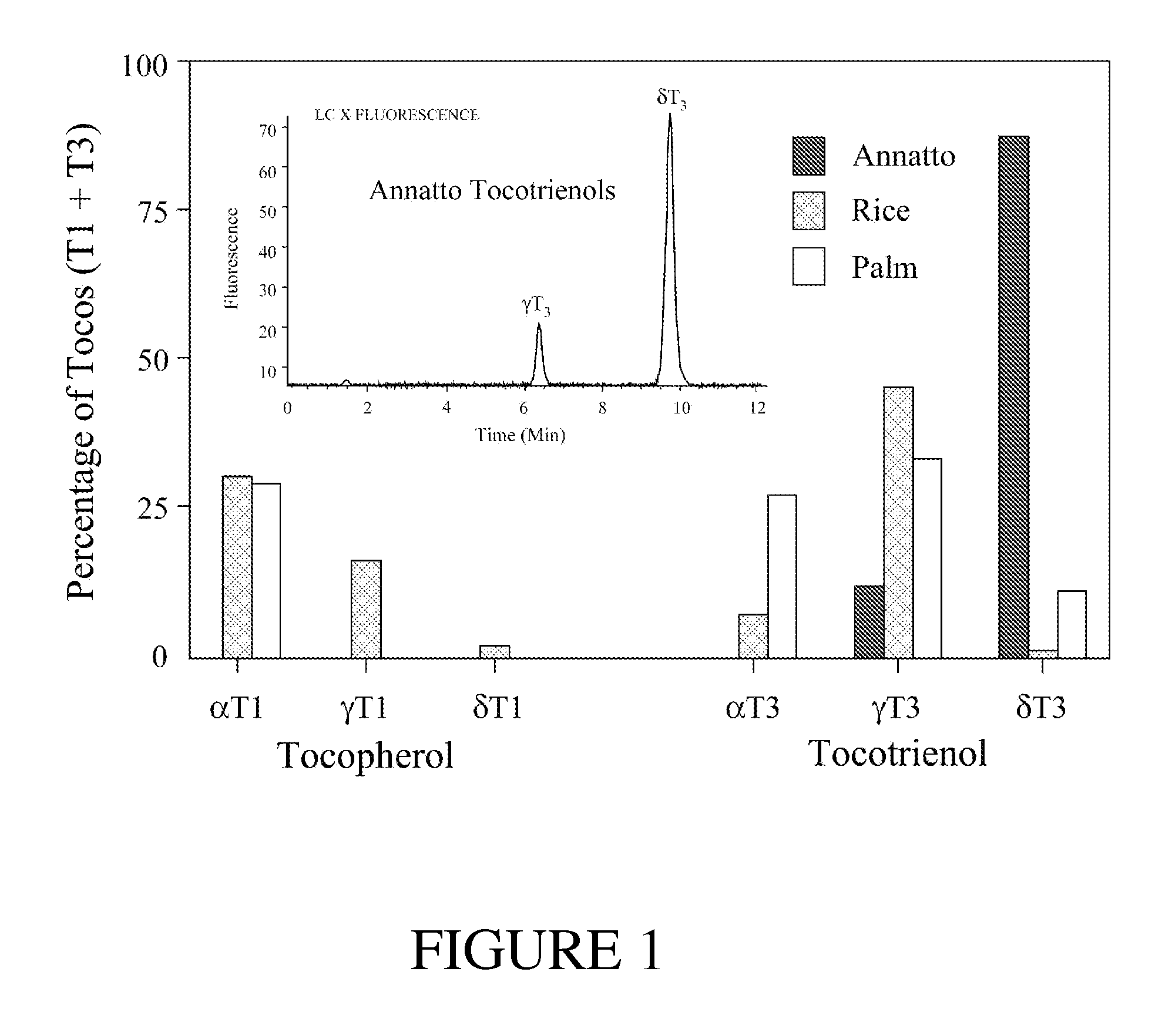
Annatto extract compositions including tocotrienols and tocopherols and methods of use
InactiveUS20050037102A1Reduce disease riskRemissionOrganic active ingredientsBiocideAlpha-TocopherolMedicine
Compositions and methods of use of annatto extracts including tocotrienols and tocopherols with an appropriate spectrum. This spectrum includes but not limited to low alpha tocopherol, high delta- and gamma-tocols, and mixtures with other extracts and / or nutrients.
Owner:TAN KS +1
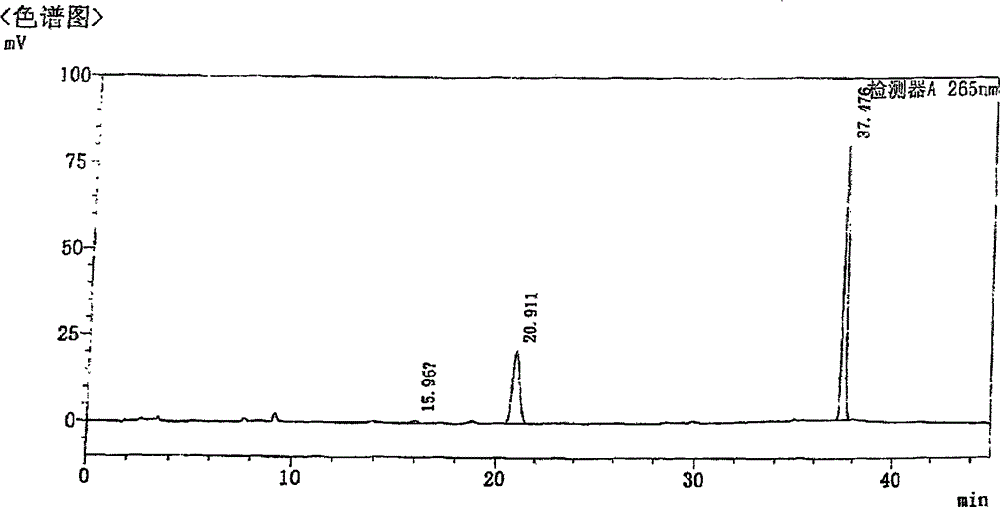
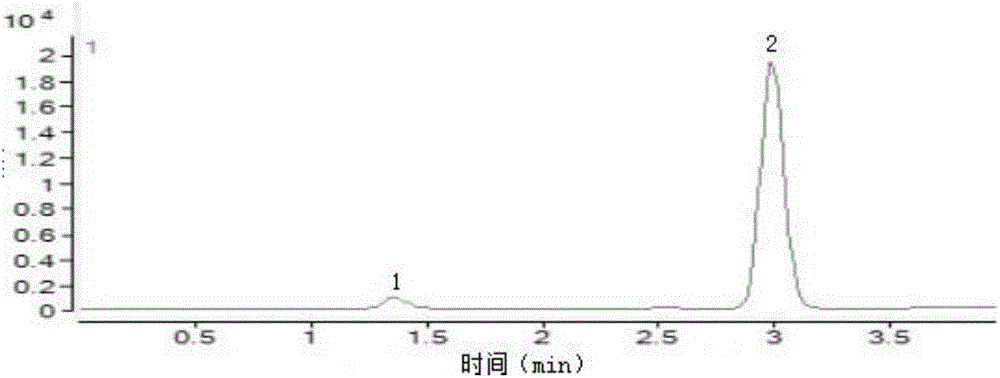
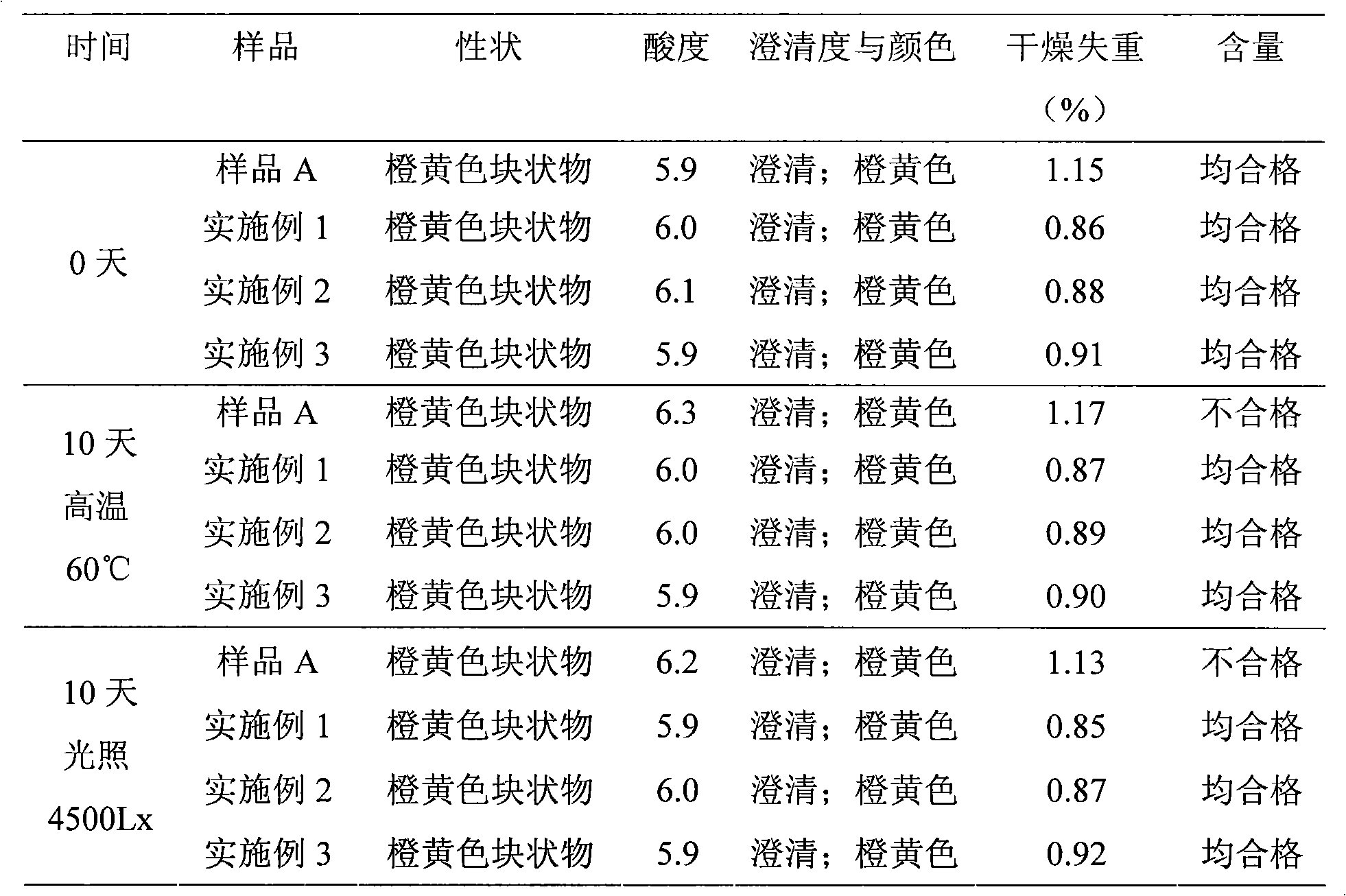
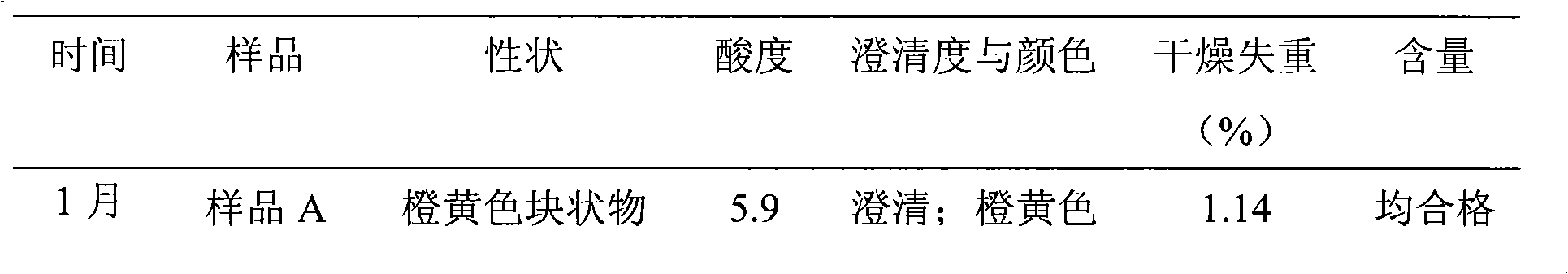
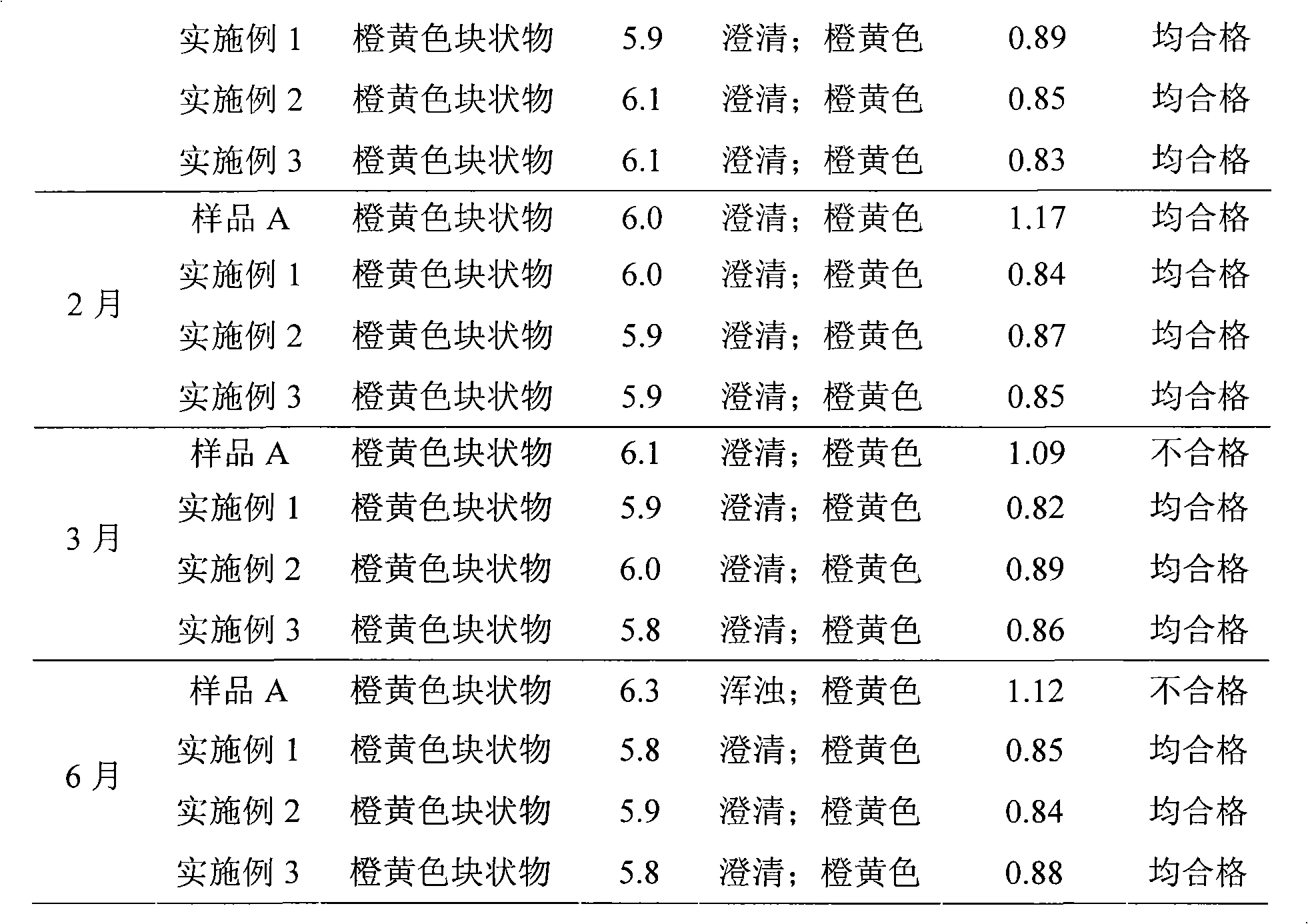
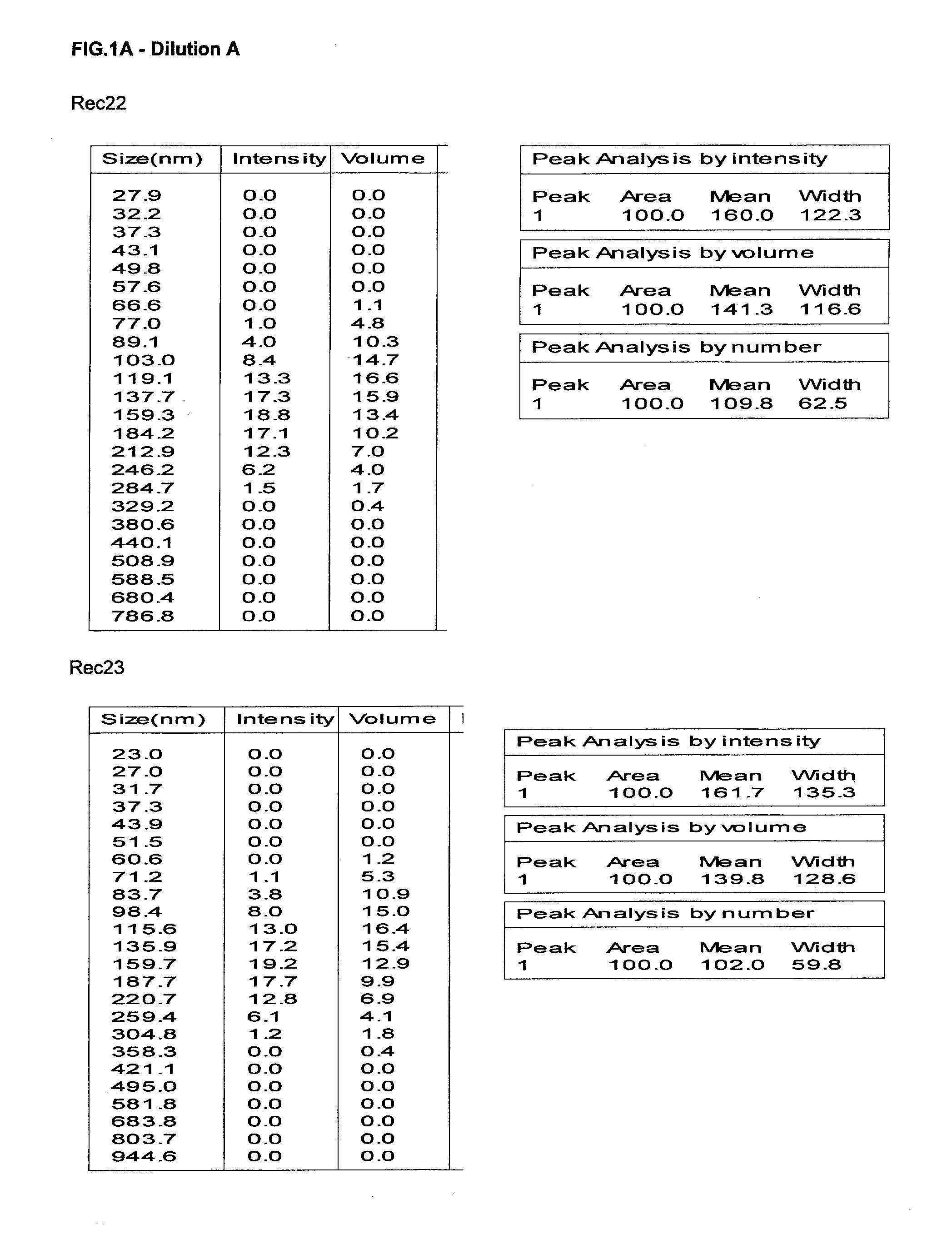
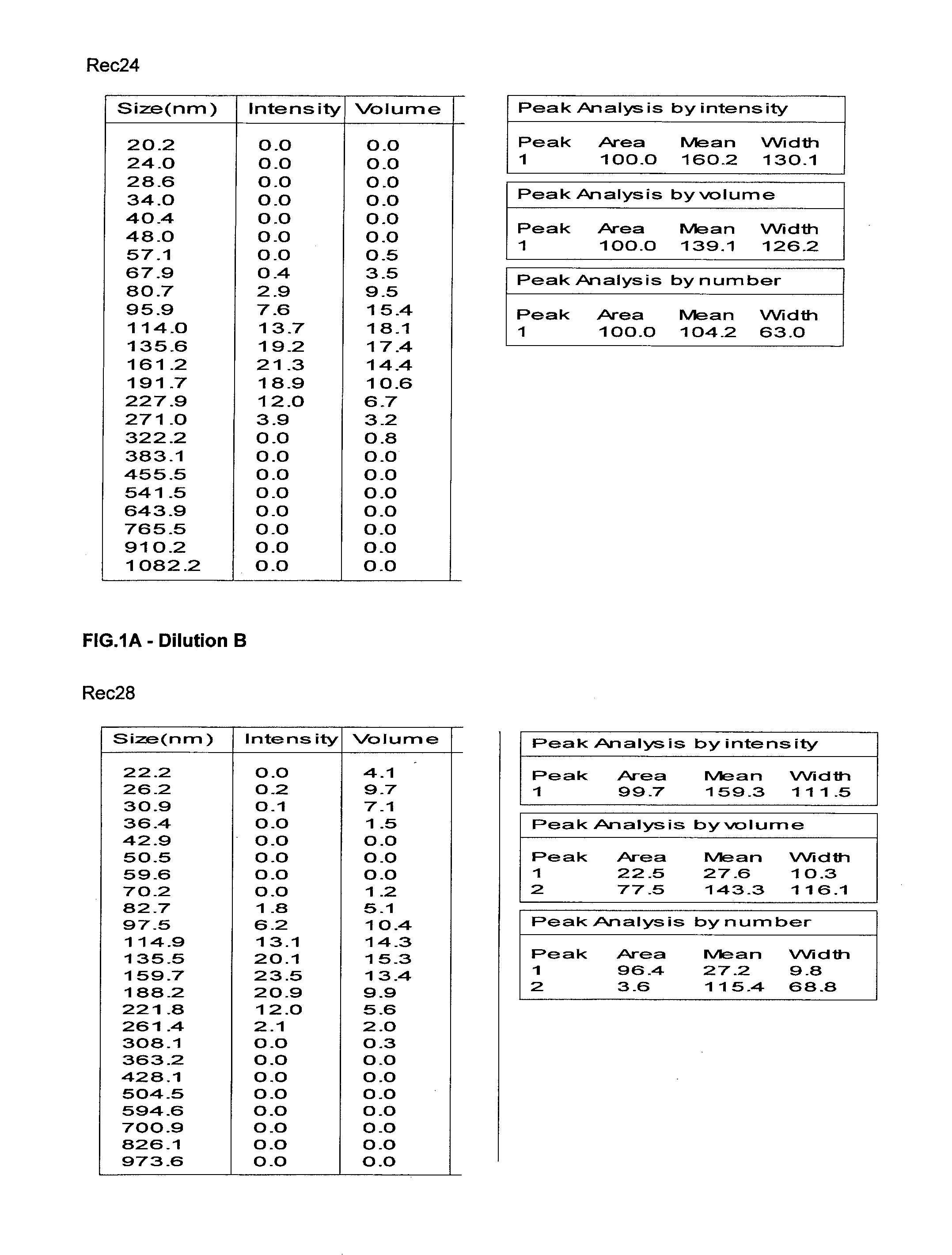
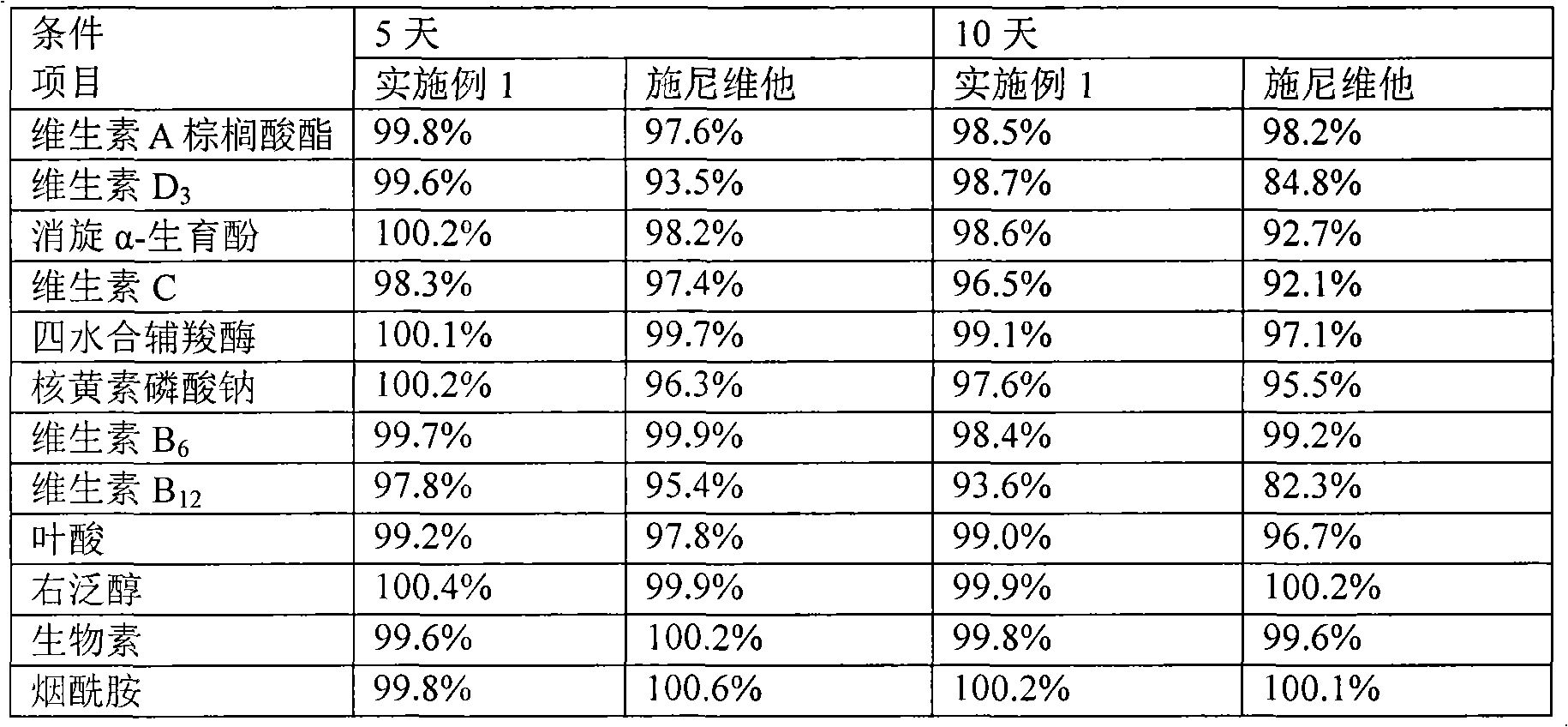
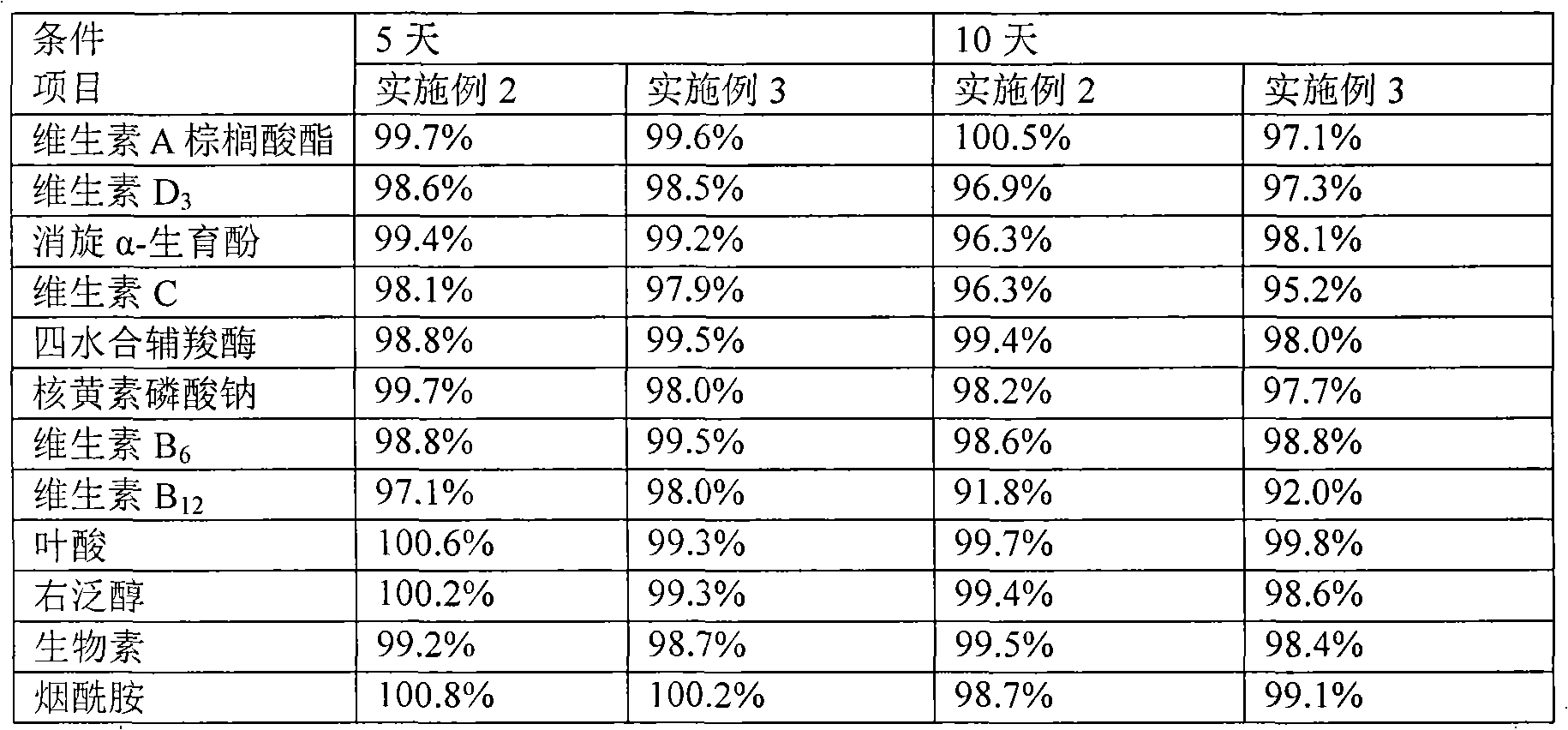
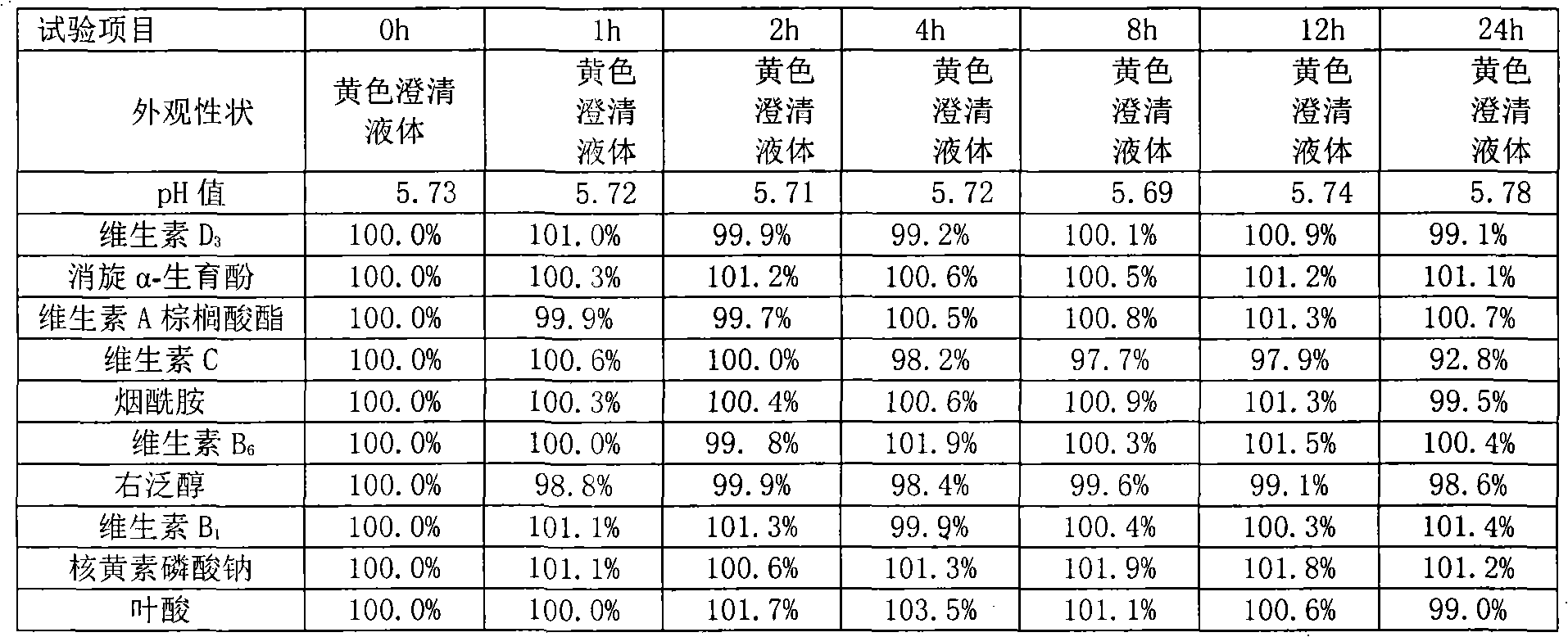
Content analysis and detection method of twelve compound vitamins for injection
InactiveCN106153796AImprove detection efficiencyHigh detection sensitivityComponent separationAlpha-TocopherolVitamin B12
The invention discloses a content analysis and detection method of twelve compound vitamins for injection. Liposoluble components including vitamin A palmitate, vitamin D3 and racemic alpha-tocopherol are detected under the same high-performance liquid phase method conditions, a test solution is prepared by extracting a preparation content by non-polar organic solvent, and the detection wavelength is 265+ / -3nm. Water-soluble components including cocarboxylase tetrahydrate, riboflavin sodium phosphate, vitamin B6, vitamin C, nicotinamide, folic acid and dexpanthenol are detected under the same high-performance liquid phase method conditions, and the detection wavelength is 210+ / -3nm. Biotin and vitamin B12 are subjected to high-performance liquid phase method based detection, the detection wavelength is 200-600nm, and the preferable detection wavelength for the vitamin B12 is 550+ / -3nm. The method has high applicability.
Owner:TIBET WEIXINKANG MEDICINE CO LTD
Annatto Extract Compositions Including Tocotrienols and Tocopherols and Methods of Use
ActiveUS20090041870A1Decreased blood levelReduce disease riskBiocideOrganic active ingredientsAlpha-TocopherolBiology
Compositions and methods of use of annatto extracts [350-450 Dalton molecular weight fraction] including tocotrienols and tocopherols with an appropriate spectrum. This spectrum includes but not limited to low alpha tocopherol, high delta- and gamma-tocols, and mixtures with other extracts [350-450 Dalton molecular weight fraction] like palm and rice and / or nutrients.
Owner:AMERICAN RIVER NUTRITION LLC
Edible emulsion containing highly unsaturated fat
An edible oil in water emulsion comprising 40-99 wt. % of a continuous aqueous phase which phase contains 0.05-15 wt. % of a protein calculated on aqueous phase and, preferably, a thickener in an amount of 0.01-3 wt. %, 1-60 wt. % of a dispersed fat phase, the fat phase comprising 0.01-0.2 wt. % of alpha-tocopherol and a triglycerides mixture containing 10-80 wt. % of mono-unsaturated fatty acid residues, 10-80 wt. % of polyunsaturated fatty acid residues, 3-15 wt. % of omega-3 fatty acid residues, the balance up to 100 wt. % consisting of saturated fatty acid residues, fatty acid residues being calculated on fat phase, which emulsion is characterized in that it contains 0.005-0.05 wt. % of delta-tocopherol, while the weight ratio of delta-tocopherol and alpha-tocopherol in the emulsion is selected from the range 5 to 0.25.
Owner:UNILEVER BESTFOODS NORTH AMERICA DISISION OF CONOPCO
Solid lipidic nanospheres suitable to a fast internalization into cells
The present invention relates to pharmaceutical compositions in form of solid lipidic nanospheres able to rapidly penetrate into the cells, comprising as an active substance a lipidic substance consisting of an ester of alpha-tocopherol or delta-tocopherol or of cholesterol or of glycerol with a carboxylic acid selected from acetic acid, propionic acid, butyric acid and succinic acid, useful in the treatment of tumoral pathologies and of Mediterranean anaemia.
Owner:GASCO MARIA ROSA
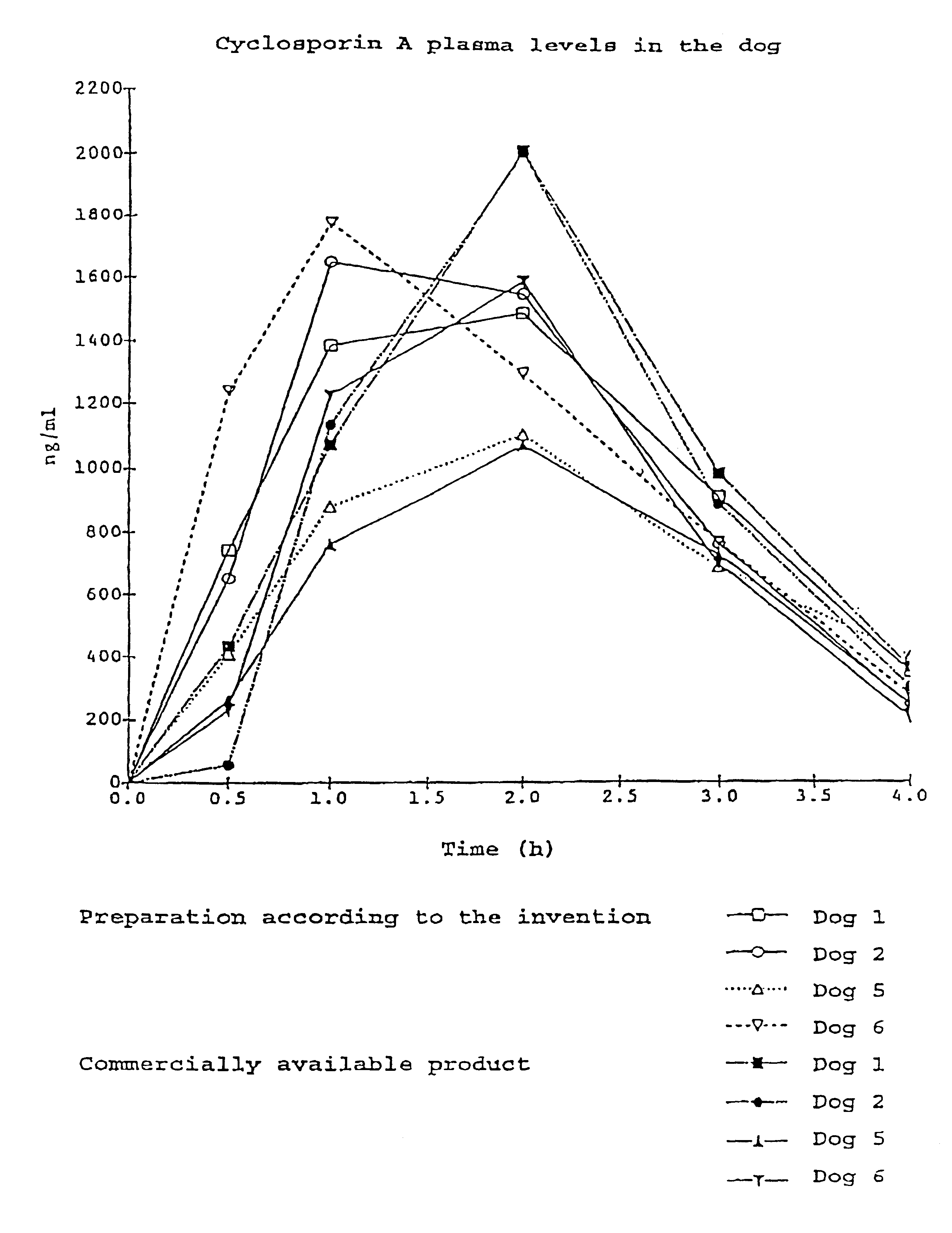
Pharmaceutical preparation with cyclosporin A
InactiveUS6696413B2Reduce impactPromote permeationBiocideOrganic active ingredientsVegetable oilAlcohol
The invention relates to a pharmaceutical preparation which consists of or contains cyclosporin A, an emulsifying alpha-tocopherol derivative, an ethoxylation product of vegetable oils, fatty acids or fats as a further emulsifier and a pharmaceutically customary alcohol.
Owner:HEXAL AG
Method of simultaneously detecting contents of vitamin A and vitamin E in blood
ActiveCN106442754AAccurate identificationStrong specificityComponent separationVitamin A RetinolAlpha-Tocopherol
The invention discloses a method of simultaneously detecting contents of vitamin A (retinol) and vitamin E (alpha-tocopherol) in blood. The method comprises the following steps: (1) centrifuging a whole blood sample, and taking a supernatant to obtain serum or plasma for standby application; (2) calibrating a standard solution; (3) pretreating the sample; and (4) automatically putting 80 microlitres of the supernatant sample in the step (3) in a sample bottle, analyzing through liquid chromatography or liquid chromatography-tandem mass spectrometry, and simultaneously quantitatively detecting the contents of the vitamin A (retinol) and the vitamin E (alpha-tocopherol) in the blood. The method of simultaneously detecting the contents of the vitamin A (retinol) and the vitamin E (alpha-tocopherol) in the blood is strong in specificity, high in accuracy and sensitivity and short in analysis time.
Owner:BEIJING HARMONY HEALTH MEDICAL DIAGNOSTICS CO LTD
Composite liposome for injection containing 12 vitamins and preparation method thereof
InactiveCN101491499ASafety proofMetabolism disorderAmide active ingredientsAlpha-TocopherolSodium phosphates
The invention relates to a liposome lyophilized preparation prepared from twelve compound vitamins. The lyophilized preparation is characterized in that the lyophilized preparation is prepared from a liposome which consists of soybean lecithin and glycocholic acid and is encapsulated with twelve vitamins of retinyl palmitate, cocarboxylase tetrahydrate, riboflavin sodium phosphate, pyridoxine hydrochloride, cyanocobalamin, cholecalciferol, ascorbic acid, racemization-alpha tocopherol, D-biotin, niacinamide, folacin and dexpanthenol.
Owner:灵康药业集团股份有限公司
Blended oil skin moisturizer and skin repairer
An organic skin moisturizer comprised of Sea Buckthorn seed oil, Camellia seed oil, Argan seed oil, Pomegranate seed oil, Rosemary essential oil, d-alpha tocopherol, Tea Tree oil, Helichrysum oil, vitamin A palmitate. A lighter organic skin moisturizer comprised of Sea Buckthorn seed oil, Camellia seed oil, Argan seed oil, Pomegranate seed oil, Meadowfoam seed oil, Jojoba oil, d-alpha tocopherol, and Rosemary essential oil. And a damaged skin moisturizer, comprised of Sea Buckthorn seed oil, Tamanu oil, Meadowfoam seed oil, d-alpha tocopherol, Tea tree oil, and Vetiver oil.
Owner:RAYMOND COBLANTZ SHERRY MAY
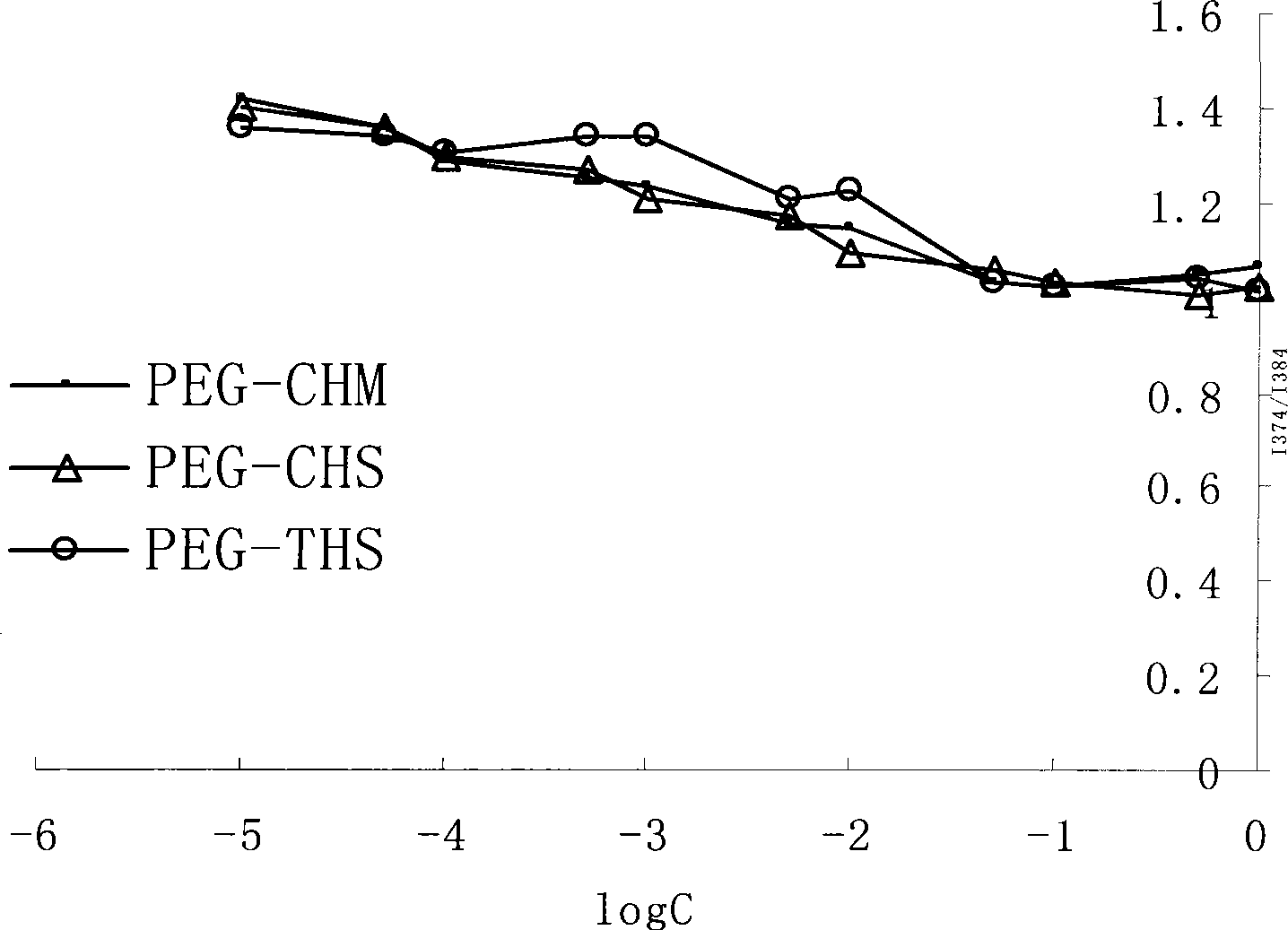
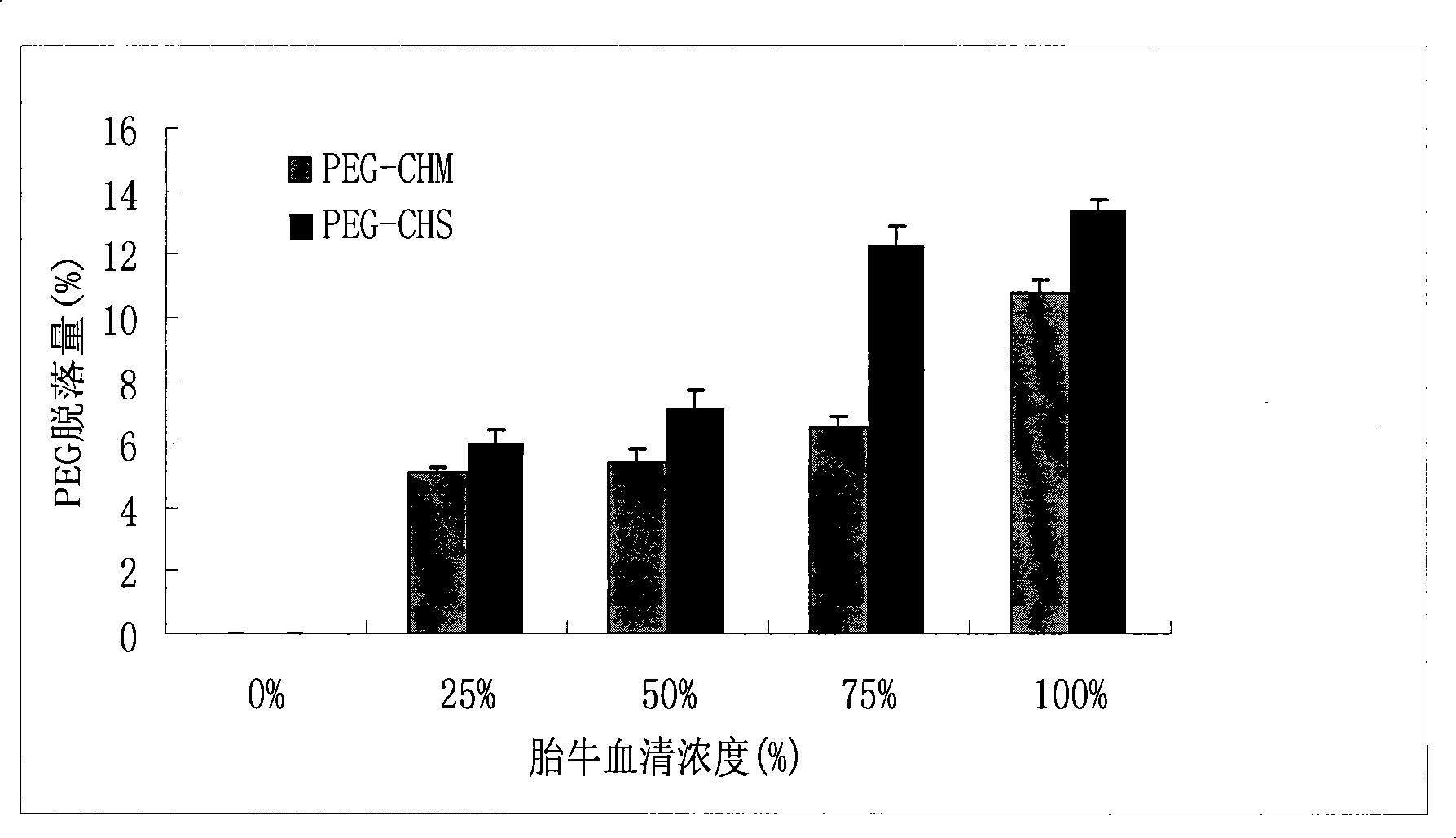
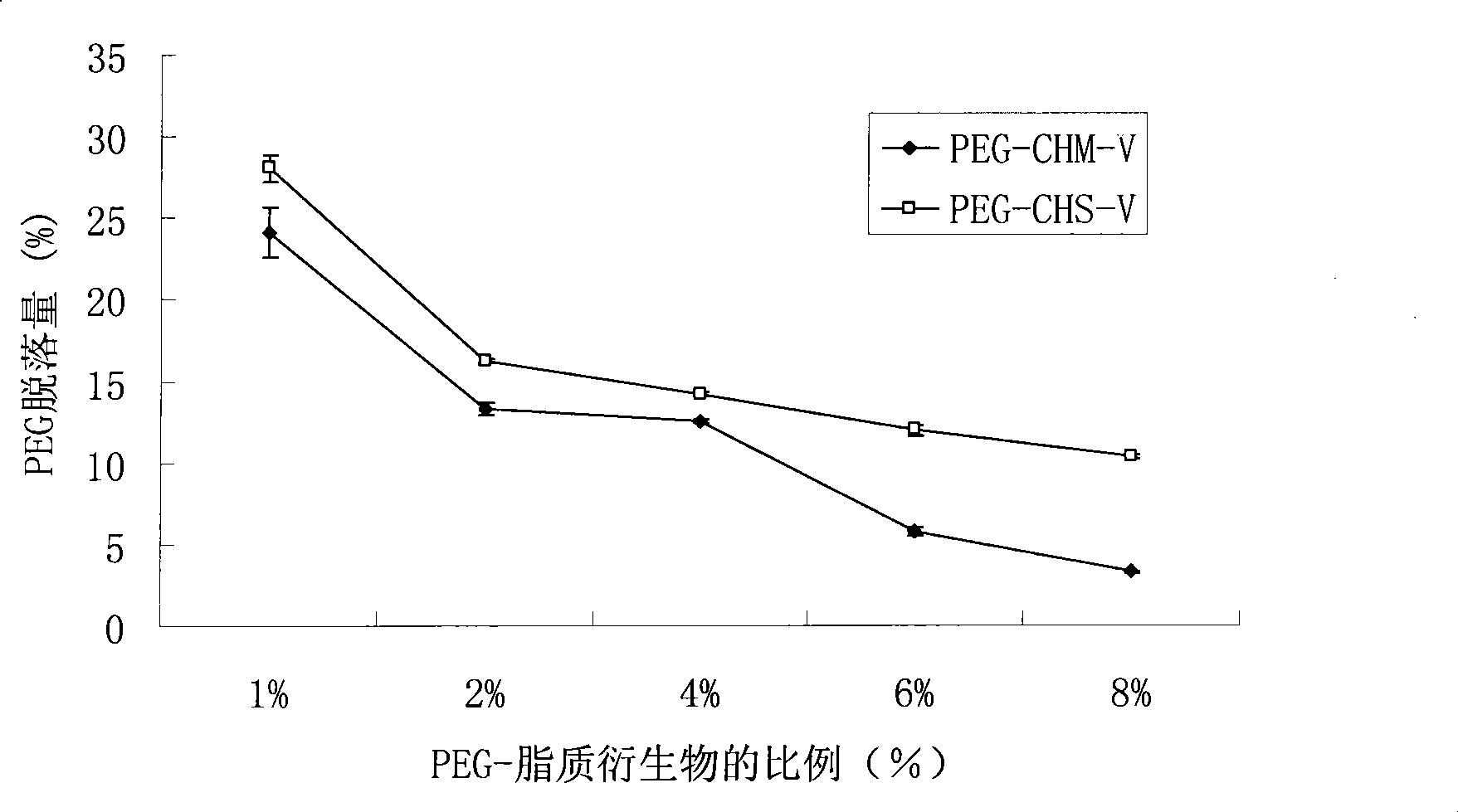
Method for preparing cleavable polyethyleneglycol lipid derivates and application
InactiveCN101468203AEliminates conjugation stepsAchieve the goal of active targetingPharmaceutical non-active ingredientsLipid formationAlpha-Tocopherol
The present invention belongs to technical field of medicine, and relates to the preparing of cleavable polyethylene glycol (PEG) lipid derivative and an application thereof in the liquid particle preparation. The general formula is as follows: CH3O(CH2CH2O)n-R-O-R, n=5-5000. The molecular weight of PEG is 300-30000. R represents one group selected from hemisuccinate group and carbomethoxy. The liposoluble fragment represented by R comprises one component selected from cholesterol, sitosterol, alpha-tocopherol. According to the invention, polyethylene glycol are connected with lipoid derivatives such as cholesterol, alpha-tocopherol, etc. through ester linkage. The cleavable PEG lipid derivative can be applied to the modification of liquid particle preparation. On one hand, the PEG lipid derivative has appropriate adhesive force of the surface of liquid particle preparation and guarantees enough holding time of PEG lipid derivative in blood. On the other hand, the PEG lipid derivative can gradually break away from the surface of preparation in the circulation process. The particle preparation which only comprises a few polyethylene glycol on the surface can combine and phagocytose the pathological cell. The medicine is delivered into the cell and therefore has the function of selectively killing the pathological cell.
Owner:SHENYANG PHARMA UNIVERSITY
Solid dispersion as well as preparation method and application thereof
ActiveCN106420633AImprove solubilityImprove bioavailabilityPharmaceutical non-active ingredientsGranular deliveryAlpha-TocopherolPolyethylene glycol
The invention relates to solid dispersion as well as a preparation method and an application thereof. The solid dispersion is prepared from indissolvable drugs, a surfactant and a water-soluble polymer material with a spray drying method after mixing and heating dissolution, wherein the surfactant is selected from at least one of sodium dodecyl sulfate, poloxamer, tween, alpha-tocopherol, succinate, polyethylene glycol, sodium cholate and polyethylene glycol / vinyl caprolactam / vinyl acetate copolymer; the water-soluble polymer material is selected from at least one of povidone, copovidone, hydroxypropyl methylcellulose and polyethylene glycol. An organic solvent is not required when the solid dispersion is prepared with the spray drying method, and the problem of organic solvent residues is solved. By means of the solid dispersion, the dissolvability of the indissolvable drugs is increased, the dissolution speed and the dissolubility are remarkably increased, and the bioavailability of the indissolvable drugs is improved.
Owner:GUANGZHOU ZHONGDA NANSHA TECH INNOVATION IND PARK +1
Alpha-tocopherol treatment for cystic fibrosis
InactiveUS20050074445A1Overcome malabsorptionImprove immunityBiocideVitamin food ingredientsBeta-CaroteneAlpha-Tocopherol
Disclosed herein is an aqueous emulsion of alpha-tocopherol and preferably other forms of vitamin E, coenzyme Q10, beta-carotene, vitamin D, and vitamin K useful for treating complications of cystic fibrosis. The aqueous emulsion improves malabsorption and immunity against infection, and reduces oxidative stress and respiratory complications in cystic fibrosis.
Owner:YASOO HEALTH
Nutritional compositions including rrr-alpha tocopherol and polyunsaturated fatty acids
InactiveUS20150025133A1Improved central nervous system maturationImproved cognitive developmentBiocideSugar food ingredientsBrain developmentIodo fatty acid
Disclosed are nutritional formulas generally, and infant formulas specifically, including a combination of RRR-alpha tocopherol, LC-PUFAs, and optionally vitamin C. The combination enhances brain development and improves cognitive performance in an individual, and specifically in an infant.
Owner:ABBOTT LAB INC
Influenza vaccine
ActiveUS20110243987A1Boosting antibodyBoosting cellular immune responseSsRNA viruses negative-senseViral antigen ingredientsAdjuvantSterol
The present invention relates to monovalent influenza vaccine formulations and vaccination regimes for immunising against influenza disease, their use in medicine, in particular their use in augmenting immune responses to various antigens, and to methods of preparation. In particular, the invention relates to monovalent influenza immunogenic compositions comprising an influenza antigen or antigenic preparation thereof from an influenza virus strain being associated with a pandemic outbreak or having the potential to be associated with a pandemic outbreak, in combination with an oil-in-water emulsion adjuvant comprising a metabolisable oil, a sterol and / or a tocopherol such as alpha tocopherol, and an emulsifying agent.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
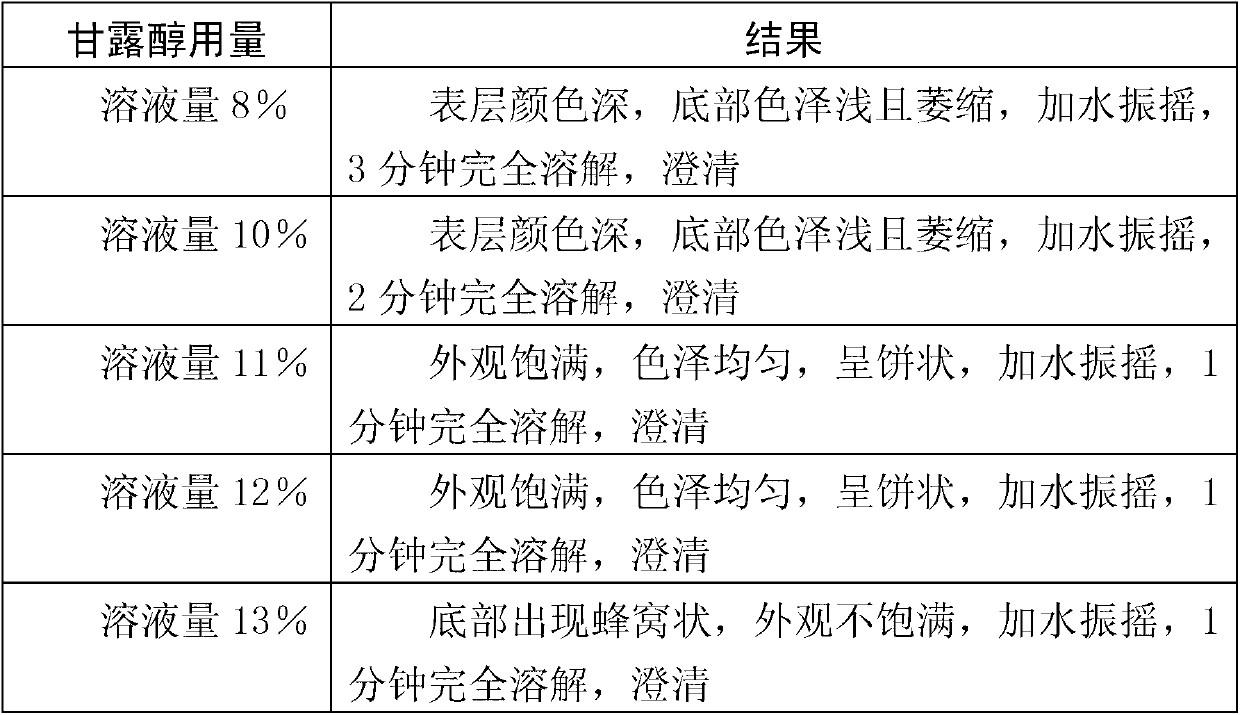
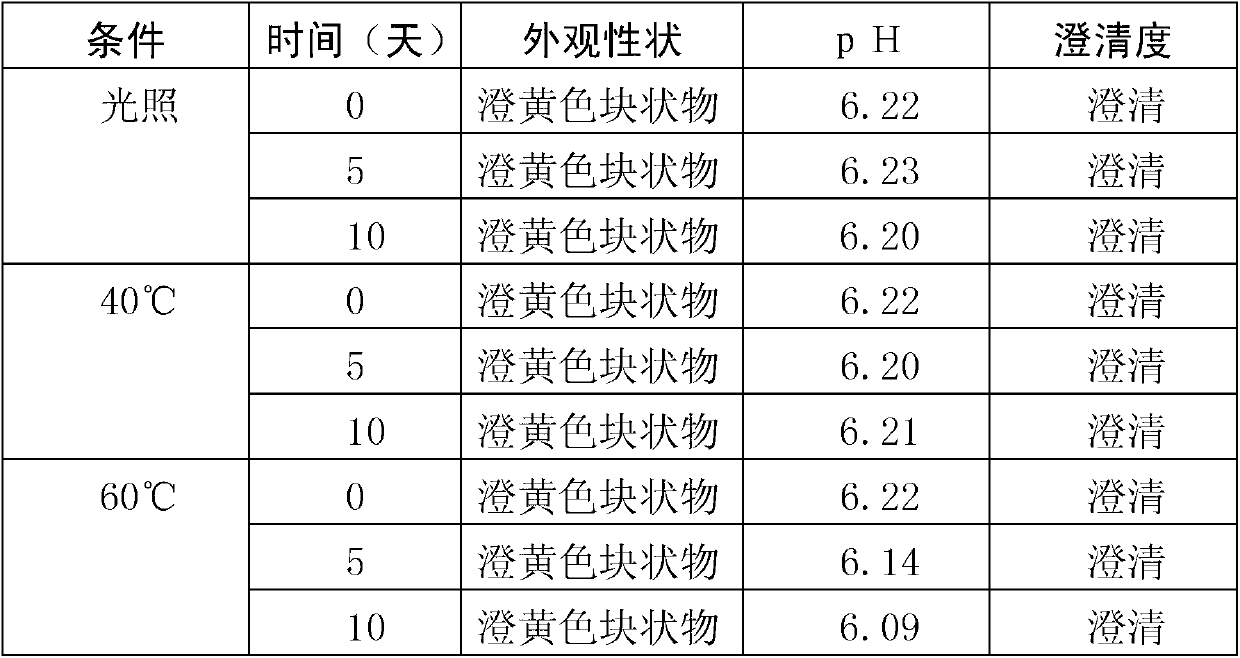
Stable complex vitamin composite and preparation method thereof
ActiveCN102068453AMetabolism disorderPharmaceutical non-active ingredientsAlpha-TocopherolVitamin B12
The invention relates to an aqueous solution for preparing a stable complex vitamin composite, a complex vitamin composite injection and a preparation method thereof. The composite is prepared from 11 vitamins, or analogs thereof, racemization alpha-tocopherol, polysorbate and medically acceptable auxiliary materials by the freeze drying technology, wherein 11 vitamins comprises vitamin A palmitate, vitamin D3, vitamin C, cocarboxylase tetrahydrate, riboflavin sodium phosphate, vitamin B6, vitamin B12, folic acid, dexpanthenol, biotin and nicotinamide. Compared with the prior art, the composite has better stability.
Owner:西藏中卫诚康药业有限公司
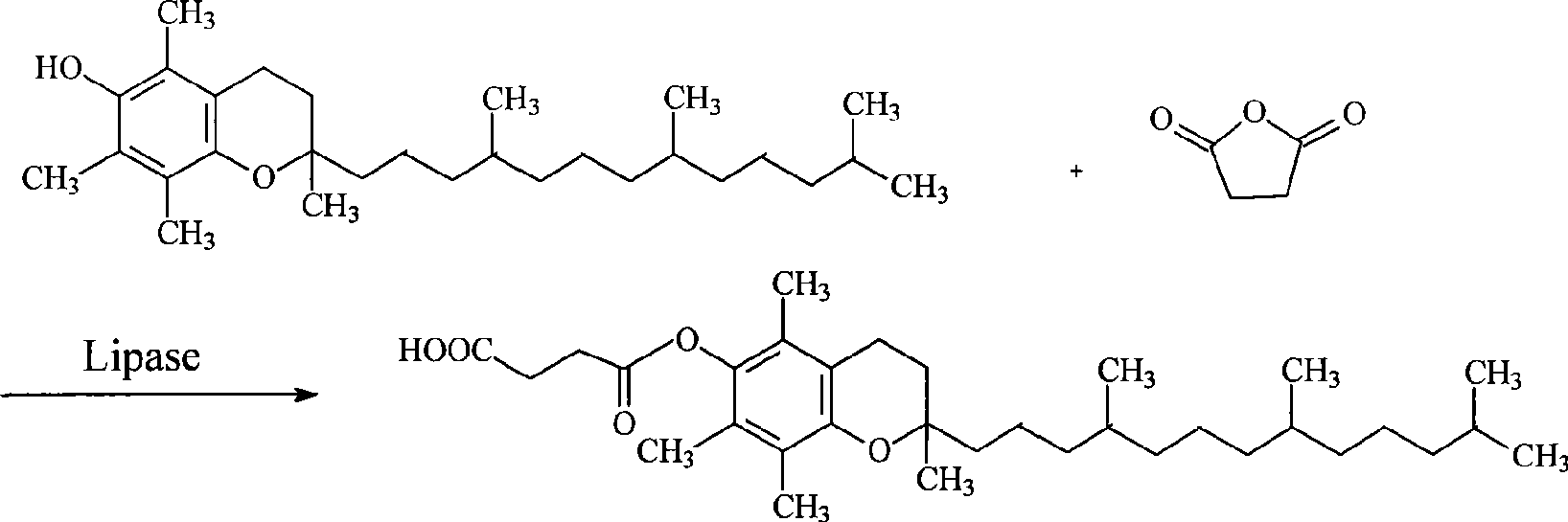
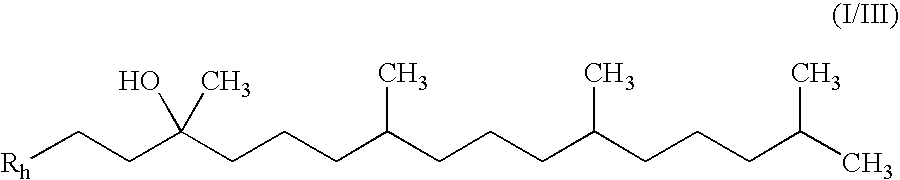
Method for synthesizing natural alpha-tocofecol tocopherol acid succinate by using lipase
A method for synthesizing natural alpha-tocopherol succinic acid monoester using lipase pertains to the field of biological chemicals, which is characterized in that the preparation method includes: adding the substrate of natural alpha-tocopherol and succinic anhydride at molar ratio of 1:1-1:8 in an organic solvent, adding lipase to start the reaction, oscillating or mixing reaction 20-72 hours under the conditions of 20-45 DEG C, wherein, the conversion rate of the alpha-tocopherol is 80-98.39%. Compared with the prior industry process in which esterification is performed at high-temperature by using high toxicity chemical catalyst under, the method has advantages of mild reaction conditions (room temperature, atmospheric pressure), low energy consumption, non-toxic catalyst, specificity, high reaction efficiency fewer accessory substances, and the like.
Owner:UNIV OF SCI & TECH BEIJING
Pharmaceutical composition of 12 complex vitamins for injection and preparation method thereof
InactiveCN103006683AHydroxy compound active ingredientsMetabolism disorderThiamine pyrophosphateAlpha-Tocopherol
The invention provides a pharmaceutical composition of 12 complex vitamins for injection and a preparation method thereof. The pharmaceutical composition of 12 complex vitamins for injection provided by the invention comprises the active ingredients of vitamin A palmitate, cholecalciferol, racemic alpha-tocopherol, ascorbic acid, nicotinamide, dexpanthenol, pyridoxine hydrochloride, riboflavin sodium phosphate, tetrahydrate thiamine pyrophosphate, folic acid, D-biotin and cyanocobalamin, and auxiliary materials namely polysorbate 80 and mannitol. The prescription provided by the invention does not contain auxiliary material glycocholic acid, and can be clinically used for people with over-high glycocholic acid.
Owner:SHANXI PUDE PHARMA CO LTD
Soybean blending edible oil and preparation process thereof
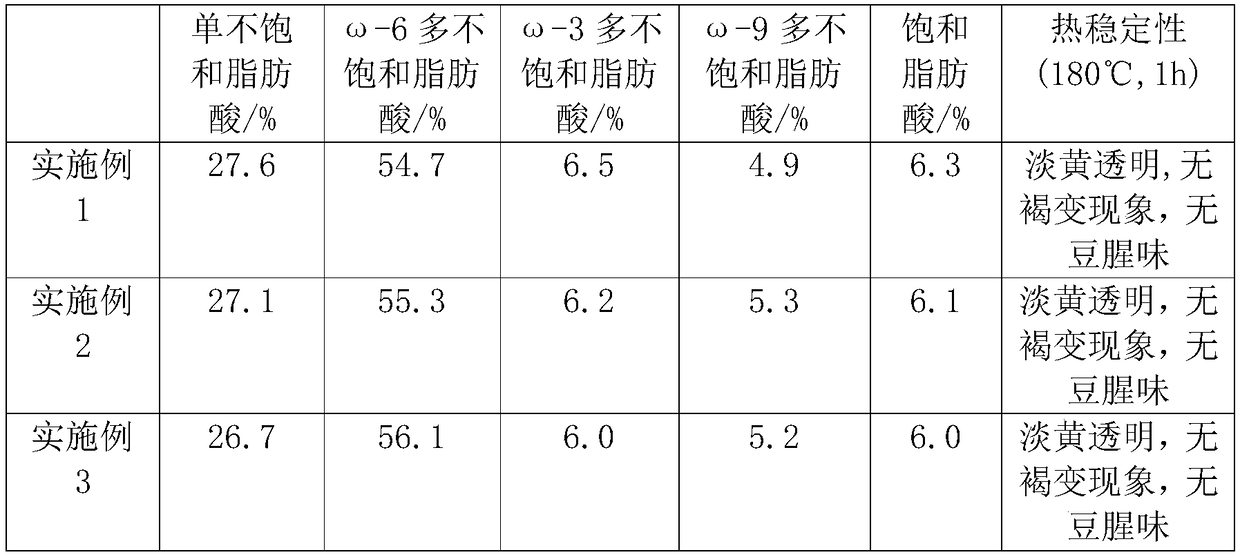
InactiveCN109042909APrevent high temperature browning phenomenonReduce dissolved oxygen contentFatty substance preservation using additivesEdible oils/fatsBiotechnologyVegetable oil
The invention discloses soybean blending edible oil and a preparation process thereof. The soybean blending edible oil is prepared from the following raw materials in parts by weight: 100 parts of soybean oil, 20-30 parts of olive oil, 15-25 parts of grape seed oil, 10-20 parts of rosehip oil, 10-15 parts sea buckthorn oil, 6-10 parts of perilla seed oil, 1-7 parts of corn germ oil, and 0.1-0.2 part of a vegetable oil modifying agent; the vegetable oil modifying agent is prepared from the following ingredients in parts by weight: 2-3 parts of glucose oxidase, 3-4 parts of o-phosphoethanolamine, 0.8-1.2 parts of alpha-tocopherol, 0.6-1 part of a rosemary extract, 1-2 parts of lycopene, 0 .3-0.5 part of catechin and 0.4-0.6 part of phytic acid. The preparation process comprises the followingsteps of preparing the vegetable oil modifying agent, preparing compound vegetable oil and preparing the soybean blending edible oil. According to the soybean blending edible oil, the vegetable oil modifying agent is utilized to passivate lipoxygenase activity, prevent the browning reaction of soybean blending oil, reduce the content of dissolved oxygen and improve the oxidation resistance and thermal stability of blending oil so as to enable the blending oil to maintain good flavor and quality.
Owner:凤台县兴永食用油有限责任公司
Redox-active therapeutics for treatment of mitochondrial diseases and other conditions and modulation of energy biomarkers
Methods of treating or suppressing mitochondrial diseases, such as Friedreich's ataxia (FRDA), Leber's Hereditary Optic Neuropathy (LHON), mitochondrial myopathy, encephalopathy, lactacidosis, stroke (MELAS), or Kearns-Sayre Syndrome (KSS) are disclosed, as well as compounds useful in the methods of the invention, such as alpha-tocopherol quinone. Methods and compounds useful in treating other disorders are also disclosed. Energy biomarkers useful in assessing the metabolic state of a subject and the efficacy of treatment are also disclosed. Methods of modulating, normalizing, or enhancing energy biomarkers, as well as compounds useful for such methods, are also disclosed.
Owner:PTC THERAPEUTICS INC
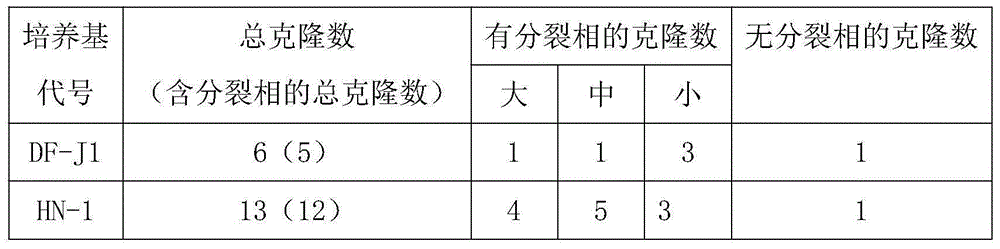
Low serum amniotic fluid cell culture medium
The invention discloses a low serum amniotic fluid cell culture medium. On the basis of mixing a basal culture medium RPMI 1640 and a basal culture medium alpha MEN according to the proportion of one to one, the components of insulin, a basic fibroblast growth factor (bFGF), an epidermal growth factor (EGF), bovine serum albumin (BSA), hydrocortisone, alpha tocopherol, NaHCO3, ferric citrate, tween 80, ethanol amine, oleic acid, linoleic acid and fetal calf serum are added, wherein addition of fetal calf serum content accounts for not more than 1% of the total volume of the low serum amniotic fluid cell culture medium. According to the low serum amniotic fluid cell culture medium, compared with an existing public technology, the serum content and the cost are reduced, the effect is significantly improved, colony forming efficiency and colony growth speed are increased obviously, in a short time, a great number of aminotic cells in a division phase can be obtained through culture, and the requirements of clinical diagnoses and scientific research can be met.
Owner:广州和能生物科技有限公司
Method for preparing low-benzopyrene high-purity d-alpha tocopherol acetate
The invention discloses a method for preparing low-benzopyrene high-purity d-alpha tocopherol acetate. The method comprises the following steps of: mixing d-alpha tocopherol, an inert organic solvent and a catalyst in a certain ratio, controlling the temperature, performing esterification reaction on the mixture and acetic anhydride, mixing obtained crude d-alpha tocopherol acetate and a polar solvent, loading to a column, adsorbing by using resin so as to remove impurities, mixing the obtained product and the inert organic solvent, adding a removal agent in a certain ratio, circulating at the constant temperature of 60 DEG C for 2 hours in an external circulation mode, filtering, concentrating, desolventizing, and performing short-path molecular distillation refinement under high vacuum conditions to obtain the low-benzopyrene high-purity d-alpha tocopherol acetate. The content of the obtained product is high, and the content of the d-alpha tocopherol acetate is more than or equal to 98 percent; the specific rotation is more than or equal to +24 degrees; the concentration of heavy metals (based on Pb) is less than or equal to 10ppm; and the concentration of benzopyrene is less than or equal to 2ppb. The quality of products meets the standards established by developed countries in Europe and America.
Owner:浙江伊宝馨生物科技股份有限公司
Micronutrient formulations and related methods of manufacture
InactiveUS20080020035A1Prevent excess productionBiocideOrganic active ingredientsAlpha-TocopherolPalmitates
A micronutrient formulation system is provided and the system comprises: a first composition comprising alpha tocopherol and derivative esters of alpha tocopherol, the derivative esters of alpha tocopherol being selected from a group consisting essentially of alpha tocopheryl acetate, alpha tocopheryl palmitate, alpha tocopheryl succinate, alpha tocopheryl nicotinate and mixtures thereof; a second composition comprising vitamin A and natural-mixed carotenoids; a third composition comprising calcium ascorbate; a fourth composition selected from a group consisting essentially of B-vitamins, selenium, zinc, magnesium, chromium and mixtures thereof; and a fifth composition selected from a group consisting essentially of alpha lipoic acid, co-enzyme Q10, L-carnitine, n-acetyl cysteine and mixtures thereof, wherein said formulation is without iron, copper and manganese.
Owner:PRASAD KEDAR +2
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com