Method for preparing cleavable polyethyleneglycol lipid derivates and application
A technology of polyethylene glycol lipid and polyethylene glycol, applied in the field of medicine, can solve problems such as affecting drug efficacy, being difficult to degrade, and falling off.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
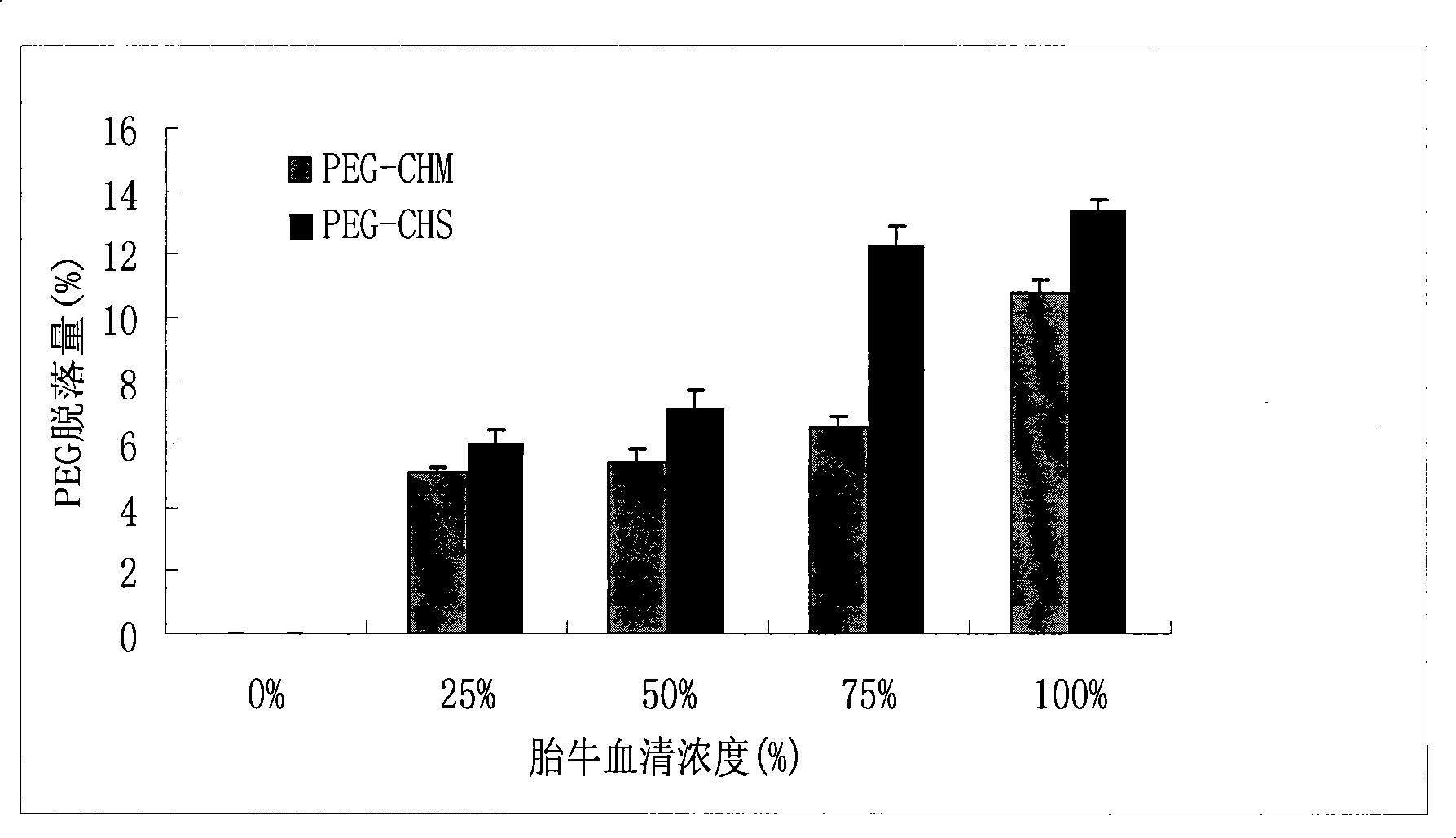
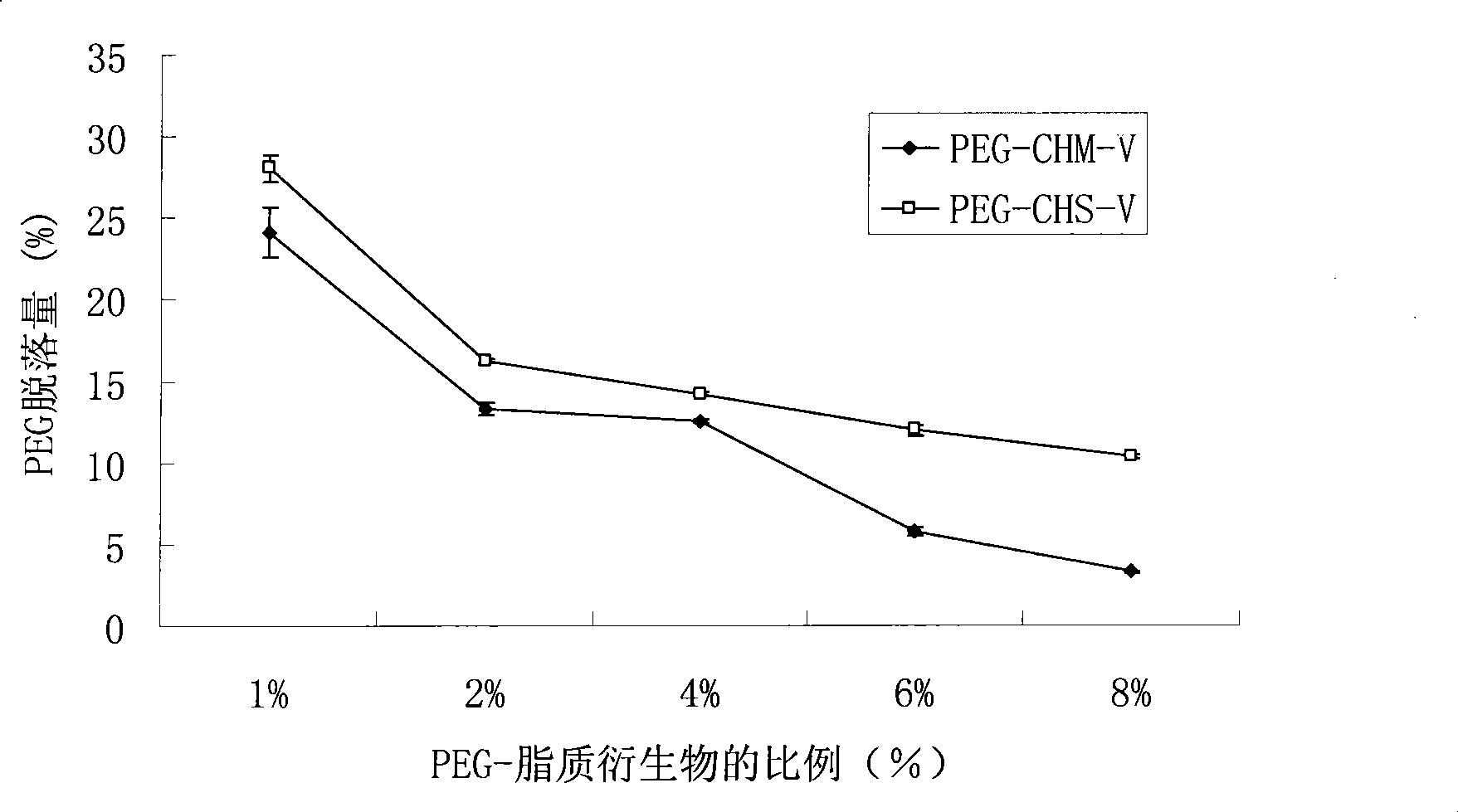
[0033] Example 1 Synthesis of polyethylene glycol-cholesterol methyl ester (PEG-CHM)
[0034] Put 1.2mmol cholesterol methyl chloromethyl and 0.8mmol monomethyl ether polyethylene glycol (molecular weight 2000) into a three-necked flask, add DMAP (0.4mmol) and triethylamine (1.08mmol) under nitrogen conditions, and add 20mL dichloromethane As the solvent, stir for 1 hour under ice-water bath conditions, remove the ice bath temperature and react for 24 hours. After the crude product is decompressed to recover the reaction solvent, add 100 mL of water, extract three times with dichloromethane, and then wash three times with ice water, saturated with chlorine Washed with sodium chloride 3 times, 2M hydrochloric acid washed 3 times, precipitated with ice ether, and recrystallized from absolute ethanol to obtain a white waxy polymer. The product obtained is PEG-CHM, IR(KBr)(cm -1 ): PEG has no carbonyl absorption peak, and the carbonyl absorption peak of CHM is at 1776cm -1 There is a ...
Embodiment 2
[0035] Example 2 Synthesis of polyethylene glycol-α-tocopherol hemisuccinate (PEG-THS)
[0036] Put 1mmol α-tocopherol hemisuccinate and 0.6mmol monomethyl ether polyethylene glycol (molecular weight 2000) into a round bottom flask, use 20mL dichloromethane as the reaction solvent, add 44mg DMAP in an ice water bath, and add 206mg after 15 minutes Dicyclohexylcarbodiimide (DCC) was used as a catalyst, reacted at room temperature for 4 hours, and filtered with suction to obtain a crude product solution. The crude product was washed 3 times with 2M hydrochloric acid and extracted 3 times, then washed 3 times with saturated sodium bicarbonate, washed 3 times with distilled water, dried by rotary evaporation, precipitated with ice ether, and recrystallized from absolute ethanol to obtain a white waxy polymer. The product is PEG-THS, IR(KBr)(cm -1 ): PEG has no carbonyl absorption peak, and the carbonyl absorption peak of THS is at 1753cm -1 And 1714cm -1 There is a carbonyl absorption...
Embodiment 3
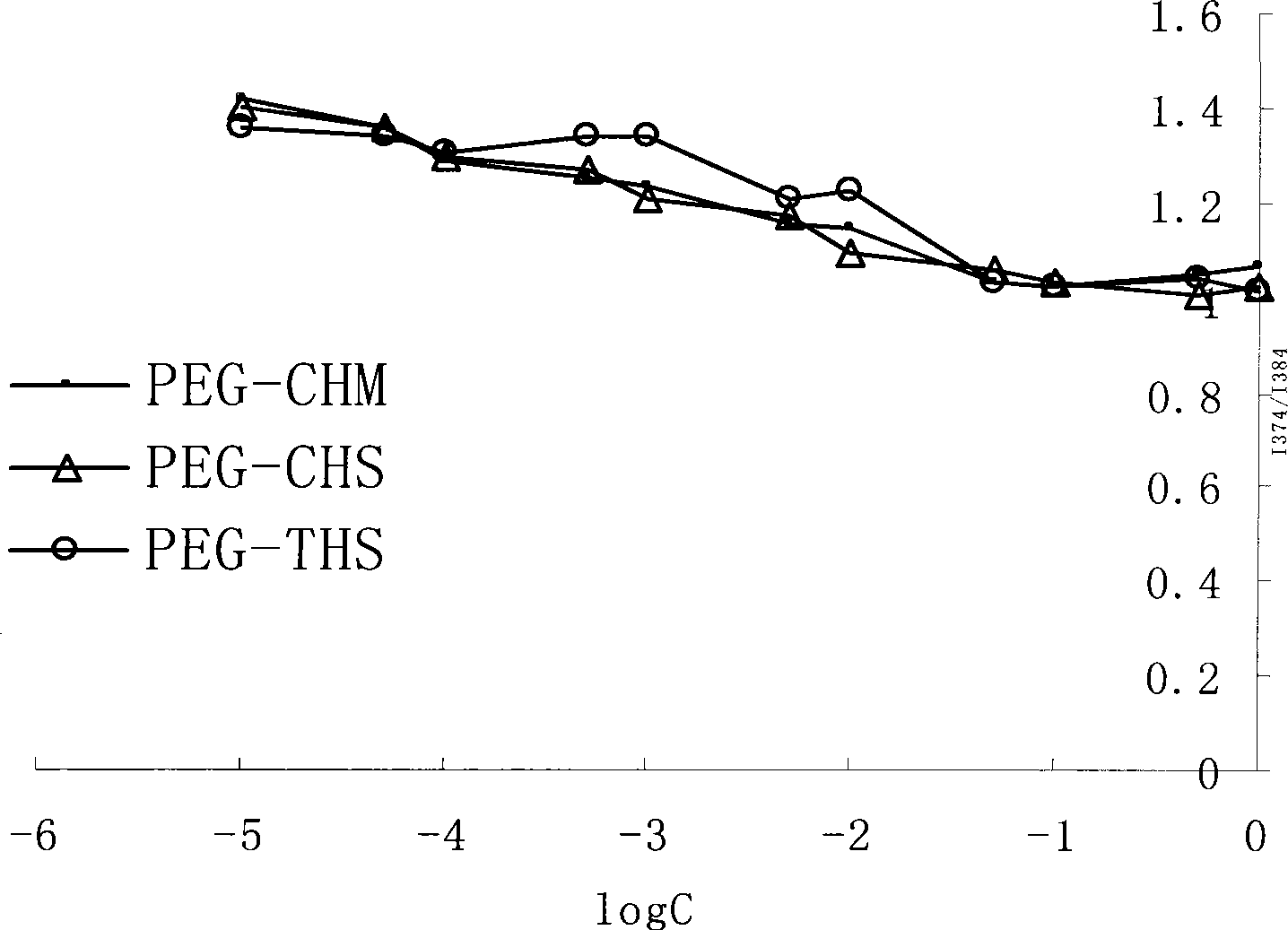
[0037] Example 3 Determination of Critical Micelle Concentration (CMC) of PEG Lipid Derivatives
[0038] Due to the hydrophilic group and lipophilic group in the molecular structure, PEG lipid derivatives can spontaneously form micelles in aqueous solution, and the CMC of PEG lipid derivatives is determined by the fluorescent probe method.
[0039] Precisely pipette 0.1mL to a concentration of 1×10 -5 Put several parts of the pyrene working solution of M in a vial, blow dry with nitrogen, accurately weigh out several portions of PEG-CHS, PEG-CHM, and PEG-THS, put them in the vial, add 10 mL of pure water, respectively, to obtain the pyrene solution The concentration is 10 -7 M(The saturated solubility of pyrene in pure water is 7×10 -7 M, the value is slightly lower than the saturated solubility), ultrasonic 4h at 60 ℃ in a water bath, placed overnight, you will get 10 -5 , 5×10 -5 , 10 -4 , 5×10 -4 , 10 -3 , 5×10 -3 , 10 -2 , 5×10 -2 , 10 -1 , 5×10 -1 , 1,5g / L solution, ready for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com