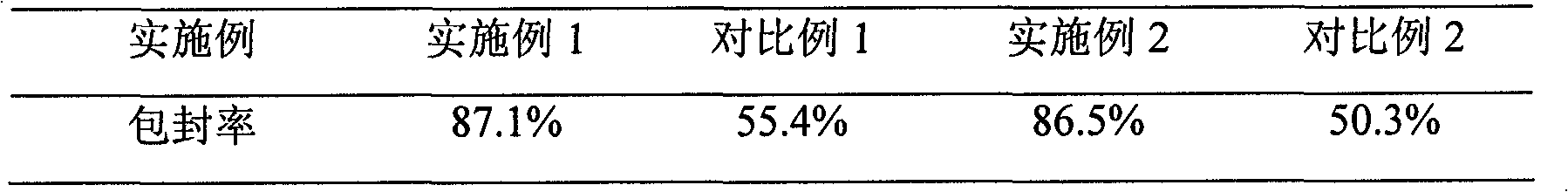
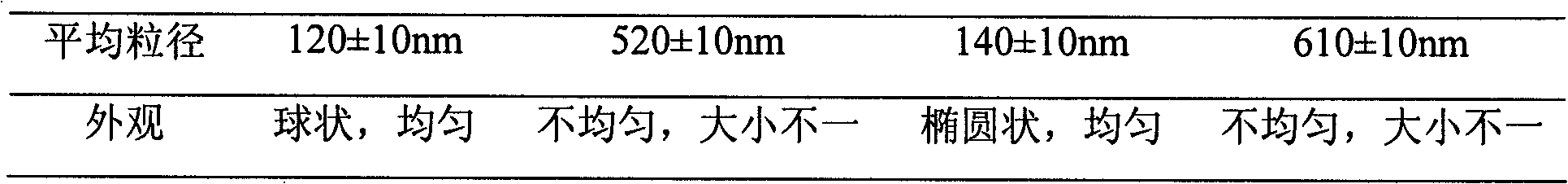
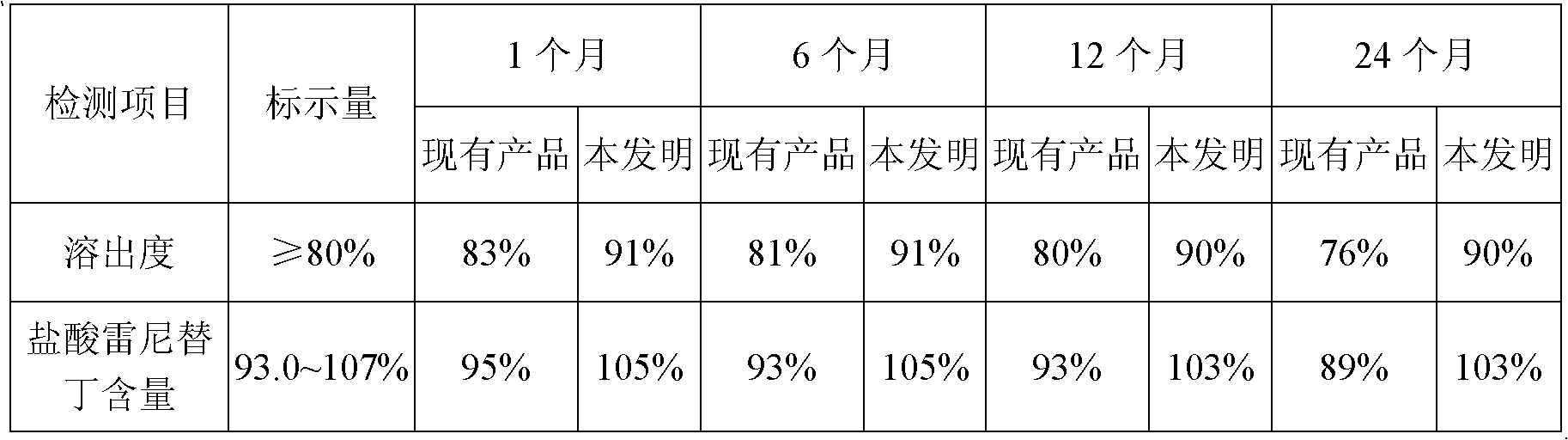
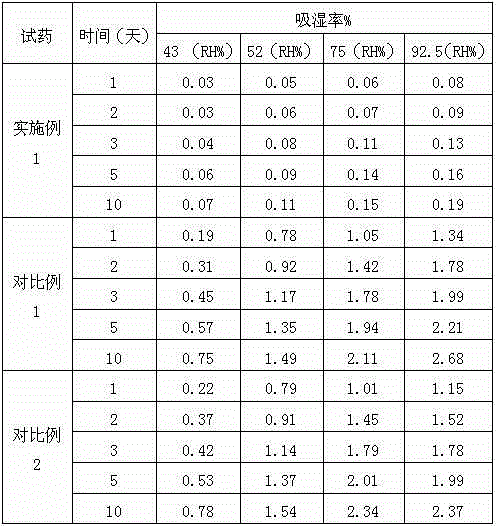
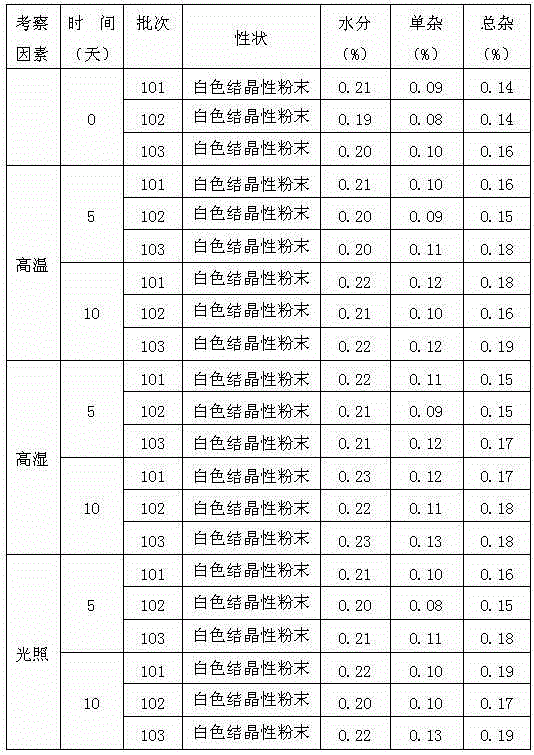
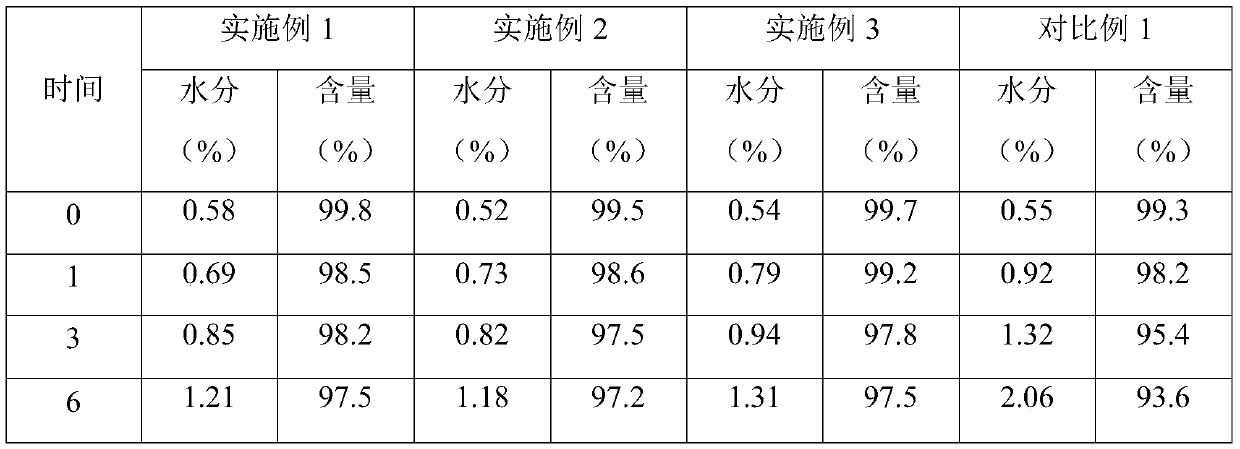
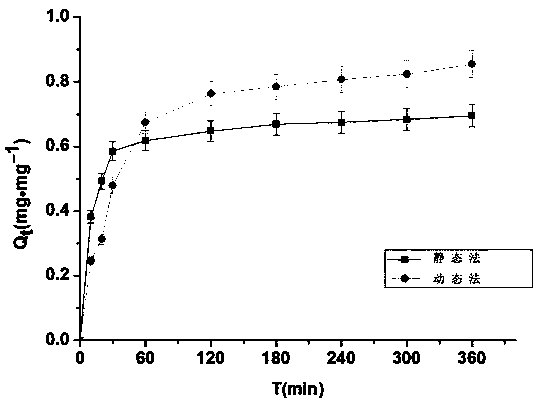
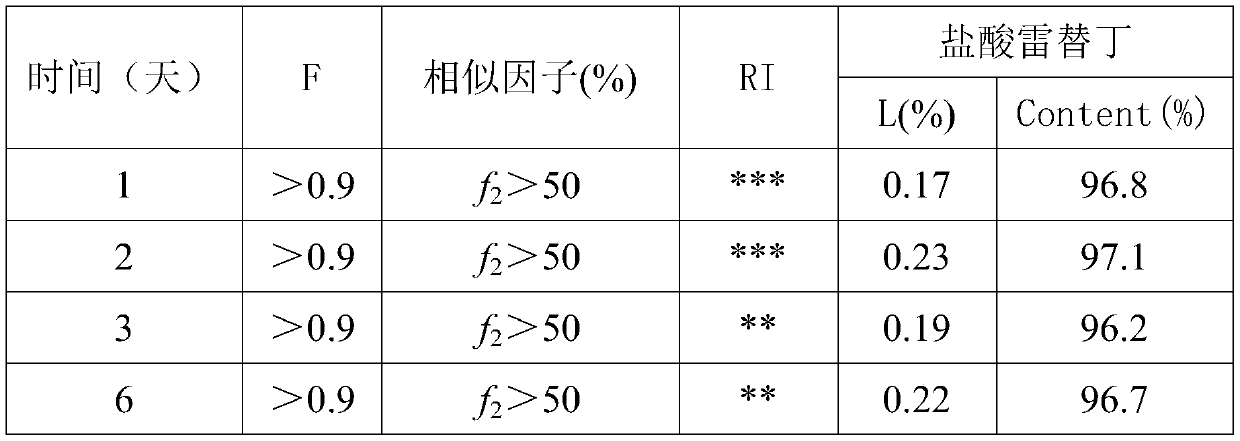
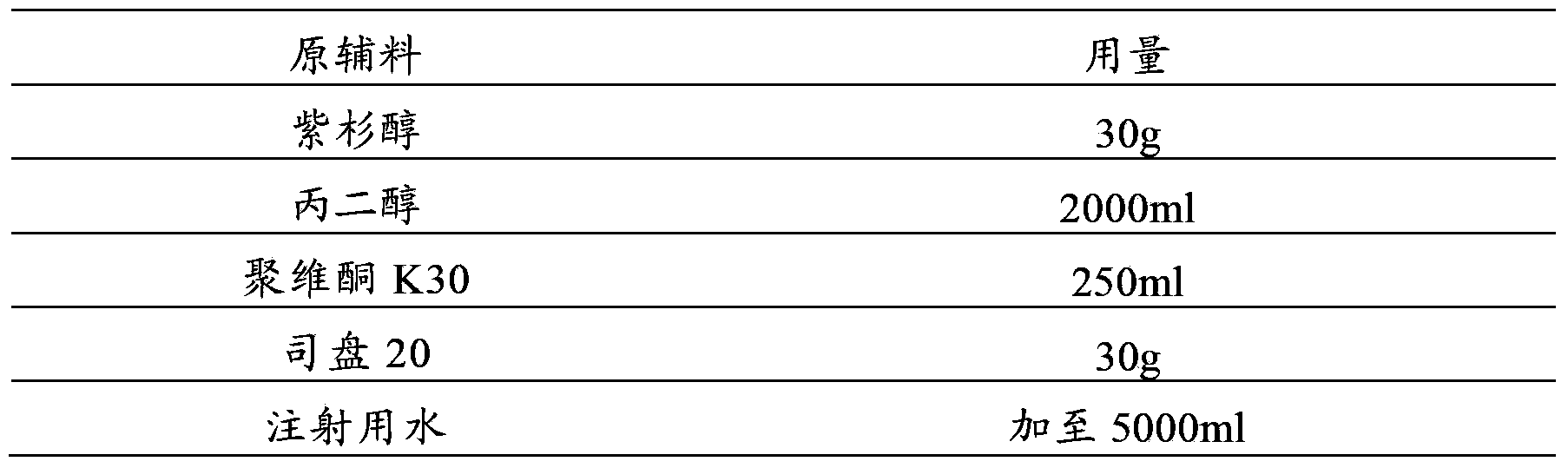
Patents
Literature
42 results about "Ranitidine Hydrochloride" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
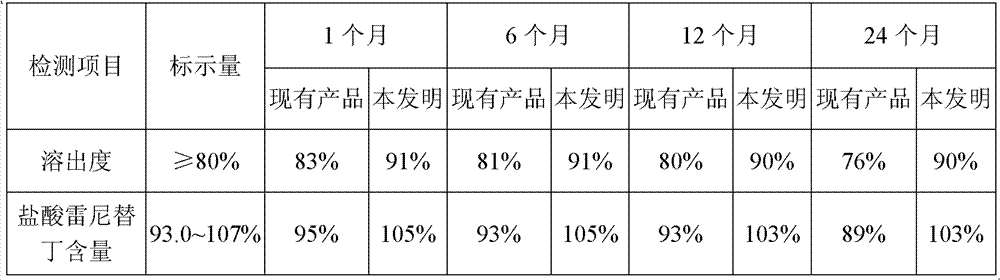
A member of the class of histamine H2-receptor antagonists with antacid activity. Ranitidine is a competitive and reversible inhibitor of the action of histamine, released by enterochromaffin-like (ECL) cells, at the histamine H2-receptors on parietal cells in the stomach, thereby inhibiting the normal and meal-stimulated secretion of stomach acid. In addition, other substances that promote acid secretion have a reduced effect on parietal cells when the H2 receptors are blocked.
Ranitidine hydrochloride lipidosome capsule and new application thereof
InactiveCN101623258AImprove efficacyImprove bioavailabilityDigestive systemAntiviralsYolkHerpetic stomatitis
The invention provides a ranitidine hydrochloride lipidosome capsule and new application thereof. Particularly, certain amounts of yolk lecithin, cholesterin, natrium glycocholicum, tween 80 and an active ingredient ranitidine hydrochloride are combined and are prepared into ranitidine hydrochloride lipidosome by film dispersion technology, and the ranitidine hydrochloride lipidosome is mixed with the general accessories of medicine to prepare the capsule. The ranitidine hydrochloride lipidosome capsule better solves the problems of easy deliquescence, moisture absorption, color change and poor stability of the ranitidine hydrochloride, increases preparation medical effect and biological availability and can be used for treating herpetic stomatitis of childrem.
Owner:HAINAN MEIDA PHARMA
Composition for treating chicken proventriculitis and preparation method thereof
InactiveCN101966178AEfficient killingEffective treatmentAntibacterial agentsDigestive systemDiseaseIrritation
The invention discloses a composition for treating chicken proventriculitis and a preparation method thereof, which aim to provide a composition for treating the infection of chicken proventriculitis by addressing both symptoms and root causes and a preparation method thereof. The composition comprises the following components in percentage by weight: 1 to 10 percent of florfenicol, 5 to 15 percent of metronidazole, 1 to 6 percent of ranitidine hydrochloride, 2 to 15 percent of taurine and the balance of glucosum anhydricum. In the composition, aiming at pathogeny, the florfenicol and the metronidazole which serve as antimicrobial medicaments are adopted to kill anaerobic bacteria and helicobacter pylori effectively, reduce the occurrence probability of the tolerance of pathogenic bacteria and improve the sensitivity; the ranitidine hydrochloride inhibits the gastric acid effect and reduces the injury of gastric acid to inflamed glandular stomachs; and the taurine has the obvious effect of inflammation resistance, has no irritation on gastrointestinal tracts, and has the effect of diminishing inflammation quickly. Aiming at the pathogeny and diseases, the medicaments are combined, so that the composition can address both symptoms and root causes effectively on antipathogen and clinical symptoms thereof and treat the chicken proventriculitis effectively.
Owner:TIANJIN SHENGJI GRP CO LTD
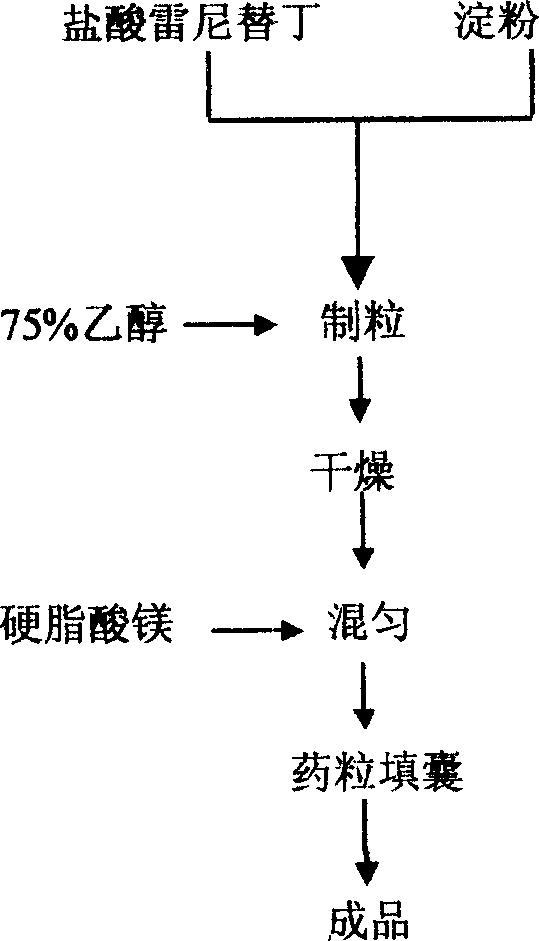
Ranitidine hydrochloride capsule producing process
InactiveCN1488345APromote absorptionFast dissolutionOrganic active ingredientsDigestive systemAlcoholRanitidine Hydrochloride
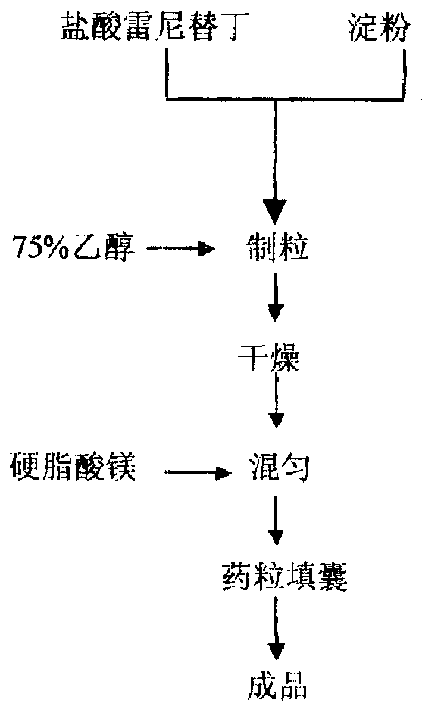
The invention discloses a Ranitidine hydrochloride capsule producing technique, raw material being Ranitidine hydrochloride, assistant material being amylum, and lubricant being magnesium stearate. Mix raw and assistant materials to make particles, then dry to mix evenly, add in the lubricant, and finally capsulize to make the finished product. After mixing raw and assistant materials, add in 50-95% alcohol solution as wetter replacing bond, to make particles, so as to make the particles looser, extremely easy to dissolve in water and having fluidity.
Owner:雅来(佛山)制药有限公司
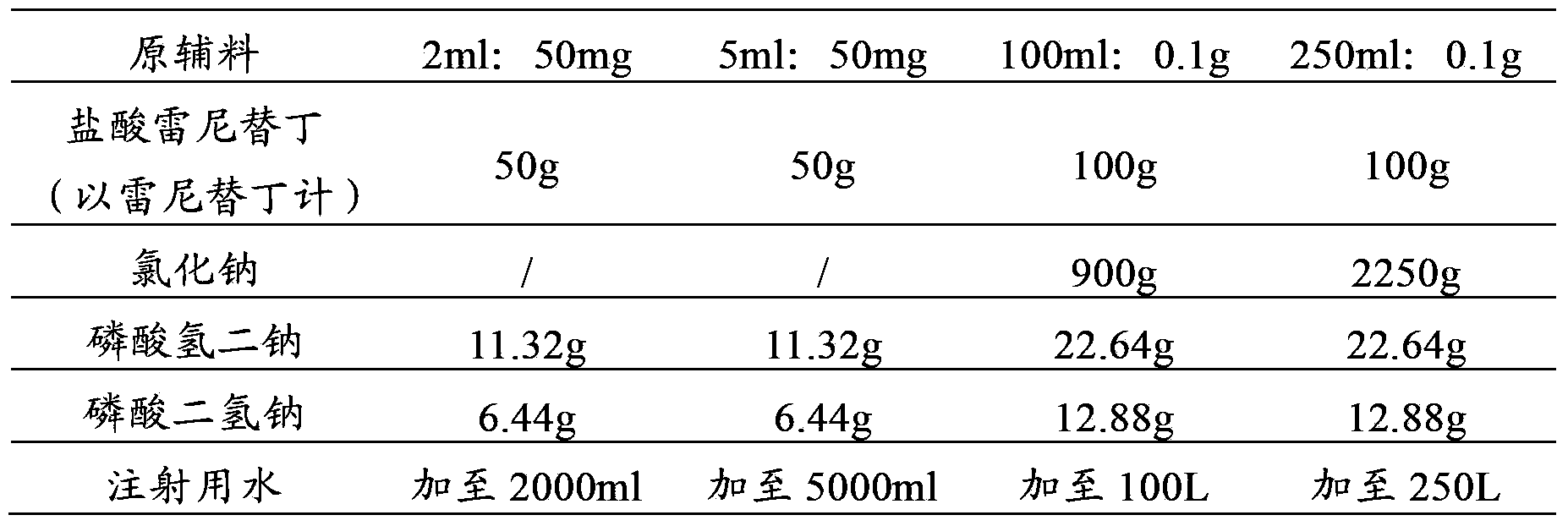
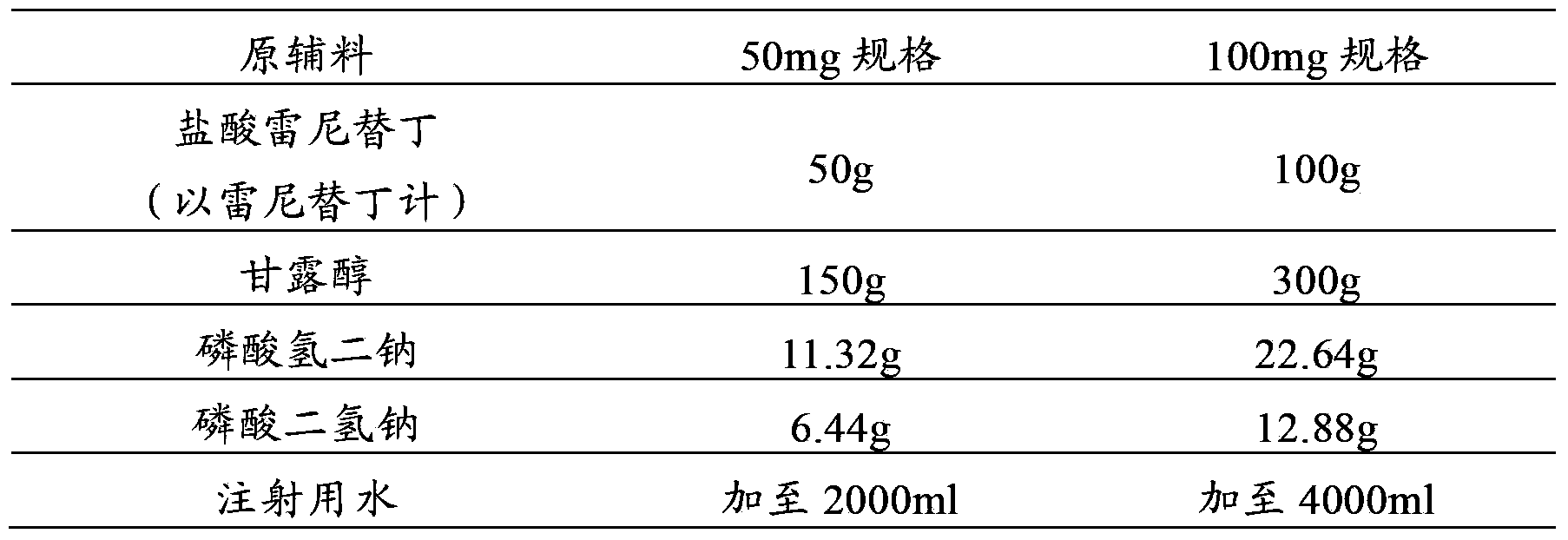
Injection prescription of freeze-drying powder of ranitidine hydrochloric acid and preparation method
InactiveCN1795849AQuality improvementAvoid decompositionPowder deliveryOrganic active ingredientsActive componentFreeze-drying
A freeze-dried powder injection of ranitidine hydrochloride is proportionally prepared from ranitidine hydrochloride, freeze-drying supporting agent, stabilizer and pH regulator through proportionally dissolving the active component in the water for injection while stirring, regulating pH value until it becomes neutral, adding the carbon for injection, stirring, filtering for removing carbon, filtering with 0.45-micron micropore film and then with 0.22-micron micropore film, bottling, freeze-drying, and vacuum sealing.
Owner:FUJIAN MINDONG REJUVENATION PHARMA
Synthesis method of ranitidine alkali and its hydrochloride
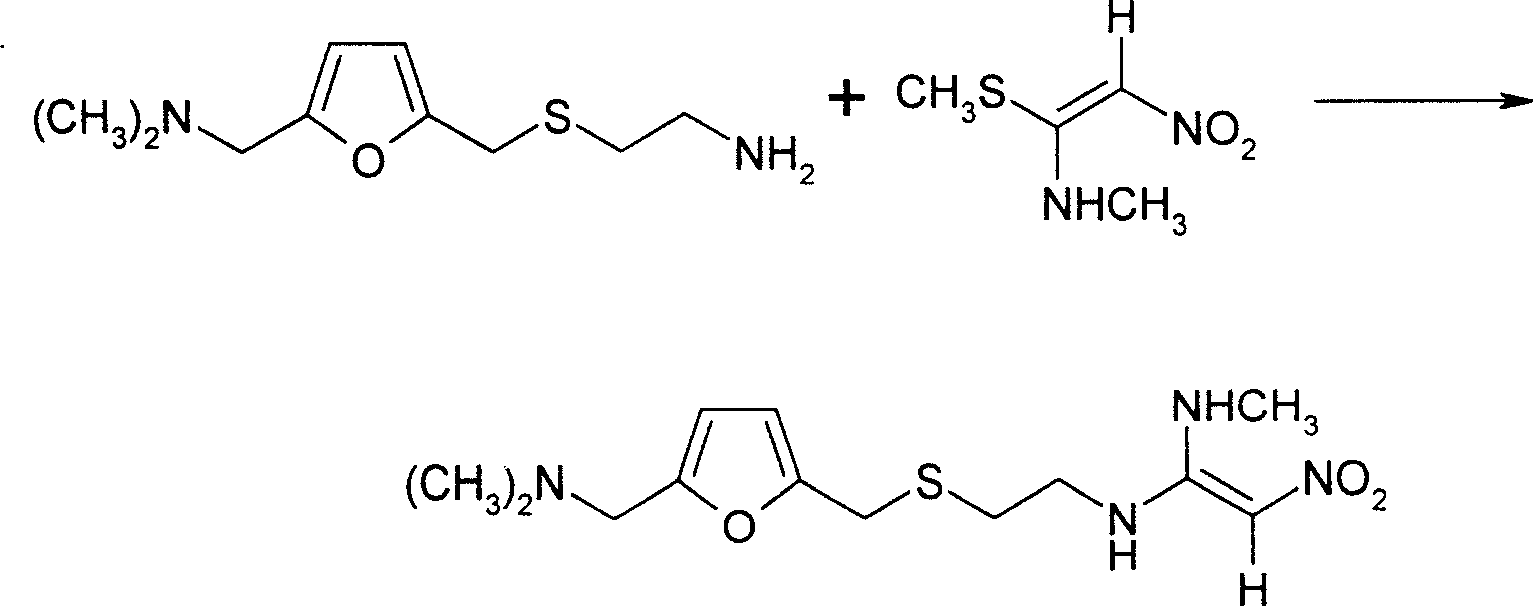
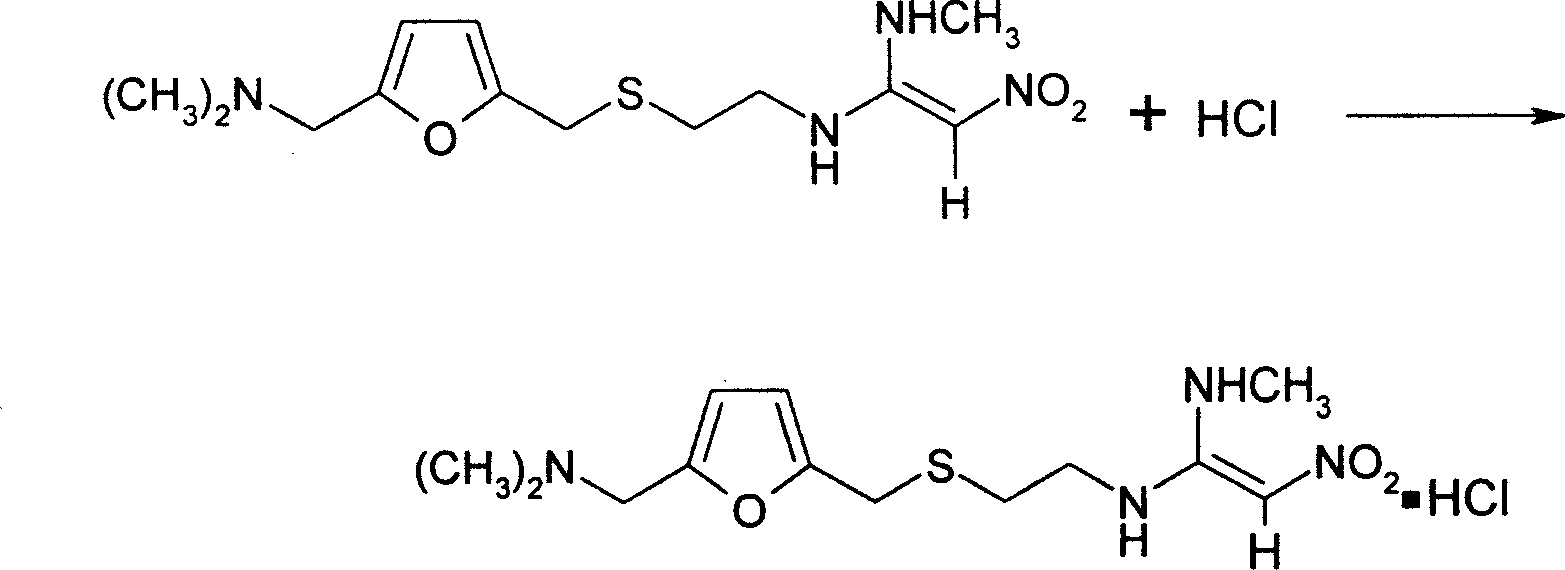
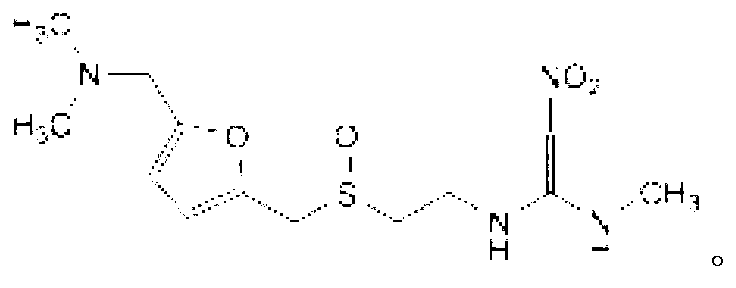
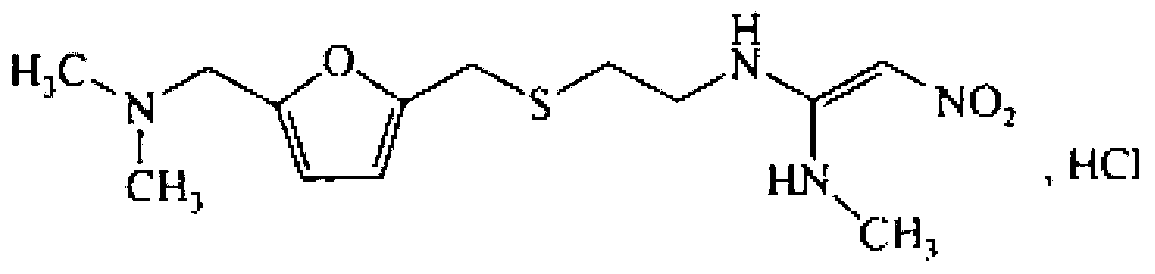
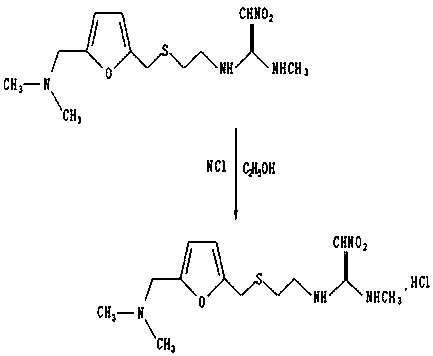
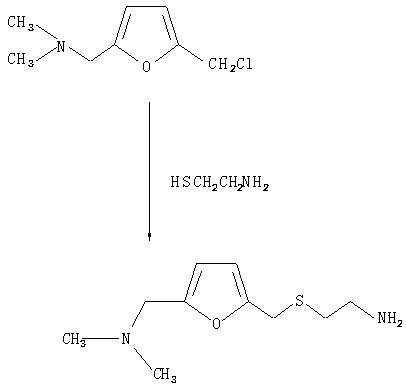
The invention provides a method for synthesis of ranitidine alkali and ranitidine hydrochloride. It is characterized in that: 2- [ [ [5- (dimethylamino) methyl- 2- furyl] methyl] sulpho] ethylamine reacts with 1- methylthio - 1- methylamino- 2- nitro ethylene directly in water phase, crystallizing and separating out ranitidine alkali. The synthesis method comprises the following steps: adding 2- [ [ [5- (dimethylamino) methyl- 2- furyl] methyl] sulpho] ethylamine and 1- methylthio- 1- methylamino- 2- nitro ethylene with a molar ratio of 1.04: 1 into water, heating to 48- 52 Deg. C, reacting 4.5 hours with a vacuum degree of 0.02- 0.05 MPa, cooling, adding sodium-hydroxide of concentration of 10% to regulate the PH value to 11.0- 11.4, filtrating and cooling the filtrate to 0- 2 Deg. C, crystallizing for 12 hours, centrifugally filtrating, and rinsing filter cake with purity water to prepare wet ranitidine alkali; dissolving ranitidine alkali in alcohol and reacting with chlorhydric acid to prepare ranitidine hydrochloride.
Owner:石药集团中诺药业(石家庄)有限公司
Solid preparation of ranitidine hydrochloride/bismuth potassium citrate medicinal composition
InactiveCN101862320AGuaranteed stabilityImprove stabilityAntibacterial agentsOrganic active ingredientsRanitidine HydrochlorideBioavailability
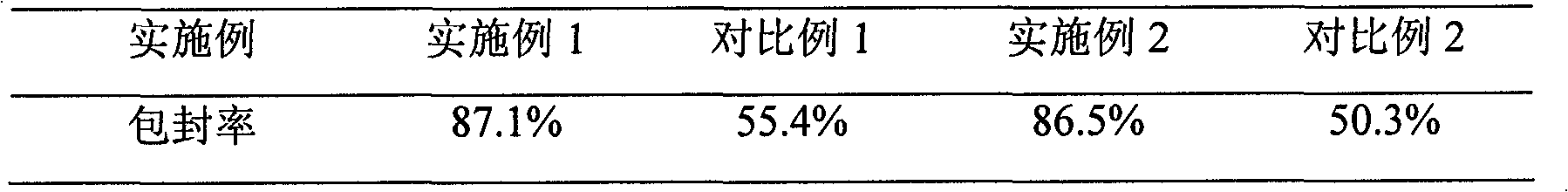
The invention relates to the solid preparation of a ranitidine hydrochloride / bismuth potassium citrate medicinal composition, which is prepared by mixing ranitidine hydrochloride / bismuth potassium citrate medicinal composition microcapsules and other pharmaceutical adjuvant required by the preparation of the solid preparation, wherein the ranitidine hydrochloride / bismuth potassium citrate medicinal composition microcapsules are prepared from ranitidine hydrochloride, bismuth potassium citrate, chitosan and sodium alginate. Compared with the prior art, the preparation of the invention has the characteristics of greatly improved stability and bioavailability, and stable and persistent release.
Owner:郝志艳
Ranitidine hydrochloride releasing-controlling dry suspension and preparing method thereof
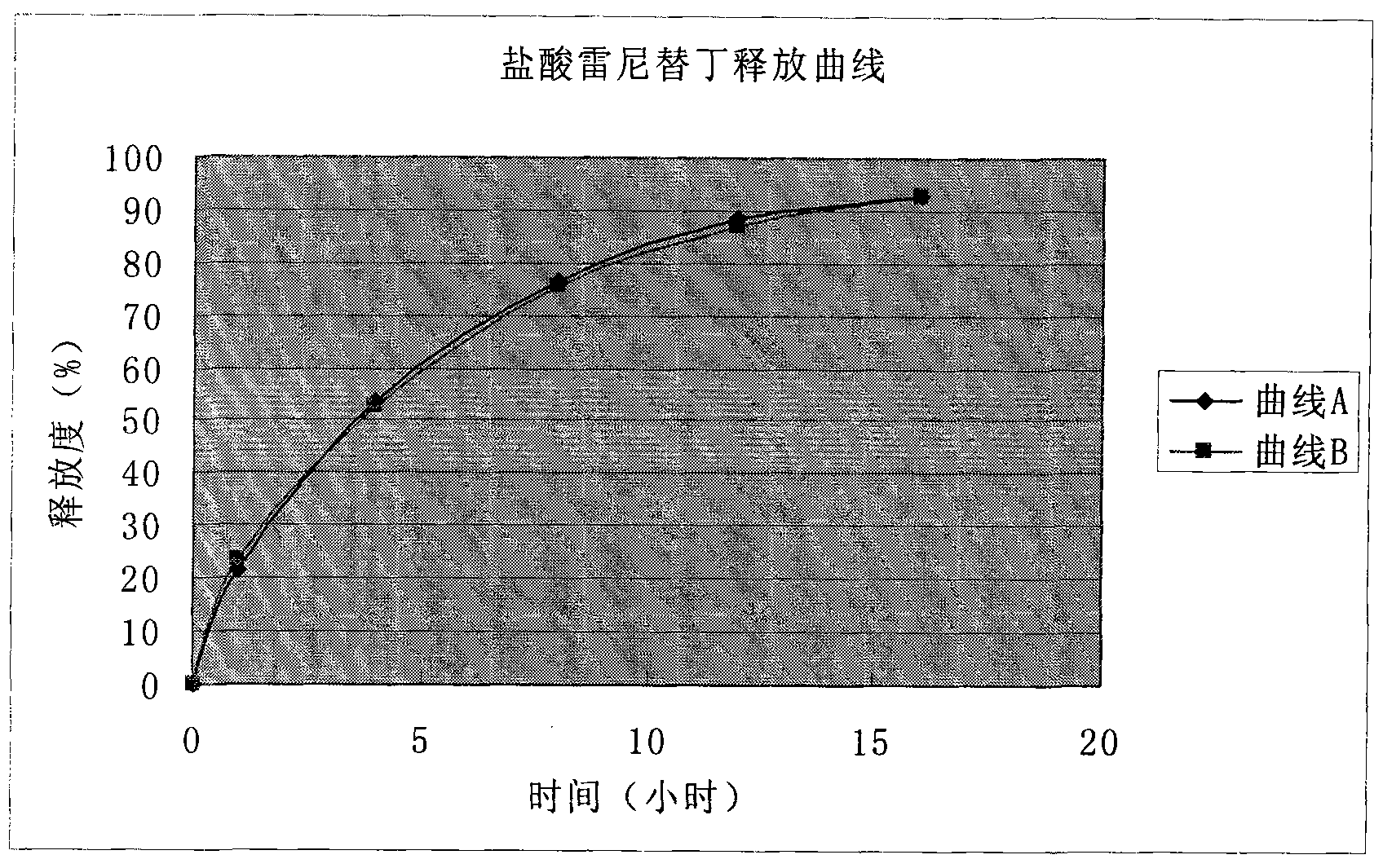
The invention discloses a ranitidine hydrochloride releasing-controlling dry suspension and a preparing method thereof. The ranitidine hydrochloride releasing-controlling dry suspension comprises ranitidine hydrochloride and polymers which can be accepted in the pharmacy and comprises, by weight, 10%-90% of ranitidine hydrochloride, 10%-90% of auxiliary materials and the balance other auxiliary materials. The auxiliary materials with the releasing controlling effect are one or more of positive ion exchange resin, methylcellulose, ethyl cellulose, acrylic resin and hydroxypropyl methylcellulose. Compared with an immediate-release preparation, the releasing-controlling preparation can keep the effective blood concentration within 24 hours, curative effects are improved, toxic and side effects are small, taking and carrying are convenient, and the taking times are reduced. Compared with a sustained-release preparation, the releasing-controlling preparation can keep the more stable blood concentration within 24 hours, curative effects are improved, and toxic and side effects are small. According to the ranitidine hydrochloride releasing-controlling dry suspension, dosing only needs to be carried out once in one day; the releasing-controlling preparation is used for treating benign gastric ulcer and duodenal ulcer in clinic.
Owner:JIANGSU SUNAN PHARMA IND CO LTD
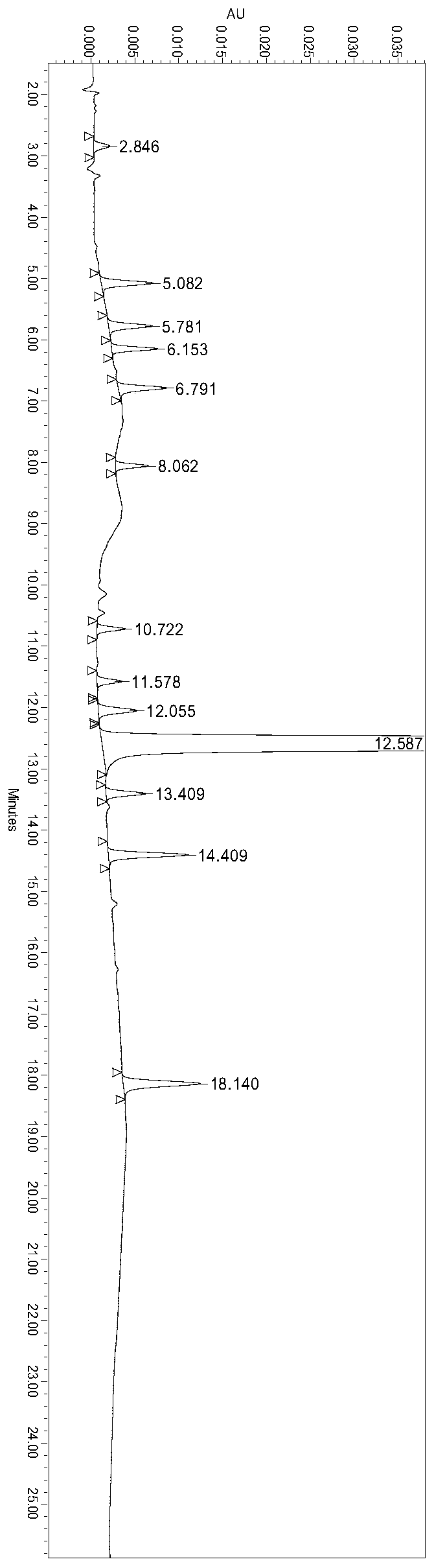
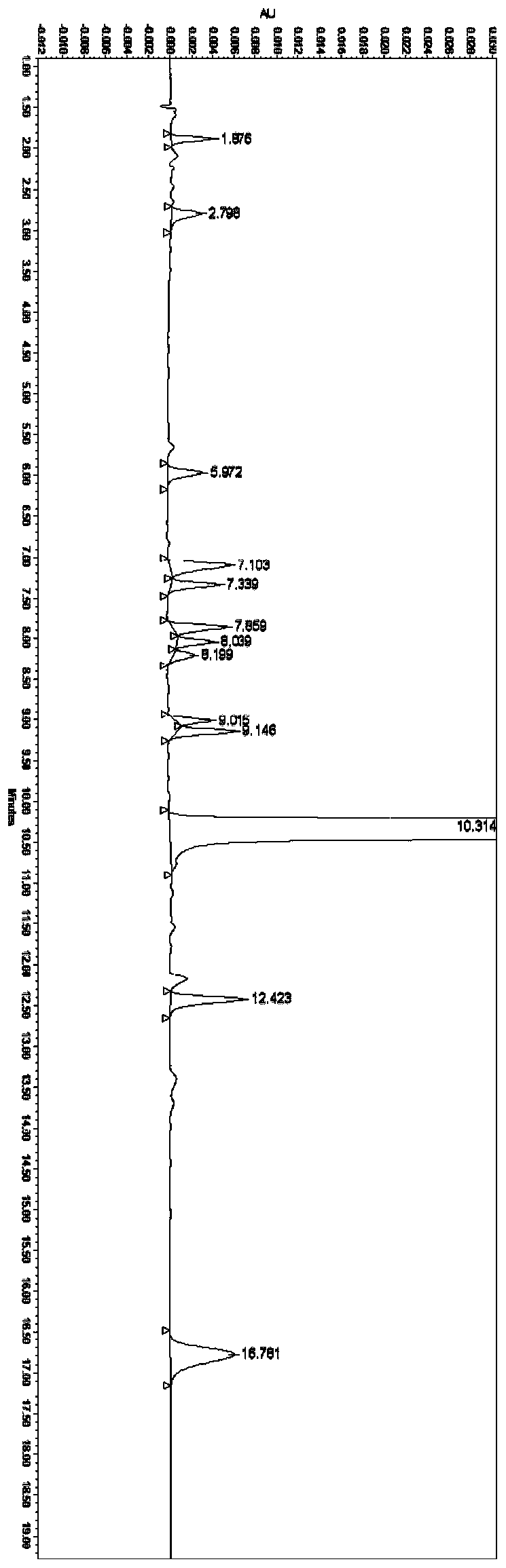
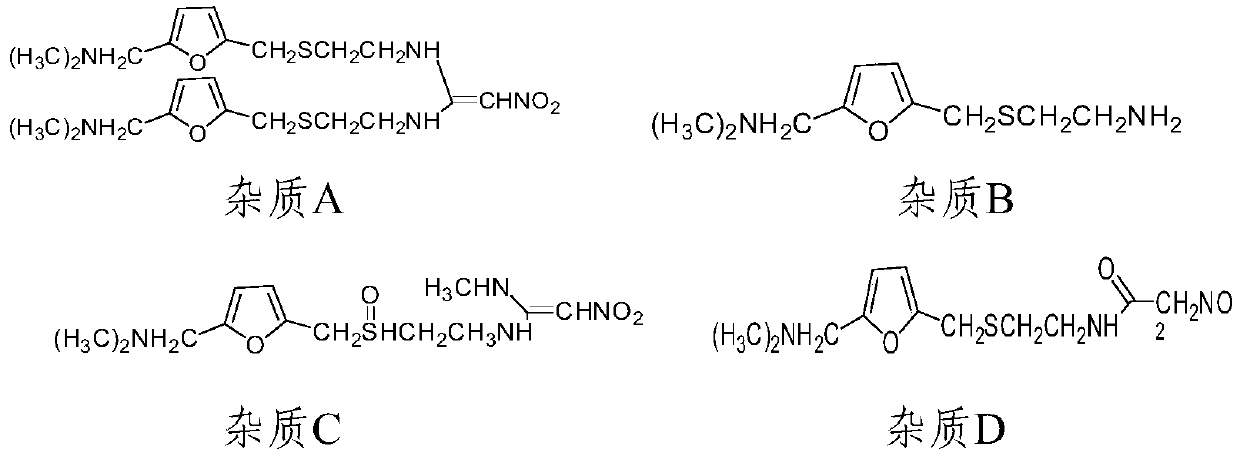
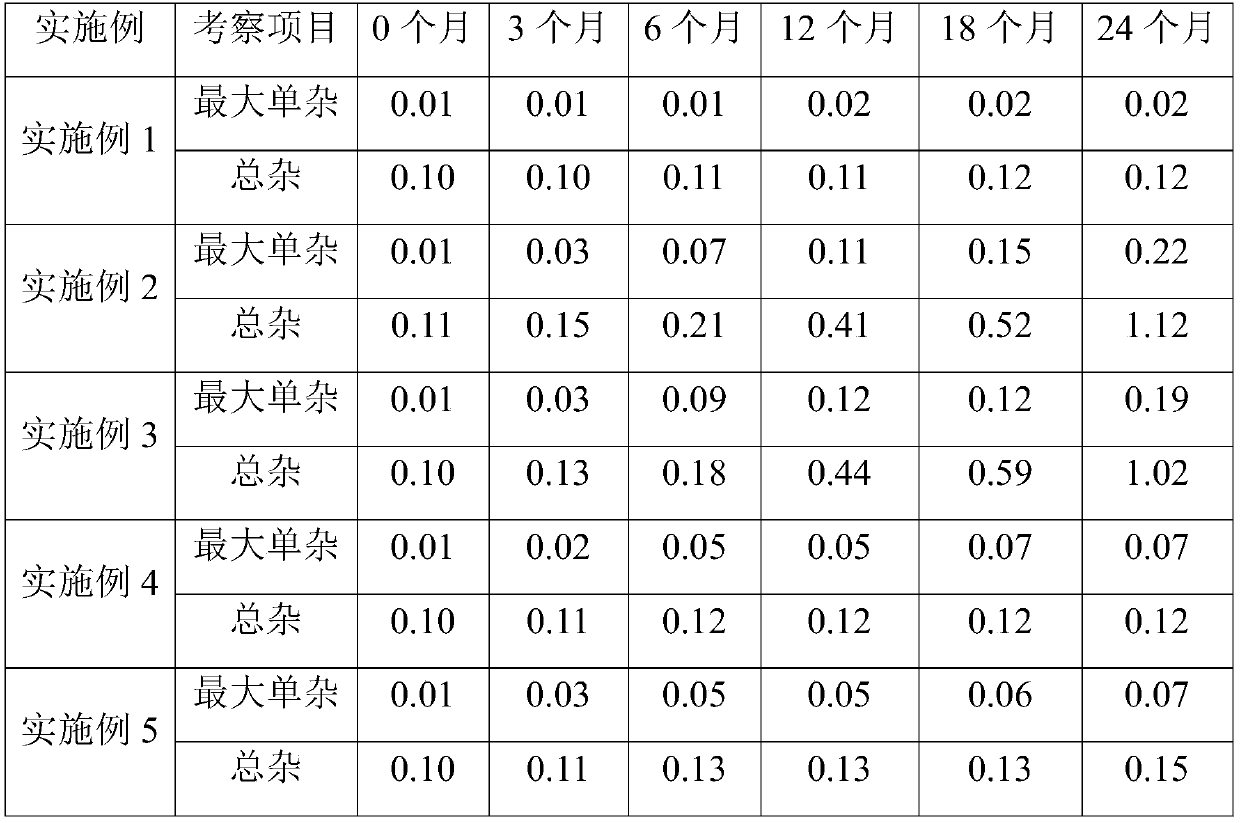
Method for determining ranitidine hydrochloride related substances through high performance liquid chromatography
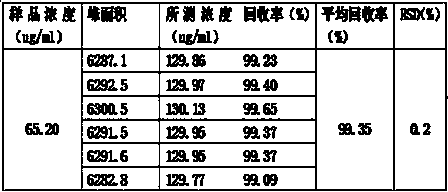
The invention relates to the field of drug detection, in particular to a method for determining ranitidine hydrochloride related substances through high performance liquid chromatography. According tothe method disclosed by the invention, octadecylsilane chemically bonded silica is used as a filler, mobile phases comprise a mobile phase A and a mobile phase B, the mobile phase A and the mobile phase B are both a mixed solution of a modified phosphate buffer solution and acetonitrile, the pH value of the modified phosphate buffer solution is 6.70+ / -0.05, the flow velocity of the mobile phasesis 1.1-1.3ml / min, and gradient elution is adopted. By using the method disclosed by the invention, ranitidine hydrochloride related substances can be separated in a high performance liquid chromatogram. By optimizing the conditions, the sensitivity and the accuracy of the detection of each component are further improved. The method enables the quality of ranitidine hydrochloride to be better controlled, has the advantages of high analysis speed, good specificity and high reproducibility, facilitates the quality detecting and monitoring of ranitidine hydrochloride, and is beneficial to safe application and popularization of ranitidine hydrochloride.
Owner:BEIJING YUEKANGKECHUANG PHARM TECH CO LTD
Ranitidine hydrochloride capsules and production method thereof
ActiveCN102138913ASolve hygroscopicityAvoid intakeOrganic active ingredientsDigestive systemMedicineRanitidine Hydrochloride
The invention discloses ranitidine hydrochloride capsules and a production method thereof. Every 1,000 capsules comprise 165 to 170 parts of ranitidine hydrochloride and 40 to 50 parts of calcium hydrophosphate. The preparation method for the capsules comprises the following steps: weighing the ranitidine hydrochloride and the calcium hydrophosphate according to the weight part; crushing the ranitidine hydrochloride and the calcium hydrophosphate respectively, adding 50 percent ethanol in a trough type mixing machine to mix for 20 to 40 minutes to prepare a soft material, and granulating; drying the prepared granules in a hot-air circulation drying oven at the temperature of lower than 70 DEG C, and controlling the moisture within 4 percent; and filling the dried granules in capsules, andpolishing to obtain the ranitidine hydrochloride capsules. The ranitidine hydrochloride capsules and the production method thereof have the advantages that: the calcium hydrophosphate is adopted as an auxiliary material, so that each quality index of the product in a valid period is ensured to be basically stable, the problem of hygroscopicity of the ranitidine hydrochloride capsules can be solved, the product stability is improved, and intake of talc powder and magnesium stearate is prevented.
Owner:JIANGSU SUNAN PHARMA IND CO LTD
Medicine ranitidine hydrochloride compound for treating stomach illness and preparation method of medicine ranitidine hydrochloride compound
ActiveCN104817523AImprove stabilityNot easy to absorb moistureOrganic chemistry methodsPharmaceutical drugRanitidine Hydrochloride
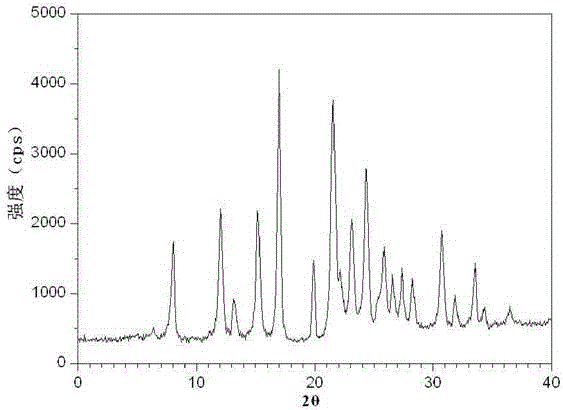
The invention discloses a medicine ranitidine hydrochloride compound for treating stomach illness and a preparation method of the of medicine ranitidine hydrochloride compound, belonging to the technical field of medicines. The X-ray powder diffraction pattern of the compound, obtained through measurement with Cu-K alpha ray, is shown in the figure 1. The new crystal form of the compound provided by the invention is different form crystal form structures in the prior art. Experiments disclose a pleasant surprise that the compound having the new crystal from structure hardly absorbs moisture and has good stability and therefore the problems that ranitidine hydrochloride is not stable in damp, heat and air and ranitidine hydrochloride products can be oxidized easily, are liable to change color after absorbing moisture and poor in stability can be solved thoroughly. The preparations of the ranitidine hydrochloride compound disclosed by the invention can be prepared conveniently.
Owner:启东市和洪农副产品专业合作社
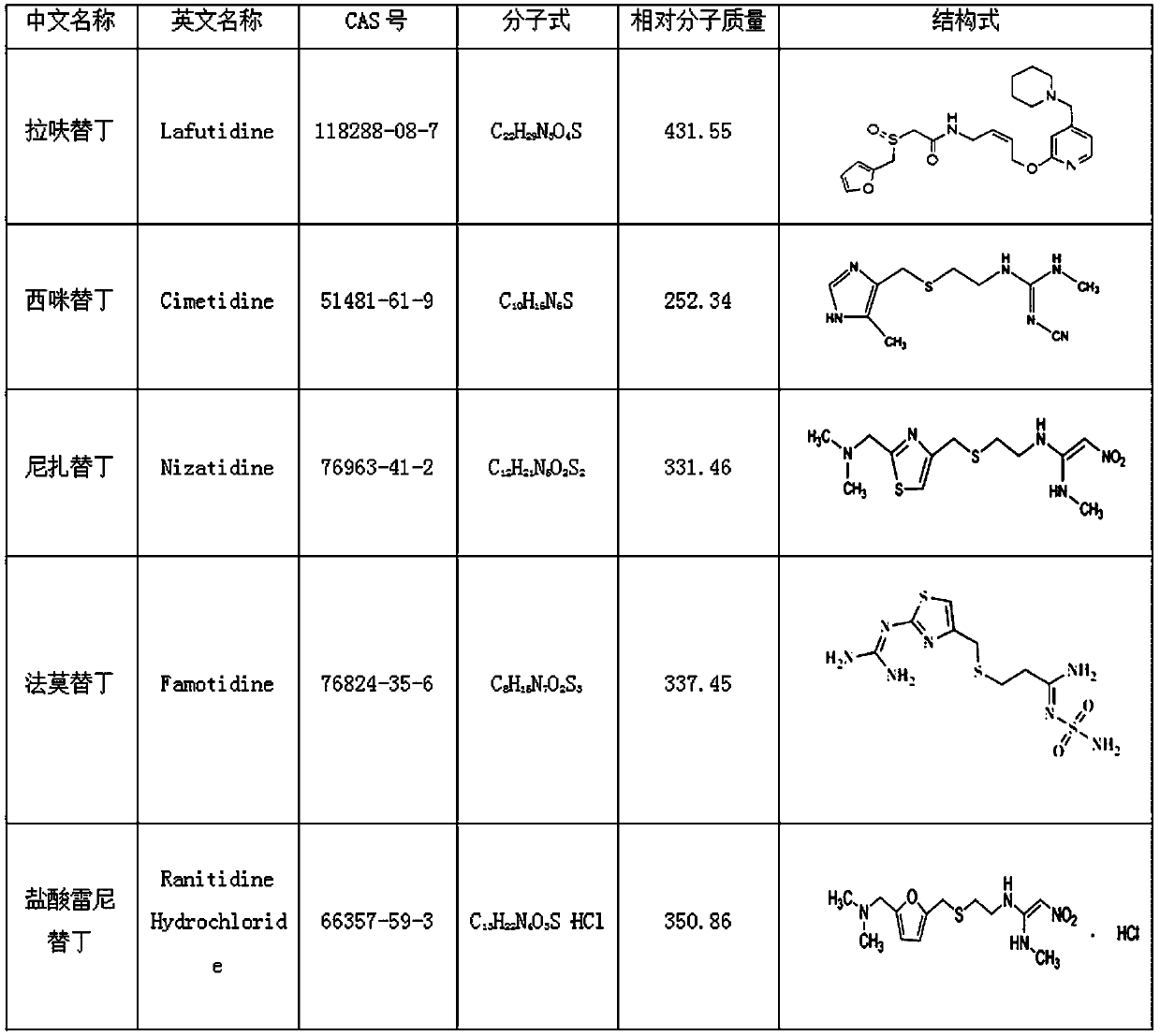
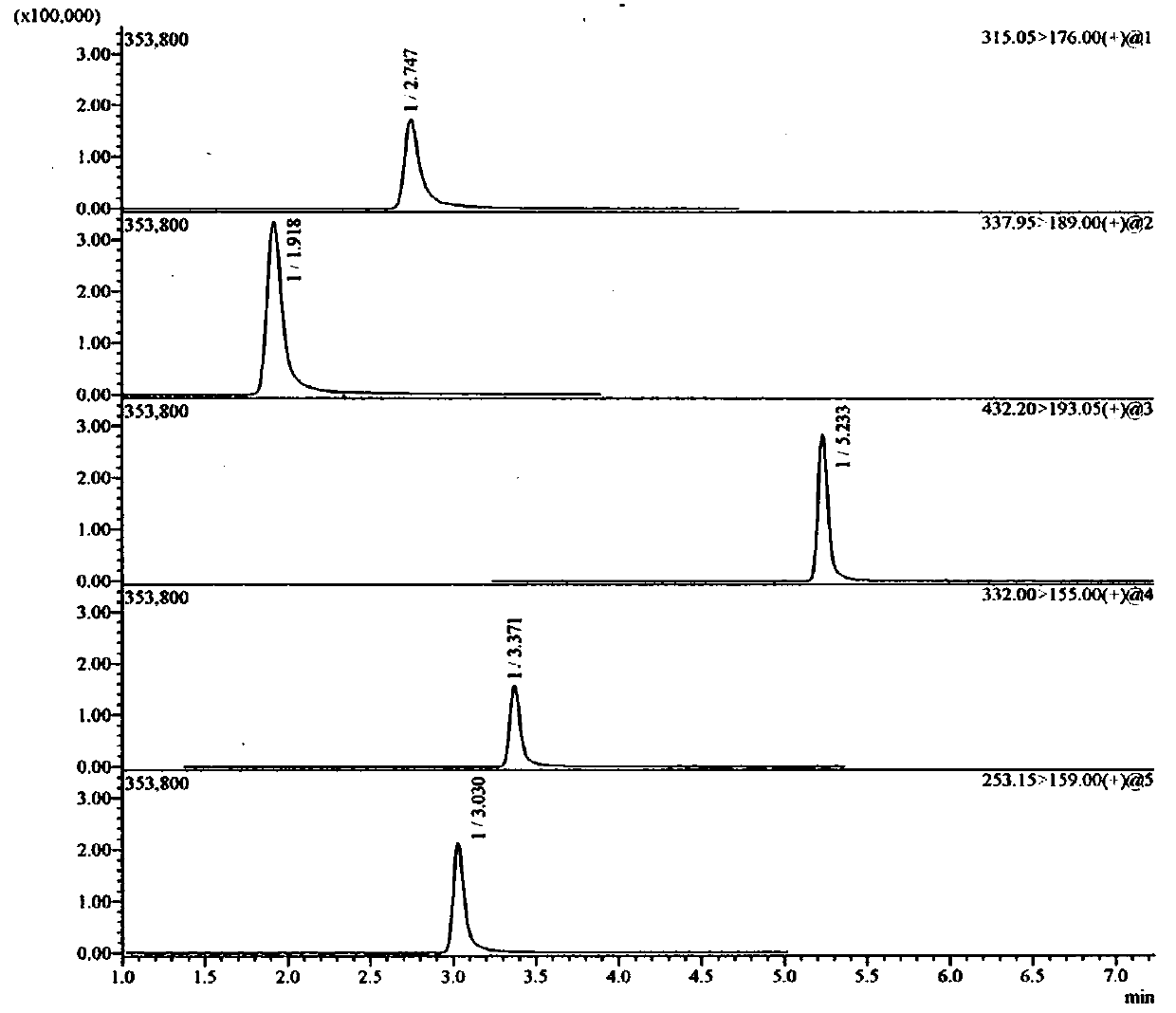
Method for detecting ranitidine hydrochloride, cimetidine, famotidine, nizatidine and lafutidine
The invention discloses a method for detecting ranitidine hydrochloride, cimetidine, famotidine, nizatidine and lafutidine. The method comprises the following steps: (1) selecting chromatographic conditions; (2) selecting mass spectrometric conditions; (3) preparing a standard solution; (4), preparing samples; (5), detecting and calculating. Tests prove that the method is rapid and high in specificity; the method is suitable for detecting the illegally added ranitidine hydrochloride, famotidine, lafutidine, nizatidine, cimetidine and other chemical medicines in Chinese patent medicines and health-care foods which have the effects of invigorating the stomach, improving the gastrointestinal function (having an auxiliary protection effect on gastric mucosal injury) and having an auxiliary protection effect on gastric mucosal injury or in other foods illegally claimed to have said functions.
Owner:太原市食品药品检验所
Method for refining ranitidine base
The invention relates to the technical field of separation and purification of fine chemicals, and more concretely relates to a method for refining ranitidine base. By using a mixed solvent prepared from an intensive polar solvent and a weak polar solvent, the dissolvability of ranitidine base and impurities in the solvent is different, so that the ranitidine base and the impurities can be effectively separated during a crystallization process, and an obtained refined product is high in purity and stable in property. After analyzing the ranitidine base refined through the method provided by the invention through a liquid chromatograph, the purity of the ranitidine base is not less than 99.0 percent, the total impurity is not larger than 0.15 percent, and the single impurity is not larger than 0.05 percent. A ranitidine hydrochloride product obtained through salifying the ranitidine base prepared and purified through the method provided by the invention has the purity reaching and beingsuperior to the quality level of an original drug, so that the safety of the drug during a use process is further improved.
Owner:SHANXI YUNPENG PHARMA
Ranitidine hydrochloride powder injection for injection
ActiveCN103315970AOrganic active ingredientsPowder deliveryHistamine h2 receptor antagonistPentagastrin stimulation test
The invention relates to a ranitidine hydrochloride powder injection for injection, specifically to a pharmaceutical composition. The ranitidine hydrochloride powder injection contains ranitidine, a freeze-drying excipient and an optional acidifying or alkalizing agent. The invention also relates to a preparation method of the pharmaceutical composition. The pharmaceutical composition, which is used as an excellent and potent histamine H2 receptor antagonist, can be used to effectively inhibit gastric acid secretion caused by stimulation of histamine, pentagastrin and carbechal, reduce gastric acid and gastric enzyme activity, and is an excellent gastropathy medicine for treating hyperacidity and heartburn. The powder injection provided by the invention has good pharmaceutical properties.
Owner:CHENGDU TIANTAISHAN PHARMA
Duck's egg membrane extraction preparation method
InactiveCN1633875AImprove immunityReduce ulcer volumeProtein composition from eggsFood preparationHydrolysateRanitidine Hydrochloride
The invention relates to a method for extracting small molecule polypeptides for medicament preparation, which comprises carrying out alkali hydrolysis to the duck's egg membrane, removing residue after the completion of hydrolysis, diluting the solution for use as medicament directly, which has the function for treating stomach ulcer. The hydrolysate can also be neutralized for alkali expelling, so as to prepare concentrated liquid or dried product after concentration.
Owner:杨秀庄
Method for preparing ranitidine hydrochloride
The invention relates to a method for preparing ranitidine hydrochloride, comprising the following steps of: (1) mixing ethanol with 2-[[[5-(dimethylamino)methyl-2-furyl]methyl]thio]ethylamine; (2) adding N-methyl-1-methylthio-2-nitro-vinylamine in reaction liquid; (3) cooling reaction liquid, and fully precipitating crystallize; (4) filtering reaction liquid, and washing, pumping and drying crystals to obtain almost white ranitidine; (5) agitating and mixing ethanol and ranitidine; (6) adding ethanol hydrochloride solution until pH value is 6-6.5, and agitating; (7) immediately adding activated carbon to decolor after the pH value becomes stable; (8) filtering reaction liquid, and cooling filtrate to 4-8 DEG C; and (9) continuously filtering, and washing, pumping and drying crystals to obtain white solid ranitidine hydrochloride. The method for preparing ranitidine hydrochloride has the advantages of simple steps, high yield and low processing cost, and is suitable for large scale production.
Owner:JIANGSU HI STONE PHARMA
Preparation method of ranitidine hydrochloride
InactiveCN107915697ASimple stepsHigh yieldOrganic chemistryHigh volume manufacturingRanitidine Hydrochloride
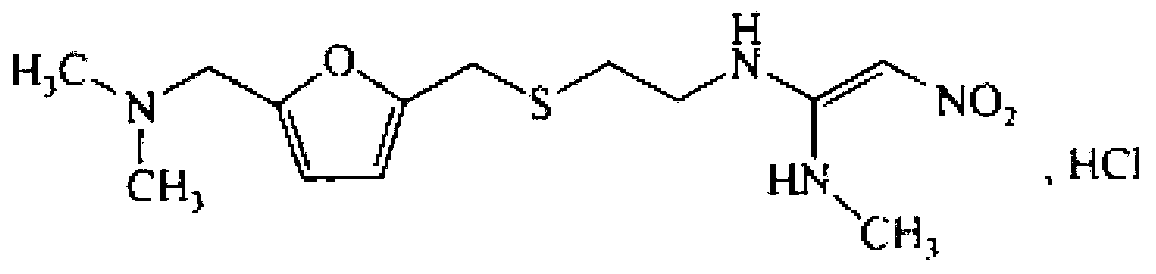
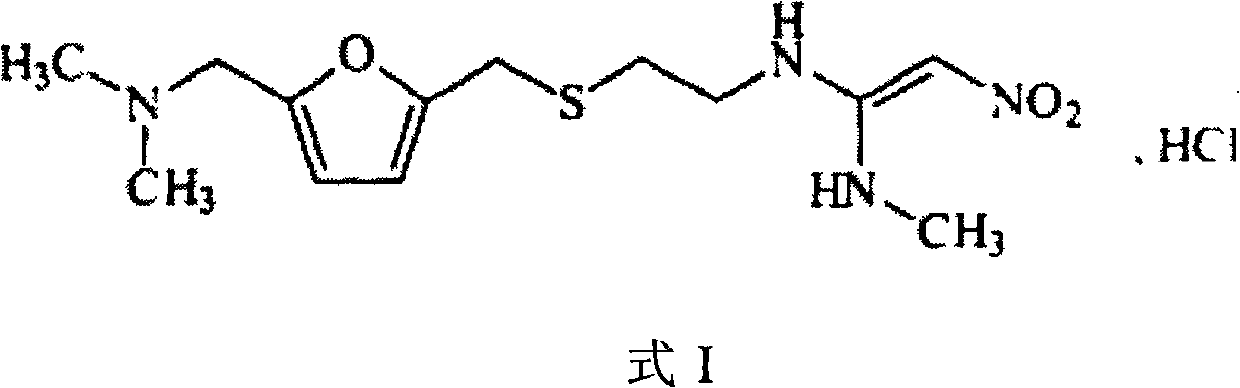
The invention discloses a preparation method of ranitidine hydrochloride, the chemical name of said ranitidine hydrochloride is N'-methyl-N-[2[[5-[(dimethylamino)methyl-2-furan Base] methyl] thio] ethyl]-2-nitro-1,1-ethylenediamine hydrochloride, the chemical structural formula of said ranitidine hydrochloride is, and the molecular formula of said ranitidine hydrochloride is C13H22N4O3S ·HCl: the invention has simple steps, higher yield than the existing level, high purity, reduced production cost, and is suitable for mass production.
Owner:孙婷婷
Method for preparing ranitidine hydrochloride capsules
InactiveCN108245492ATightly boundEasy to wrapOrganic active ingredientsCapsule deliveryMedicineRanitidine Hydrochloride
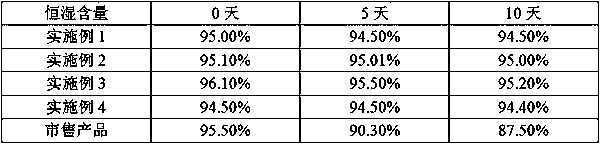
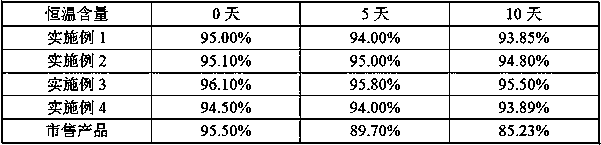
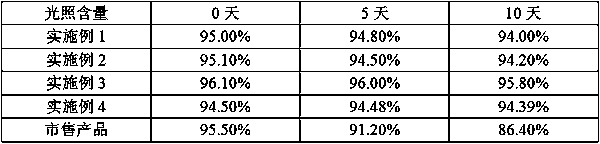
The invention discloses a method for preparing ranitidine hydrochloride capsules. Each capsule contains 10-20 parts of a bulk drug and 10-100 parts of starch. 10-20 parts by weight of the bulk drug and 10-100 parts by weight of the starch are mixed and ground in a mortar, microwave heating is carried out several times during the mixing and grinding, and capsules are filled with the obtained mixedranitidine hydrochloride drug powder to prepare the ranitidine hydrochloride capsules. The ranitidine hydrochloride capsules only adopt the single-component starch auxiliary material under above technologic conditions, so the ranitidine hydrochloride capsules have a good stability in influence factor experiments prescribed in pharmacopeia.
Owner:HUNAN ER KANG PHARMA
Preparation method of ranitidine hydrochloride tablets
InactiveCN109700775ASolve moisture absorptionPrevent drynessOrganic active ingredientsDigestive systemHydrogen phosphateMagnesium stearate
The invention belongs to the technical field of pharmaceutical preparations, and in particular relates to a preparation method of ranitidine hydrochloride tablets. The method selects auxiliary materials which are suitable for direct tabletting and have moisture-proof function, such as microcrystalline cellulose, anhydrous calcium hydrogen phosphate, magnesium stearate and silicon dioxide, adopts adirect tabletting process at the same time, solves the problem of moisture absorption of the tablet core, avoids the drying process of particles after wet granulation, and improves product quality and production efficiency. The process is simple, easy to implement, and suitable for large-scale production.
Owner:REYOUNG PHARMA
Formula and preparation method for ranitidine hydrochloride slow release suspension
InactiveCN110327295AHigh drug loading rateMask bitternessOrganic active ingredientsDigestive systemDynamic methodIrritation
The invention relates to the technical field of medicines, and discloses a formula and a preparation method for a ranitidine hydrochloride slow release suspension. The slow release suspension comprises the following ingredients in parts by weight: 20-80% of ranitidine hydrochloride medicine carrying resin micro-capsule, 20-80% of slow release auxiliary material and the balance of other auxiliary materials. Since a dynamic method is adopted to prepare the ranitidine hydrochloride medicine resin, the medicine carrying rate of the medicine resin can be obviously improved. Through reasonable configuration, the ranitidine hydrochloride is prepared into the oral liquid slow release suspension. According to different dosage requirements, the slow release suspension is taken according to dosages,and the slow release suspension has the advantages of large distribution area in gastrointestinal tracts, high adsorption speed, stable blood concentration and high bioavailability, and can reduce irritation to gastrointestinal tracts and the influence on in-vivo behaviors by a gastric emptying rate. Meanwhile, ranitidine and ion exchange resin generate ion exchange, so that a medicine enters a skeleton to cover the bitter taste of the ranitidine hydrochloride.
Owner:WUYI UNIV
Pharmaceutical composition of paclitaxel and ranitidine hydrochloride
InactiveCN103393633ASolve the problem of water solubilityFix stability issuesOrganic active ingredientsPowder deliveryPaclitaxel InjectionRanitidine Hydrochloride
The invention relates to a pharmaceutical composition of paclitaxel and ranitidine hydrochloride, and especially relates to an application product of the pharmaceutical composition, wherein the application product comprises paclitaxel injection and an injection containing ranitidine hydrochloride. Administration comprises following steps, the injection containing ranitidine hydrochloride is applied firstly by intravenous injection, the paclitaxel injection is dissolved and diluted by normal saline or 5% glucose and sodium chloride solution, and then the diluted paclitaxel injection is applied by intravenous drop infusion.
Owner:HAINAN LINGKANG PHARMA CO LTD
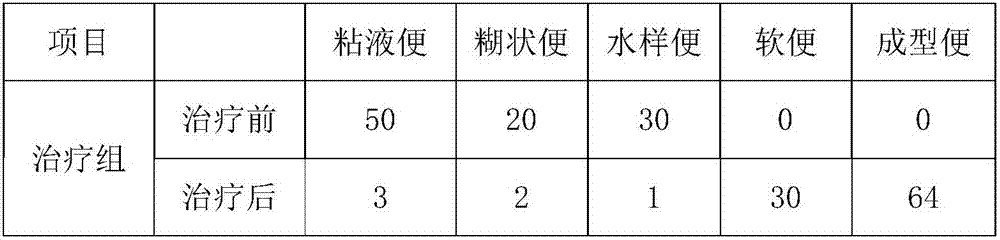
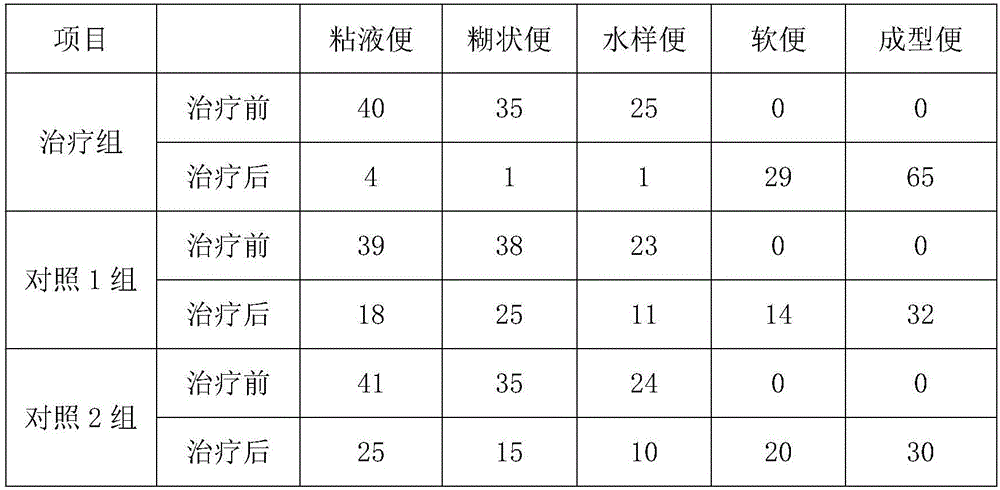
Pharmaceutical composition for treating chronic gastroenteritis and preparation method thereof
InactiveCN107441478ASmall side effectsNo complicationsHydroxy compound active ingredientsDigestive systemDiseaseArginine
The invention discloses a pharmaceutical composition for treating chronic gastroenteritis, which is prepared from the following raw materials: belladonna and aluminium dispersible tablets, levocarnitine, bismuth potassium citrate, metronidazole, ranitidine hydrochloride, vitamin B, lovastatin, niacin, calcium pantothenate, niacinamide, L-arginine, jujube polysaccharide, cassia seed, fructus crataegi, radix et rhizoma salviae miltiorrhizae, radix et rhizoma rhei, ginger, tartary buckwheat, radix puerariae lobatae, leucocyanidin, vitexin, astragalin, cryptoxanthin and quercetin. The pharmaceutical composition for treating chronic gastroenteritis has the synergistic effects through the compounding of the raw materials, has obvious anti-inflammatory activity, and has the advantages of both symptoms and roots treatment of the disease, obvious curative effects, convenient taking, no recurrence and no rebound after cure, little toxic and side effects, no complication, long-term use, less residues and low price, so that the production cost is reduced and the waste of resources is avoided.
Owner:苏州和必尔斯电子科技有限公司
Western medicine preparation for treating chronic gastroenteritis
InactiveCN106729072AGood effectEasy to takeDigestive systemAluminium/calcium/magnesium active ingredientsSide effectAstrovirus gastroenteritis
The invention discloses a western medicine preparation for treating chronic gastroenteritis. The western medicine preparation is prepared from the following raw materials in parts by weight: 5 to 10 parts of Si Dashu dispersing tablets, 5 to 10 parts of levocarnitine, 0.1 to 1 part of vitamin H, 2 to 5 parts of rebaudioside A, 3 to 8 parts of saleratus, 0.1 to 0.5 part of oleum menthae, 1 to 5 parts of oat beta-glucan, 2 to 5 parts of taurine, 5 to 10 parts of glutamine, 5 to 15 parts of plant protein powder, 2 to 10 parts of matrine, 2 to 10 parts of cucurbitacine, 2 to 5 parts of acetylkitasamycin, 1 to 5 parts of ranitidine hydrochloride, 40 to 80 parts of ethyl alcohol and 50 to 100 parts of distilled water. The western medicine preparation for treating the chronic gastroenteritis has the advantages that the synergistic effect is achieved through raw materials compounding; the obvious anti-inflammatory activity is realized; the effect of treating both symptoms and root causes is achieved; the effect is obvious; the taking is convenient; no recurrence occurs after the curing. The western medicine preparation for treating the chronic gastroenteritis has low toxic and side effects and no complications.
Owner:郑州莉迪亚医药科技有限公司
Western medicine composition for treating chronic gastroenteritis
InactiveCN106729603ASmall side effectsNo complicationsHydrolysed protein ingredientsInorganic phosphorous active ingredientsSide effectAluminium phosphate
The invention discloses a pharmaceutical composition for treating chronic gastroenteritis. The pharmaceutical composition is prepared from the following raw materials in parts by weight: 5-10 parts of rabeprazole, 5-10 parts of levocarnitine, 0.1-1 part of vitamin H, 2-5 parts of rebaudioside A, 3-8 parts of aluminium phosphate, 0.1-0.5 part of allicin, 1-5 parts of chitosan, 2-5 parts of taurine, 5-10 parts of glutamine, 5-15 parts of an earthworm protein powder, 2-10 parts of dendrobine, 2-10 parts of artemisinin, 2-5 parts of acetylkitasamycin, 1-5 parts of ranitidine hydrochloride, 40-80 parts of ethyl alcohol and 50-100 parts of distilled water. The raw materials are compounded to achieve a synergistic effect, and the pharmaceutical composition for treating chronic gastroenteritis has remarkable anti-inflammatory activity, can treat both symptoms and root causes, is remarkable in effect and convenient to take, ensures no recurrence after curing, and is small in toxic and / or side effect and free from complications.
Owner:HENAN SHUIJINGTOU CULTURAL MEDIA CO LTD
Medicine for treating stomach illness
InactiveCN104983748ASimple preparation processReduce manufacturing costOrganic active ingredientsAntipyreticLiver stomachAbdomen diseases
The invention discloses a medicine for treating stomach illness. The medicine is processed by mixing raw materials, and comprises, by weight, 140 mg-150 mg of metoclopramide tablets, 2200 mg-2500 mg of a furazolidone tablets, 2200 mg-2600 mg of cimetidine tablets, 1650 mg-2100 mg of ranitidine hydrochloride capsules, 16800 mg-18000 mg of yeast tablets and 3600 mg-4000 mg of Yunnan Baiyao powder. When the medicine is prepared, the above raw materials are mixed and polished into powder; and after the powder is qualified through microbiological detection, filling of the powder is carried out in a manner of a capsule. When the medicine is prepared, common Western medicine materials serve as the raw materials, the preparing process is simple, and the production cost is low; the product has the effects of supporting the healthy energy and stopping acid pains and is mainly used for treating the stomach illness such as gastral cavity pains, hyperacidity, congestion and edema which are caused by liver-stomach disharmony; and symptoms such as pains, giddiness, choking sensation in chest and bleeding which are caused by the stomach illness can be reduced.
Owner:梁美赠
Compound preparation containing ranitidine hydrochloride and troxipide and application thereof
InactiveCN102552257AOrally effectiveClose to peak timeOrganic active ingredientsDigestive systemIntestinal structureDisease
The invention provides a compound preparation containing ranitidine hydrochloride and troxipide and application thereof. The matching of ranitidine hydrochloride and troxipide according to weight ratio ranges from 6:1 to 1:6. Compared with the prior art, the compound preparation has the following advantages and active effects: ranitidine hydrochloride and troxipide have a pharmacology function and a coordinate effect, not only can treat stomach and intestine diseases including heartburn, gastropathy acid return and the like caused by gastric mucosal damage and too much gastric acid during acute-outbreak periods of acute gastritis and chronic gastritis, but also reinforce defense factors, improve recovery of ulcer diseases and metabolism of blood circulation of gastric mucosa at the ulcer parts, and enable gastric mucosa tissue components to be normalized. The effective dosage of ranitidine hydrochloride and troxipide in the compound preparation can be ensured, and the compound preparation further has the advantages of being effective in oral taking, similar in peak time and rapid in absorption.
Owner:PEKING UNIV FOUNDER GRP CO LTD +2
Ranitidine hydrochloride capsules and production method thereof
ActiveCN102138913BSolve hygroscopicityAvoid intakeOrganic active ingredientsDigestive systemPrillMedicine
The invention discloses ranitidine hydrochloride capsules and a production method thereof. Every 1,000 capsules comprise 165 to 170 parts of ranitidine hydrochloride and 40 to 50 parts of calcium hydrophosphate. The preparation method for the capsules comprises the following steps: weighing the ranitidine hydrochloride and the calcium hydrophosphate according to the weight part; crushing the ranitidine hydrochloride and the calcium hydrophosphate respectively, adding 50 percent ethanol in a trough type mixing machine to mix for 20 to 40 minutes to prepare a soft material, and granulating; drying the prepared granules in a hot-air circulation drying oven at the temperature of lower than 70 DEG C, and controlling the moisture within 4 percent; and filling the dried granules in capsules, andpolishing to obtain the ranitidine hydrochloride capsules. The ranitidine hydrochloride capsules and the production method thereof have the advantages that: the calcium hydrophosphate is adopted as an auxiliary material, so that each quality index of the product in a valid period is ensured to be basically stable, the problem of hygroscopicity of the ranitidine hydrochloride capsules can be solved, the product stability is improved, and intake of talc powder and magnesium stearate is prevented.
Owner:JIANGSU SUNAN PHARMA IND CO LTD
Ranitidine pharmaceutical composition and preparation method thereof
ActiveCN107714687AGood release effectImprove stabilityOrganic active ingredientsDigestive systemRanitidine HydrochlorideLubricant
The invention relates to a ranitidine pharmaceutical composition and a preparation method of the ranitidine pharmaceutical composition. The ranitidine pharmaceutical composition comprises ranitidine hydrochloride, an excipient, a stabilizer and a lubricant. The composition is excellent in preparation stability and release behavior, is quite suitable for clinical application, and improves the safety of drug use for a patient.
Owner:武汉九州钰民医药科技有限公司
Ranitidine hydrochloride lipidosome capsule and new application thereof
InactiveCN101623258BImprove efficacyImprove bioavailabilityDigestive systemAntiviralsHerpetic stomatitisYolk
Owner:HAINAN MEIDA PHARMA
Ranitidine hydrochloride controlled-release dry suspension and preparation method thereof
The invention discloses a ranitidine hydrochloride releasing-controlling dry suspension and a preparing method thereof. The ranitidine hydrochloride releasing-controlling dry suspension comprises ranitidine hydrochloride and polymers which can be accepted in the pharmacy and comprises, by weight, 10%-90% of ranitidine hydrochloride, 10%-90% of auxiliary materials and the balance other auxiliary materials. The auxiliary materials with the releasing controlling effect are one or more of positive ion exchange resin, methylcellulose, ethyl cellulose, acrylic resin and hydroxypropyl methylcellulose. Compared with an immediate-release preparation, the releasing-controlling preparation can keep the effective blood concentration within 24 hours, curative effects are improved, toxic and side effects are small, taking and carrying are convenient, and the taking times are reduced. Compared with a sustained-release preparation, the releasing-controlling preparation can keep the more stable blood concentration within 24 hours, curative effects are improved, and toxic and side effects are small. According to the ranitidine hydrochloride releasing-controlling dry suspension, dosing only needs to be carried out once in one day; the releasing-controlling preparation is used for treating benign gastric ulcer and duodenal ulcer in clinic.
Owner:JIANGSU SUNAN PHARMA IND CO LTD
Medicinal composite prepn. contg ranitidine hydrochloride and sucralfate
InactiveCN1785198AGood treatment effectOrganic active ingredientsDigestive systemSucrose sulfateGastritis
A compound medicine for treating pyrosis, gastritis, indigestion, gastrointestinal diseases, etc is proportionally prepared from ranitidine hydrochloride and sucralfate.
Owner:吕宝光 +1
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com