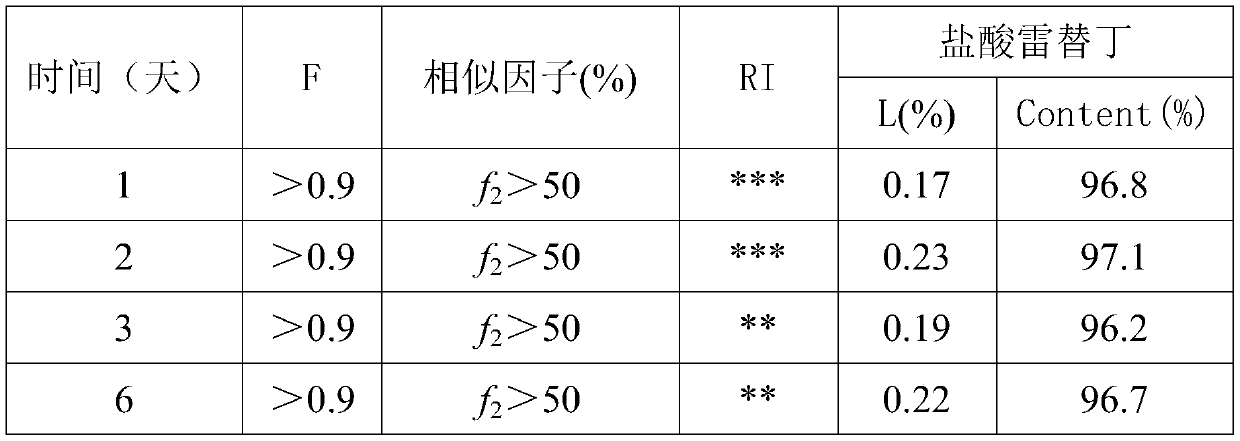
Formula and preparation method for ranitidine hydrochloride slow release suspension
A technology of ranitidine hydrochloride and sustained-release suspension, which is applied in the field of medicine, can solve the problems of poor patient compliance, inconvenient administration, and odor, and achieve stable blood drug concentration, high bioavailability, and large distribution area. big effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] The preparation of the drug-loaded resin containing ranitidine hydrochloride by dynamic method comprises the following steps:
[0031] S1: Weigh 500 mg of cation exchange resin, pack it into a column with a wet method, put it into a dynamic exchange column with a diameter of 2 cm, spread it on the bottom of the dynamic exchange column, and remove the air in it;
[0032] S2: 5mg·mL at room temperature -1 The ranitidine hydrochloride aqueous solution is poured into the exchange column from top to bottom;
[0033] S3: Adjust the lower hole plug to make it flow at 2mL·min -1 Keep the solution temperature at 25.0°C±0.5°C under constant flow rate;
[0034] S4: Take samples every 20 minutes and perform UV measurement, when the drug concentration no longer changes with time, it will reach equilibrium;
[0035] S5: After the drug loading is completed, the wet resin is washed three times with deionized water to remove the free drug on its surface;
[0036] S6: put in an oven ...
Embodiment 2
[0052] The preparation of the drug-loaded resin containing ranitidine hydrochloride by dynamic method comprises the following steps:
[0053] S1: Weigh 600mg of cation exchange resin, pack it into a column with a wet method, put it into a dynamic exchange column with a diameter of 2.5cm, spread it on the bottom of the dynamic exchange column, and remove the air in it;
[0054] S2: 3 mg·mL at room temperature -1 The ranitidine hydrochloride aqueous solution is poured into the exchange column from top to bottom;
[0055] S3: Adjust the lower hole plug to make it flow at 1mL min -1 Keep the solution temperature at 25.0°C±0.5°C under constant flow rate;
[0056] S4: Take samples every 20 minutes and perform UV measurement, when the drug concentration no longer changes with time, it will reach equilibrium;
[0057] S5: After the drug loading is completed, the wet resin is washed three times with deionized water to remove the free drug on its surface;
[0058]S6: put in an oven ...
Embodiment 3
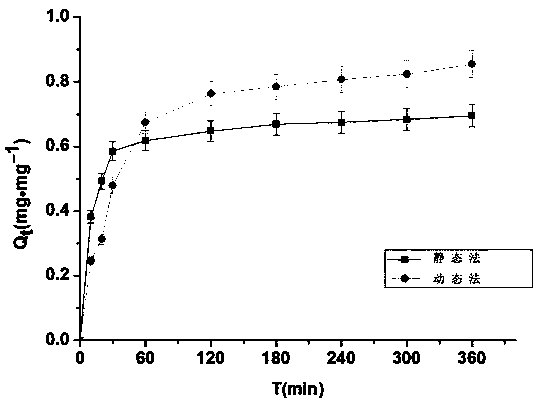
[0074] Comparison of the drug loading capacity of ranitidine hydrochloride drug-loaded resin by dynamic method and static method:
[0075] (1) Static preparation method:
[0076] Take 500mg blank resin and add to 5mg·mL -1 The ranitidine hydrochloride solution was magnetically stirred at room temperature (25°C), sampled at fixed points, and the drug loading (Qt) was plotted against the exchange time t, and the drug utilization rate was calculated.
[0077] (2) Dynamic preparation method:
[0078] Take 500mg of blank resin, wet-pack the column, spread it on the bottom of the exchange column, remove the air in it, and put 5mg·mL at room temperature (25°C) -1 The L ranitidine hydrochloride solution was poured into the exchange column from top to bottom, and its flow rate was adjusted to be 2ml / min. Samples were taken at fixed points, and the drug loading (Qt) was plotted against the exchange time t, and the drug utilization rate was calculated.
[0079] The result is as figur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com