Patents
Literature
65 results about "Cryptoxanthin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
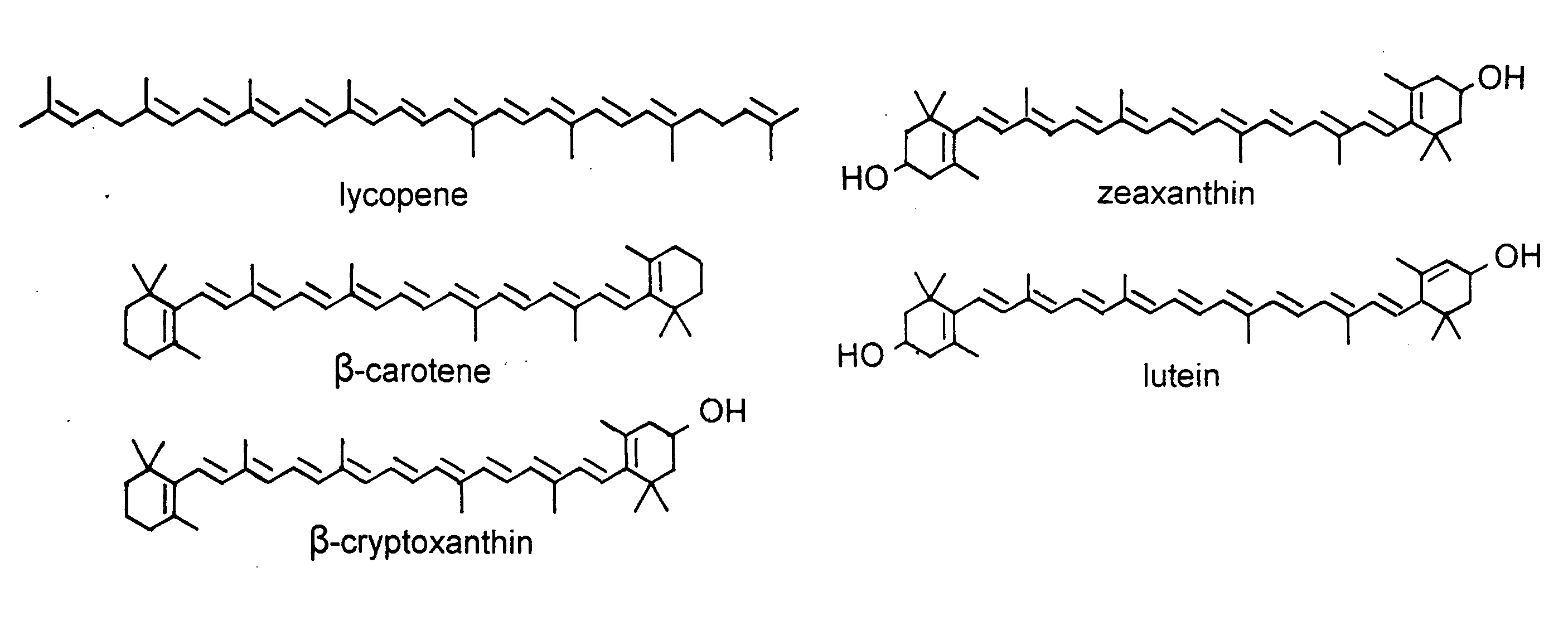
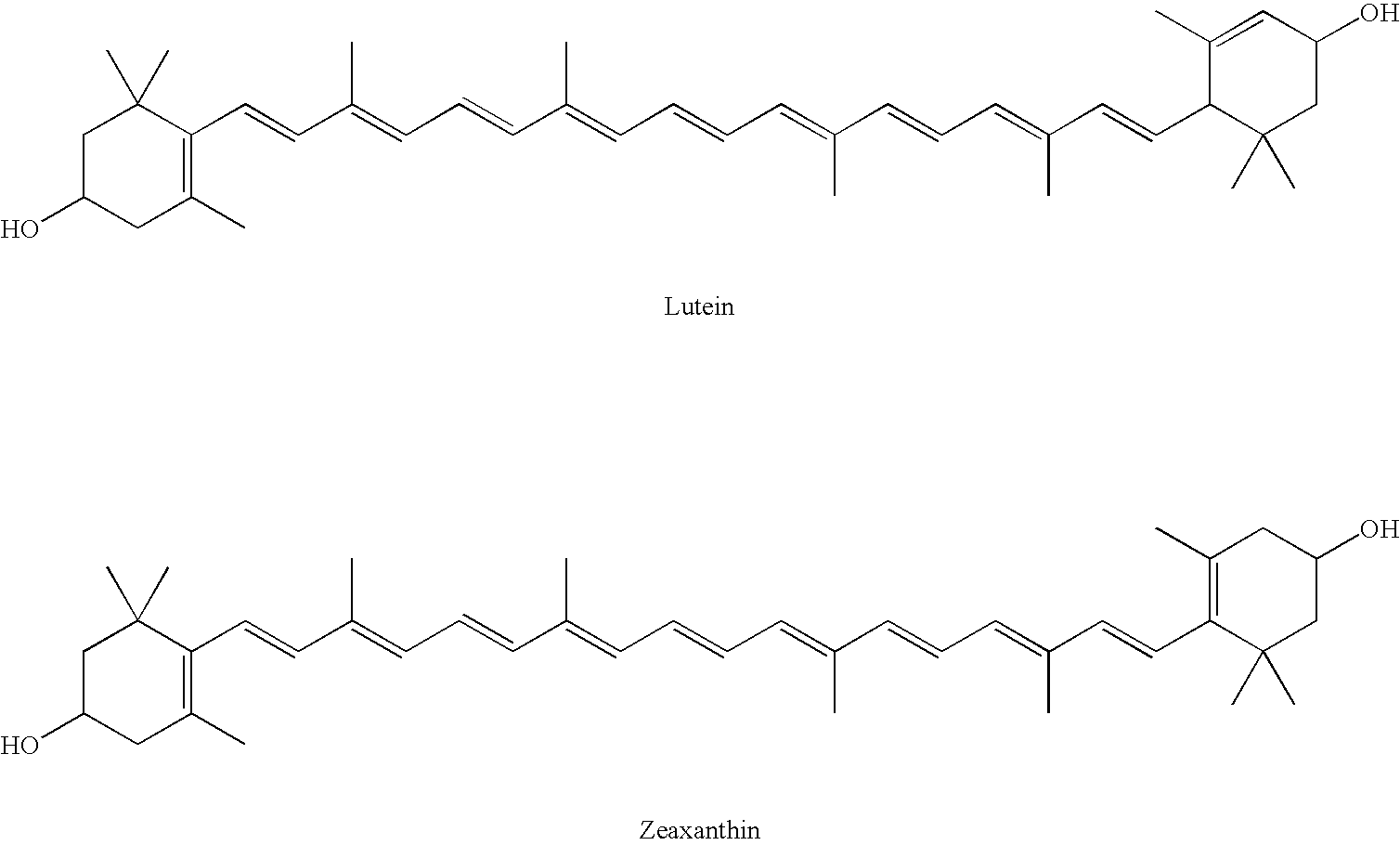
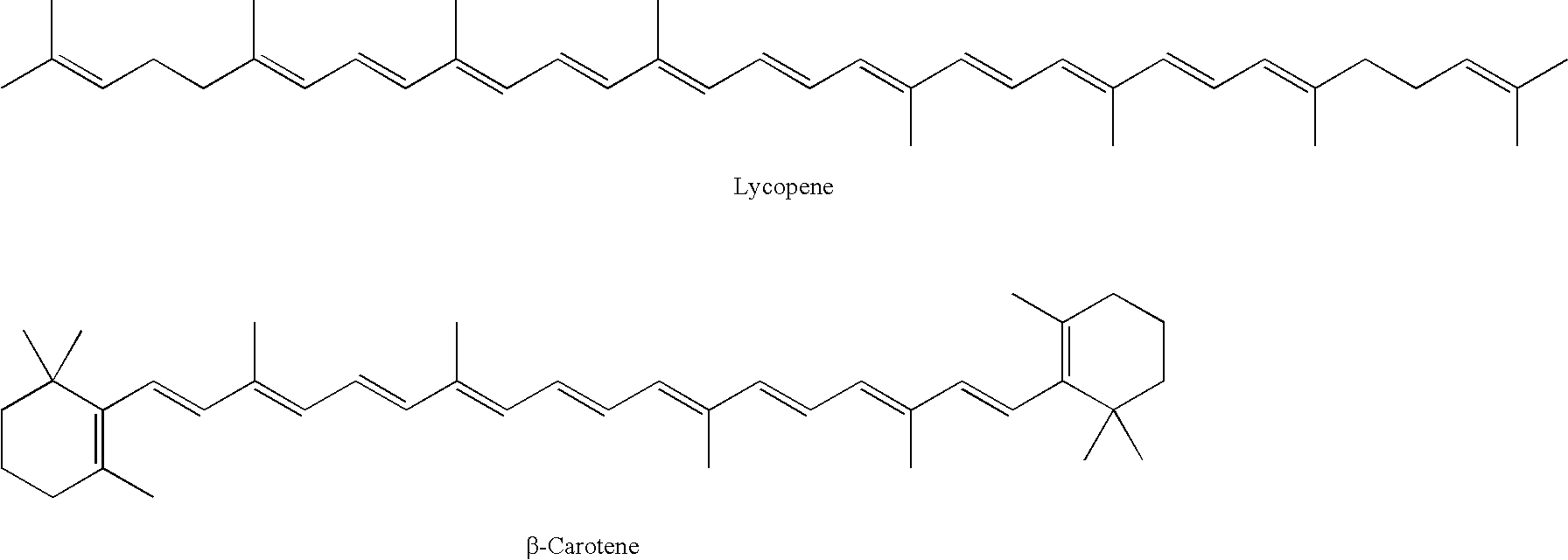
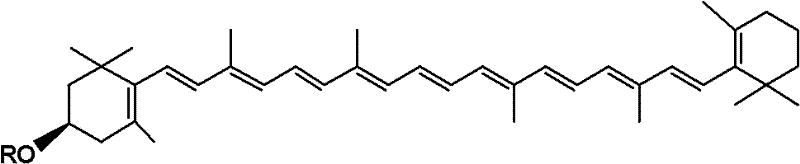
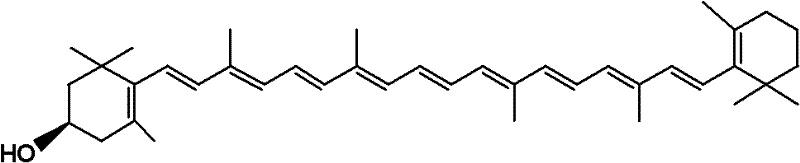
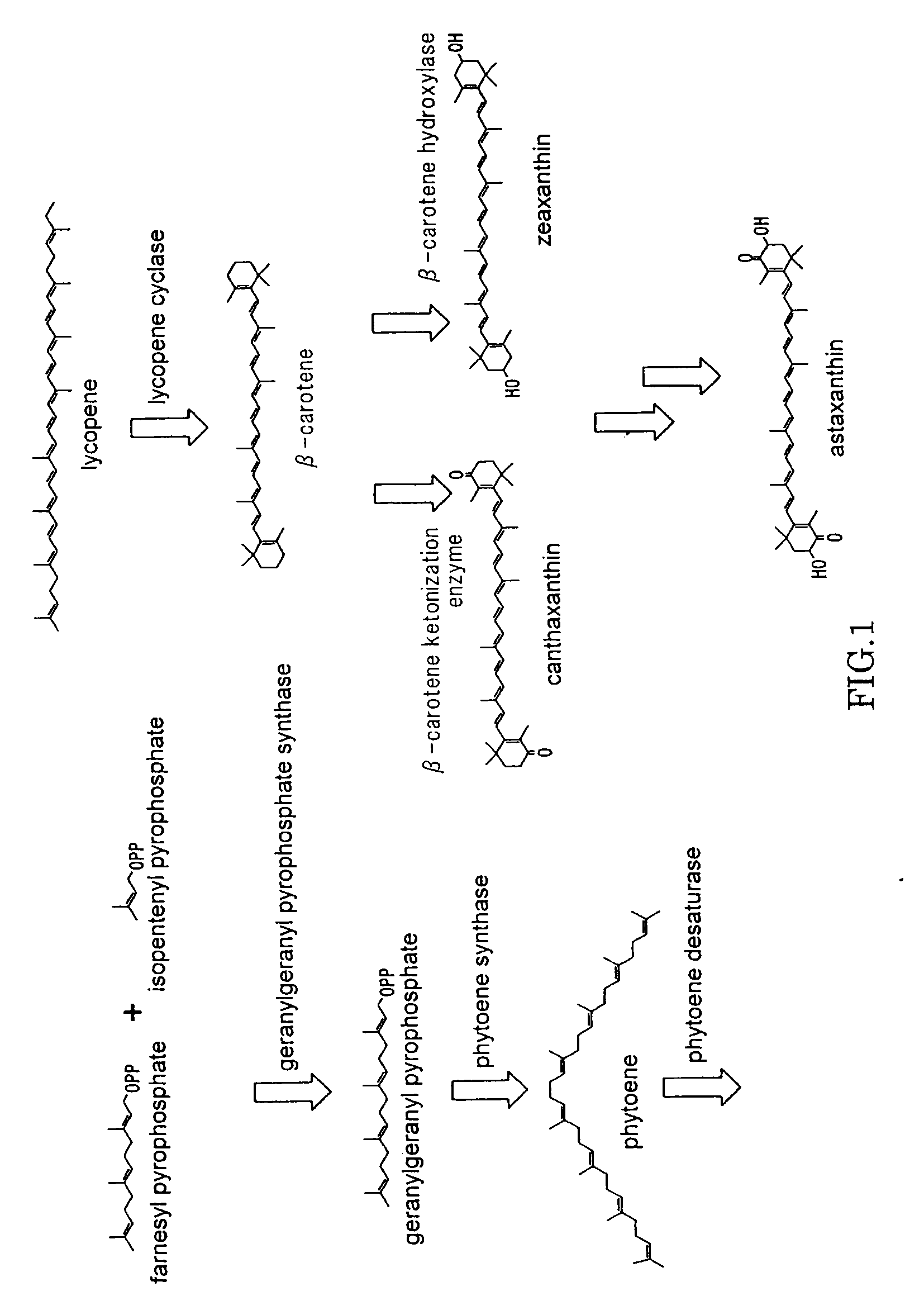
Cryptoxanthin is a natural carotenoid pigment. It has been isolated from a variety of sources including the petals and flowers of plants in the genus Physalis, orange rind, papaya, egg yolk, butter, apples, and bovine blood serum.
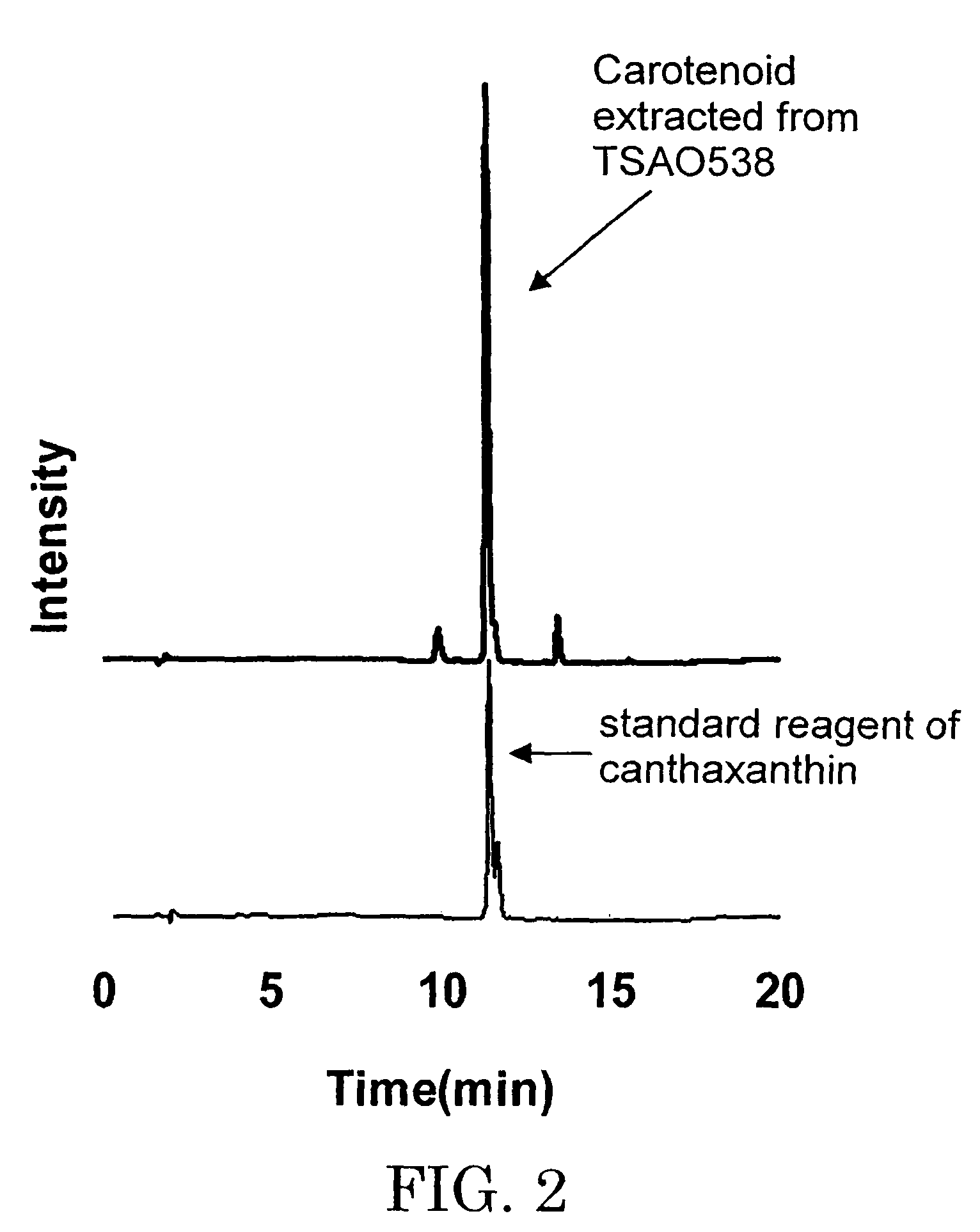
Liquid diary products containing lutein ester and cryptoxanthin and production method thereof
ActiveCN101675747AAntioxidantGood for healthMilk preparationFood preparationAntioxidantCryptoxanthin
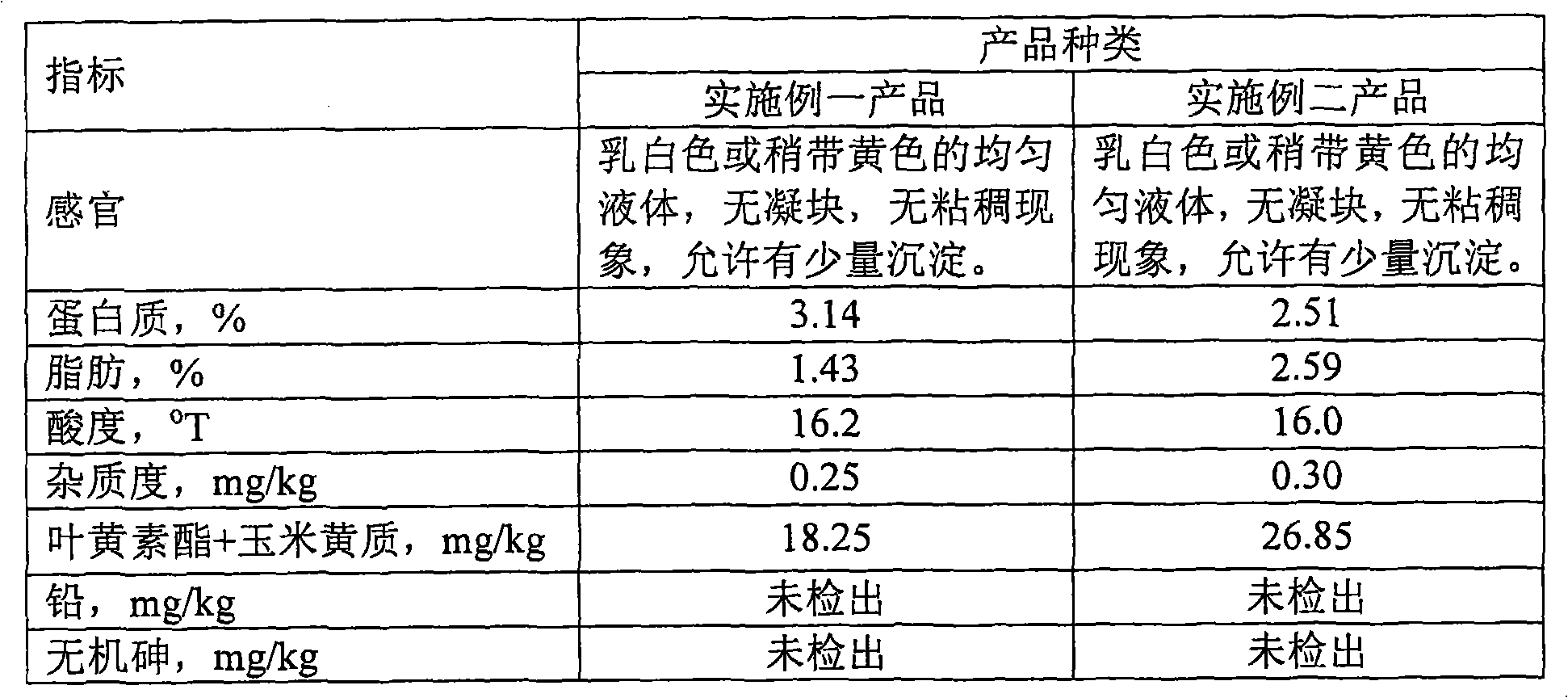
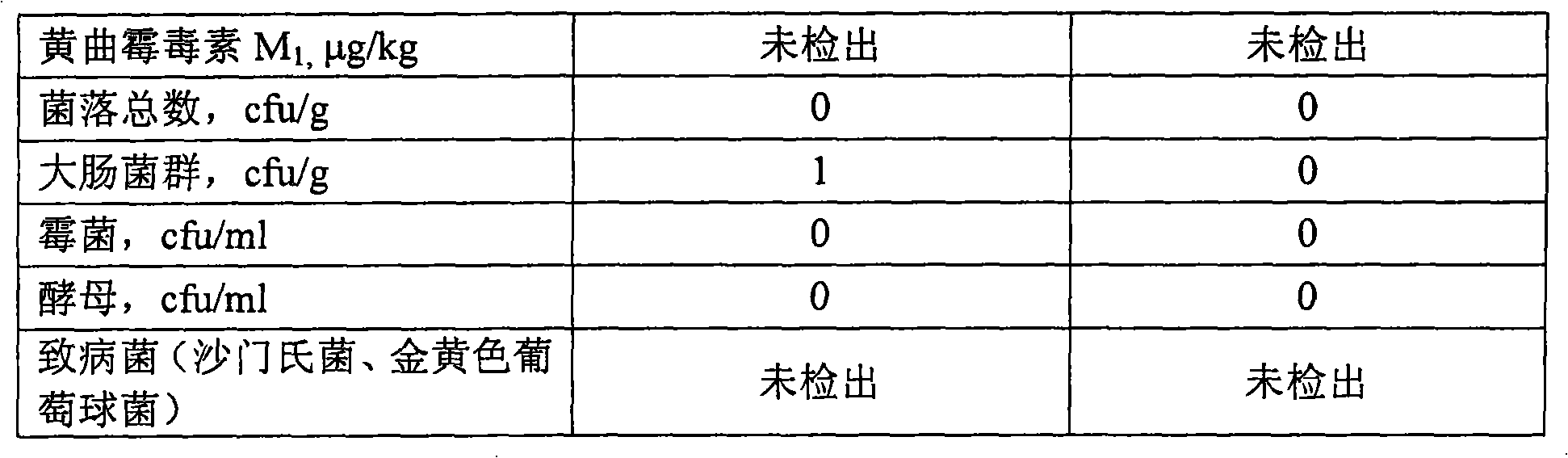
The invention provides a liquid diary products containing lutein ester and cryptpxanthin, using the total weight of the liquid diary products as reference, wherein milk >=35%; lutein ester and cryptpxanthin 0.0008%-0.005%; antioxidant 0.001-0.002%; emulsifier 0.01-0.30%. The liquid diary products are prepared by addinglutein ester and cryptpxanthin into the diary and / or water; shearing and stirring up the mixture for 15-30 minutes by a high-speed shearing stirrer and performing homogenization-treatment of the stirred-up mixture. The invention ensures the good stability and taste of the liquiddiary products in shelf life, using a reasonable formula and proper technology.
Owner:INNER MONGOLIA YILI INDUSTRIAL GROUP CO LTD
Isolation and purification of carotenoids from marigold flowers
ActiveUS7622599B2Facilitate extractabilityConvenient densityOrganic chemistryFatty-oils/fats productionHigh concentrationPetal
Owner:KATRA PHYTOCHEM PRIVATE
Oral contraceptive multivitamin compound and methods of administration
A pharmaceutical preparation comprising progestin, estrogen and a multivitamin agent. The progestin may be between 0 and 0.50 mg Desogesterel and the estrogen may be between 0 and 0.2 mg Ethinyl Estradiol. The multivitamin agent may be any combination of alpha carotene, beta carotene, biotin, bioflavonoid, calcium, chasteberry fruit, chromium, copper, coenzyme Q10, cryptoxanthin, dong quai root, folic acid, ginkgo biloba, garlic, grape seed extract, green tea extract, hesperedin, iodine, iron, lutein, malic acid, manganese, magnesium, milk thistle, molybdenum, niacin, panthothenic acid, potassium, pyridoxine HCL, quercetin, riboflavin, rutan, selenium, thiamine, vitamin A, vitamin B2, vitamin B6, vitamin B12, vitamin C, vitamin D, vitamin E, vitamin K, zinc, zoexanthin and any combination thereof.
Owner:HELLER MARGARET
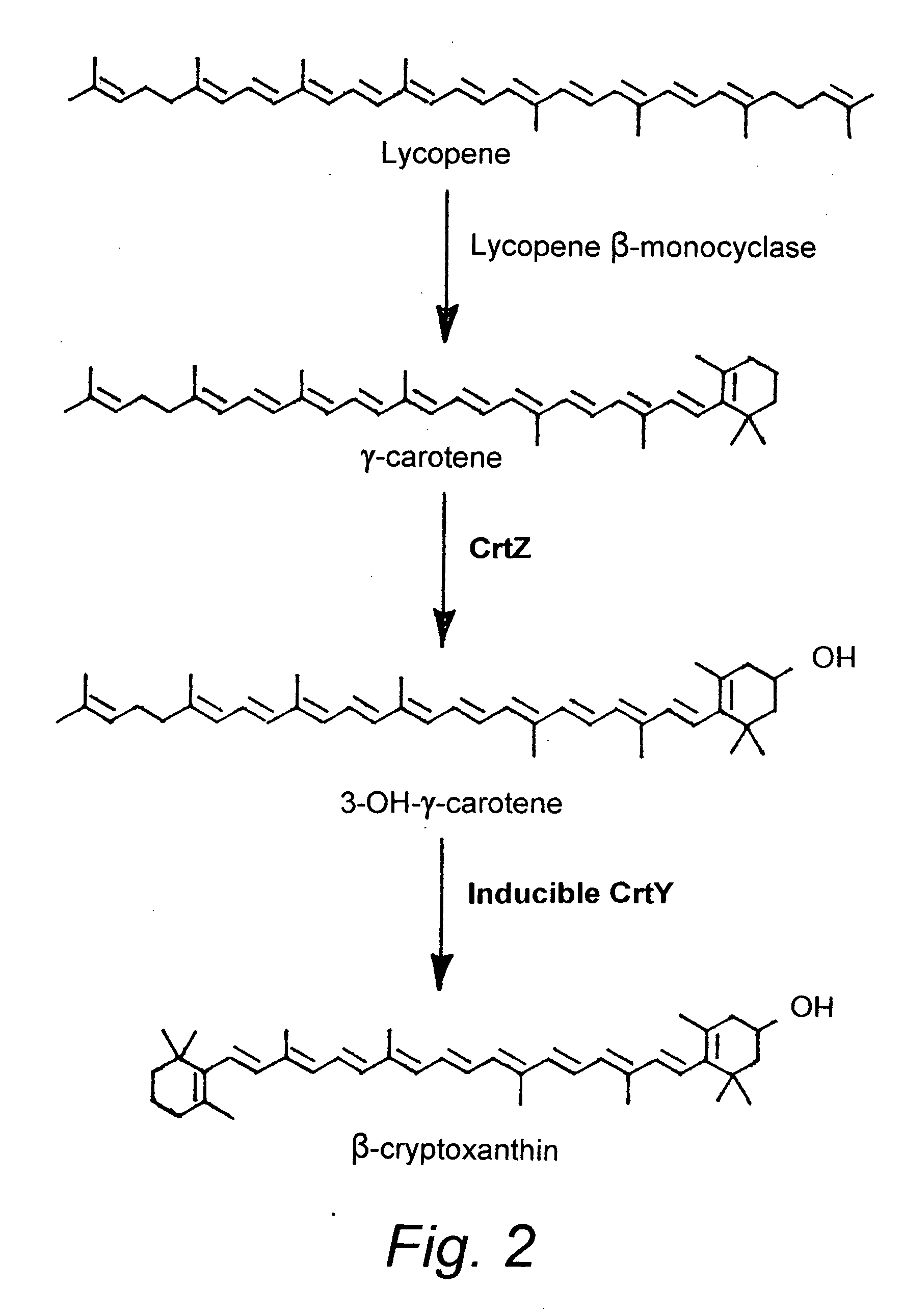
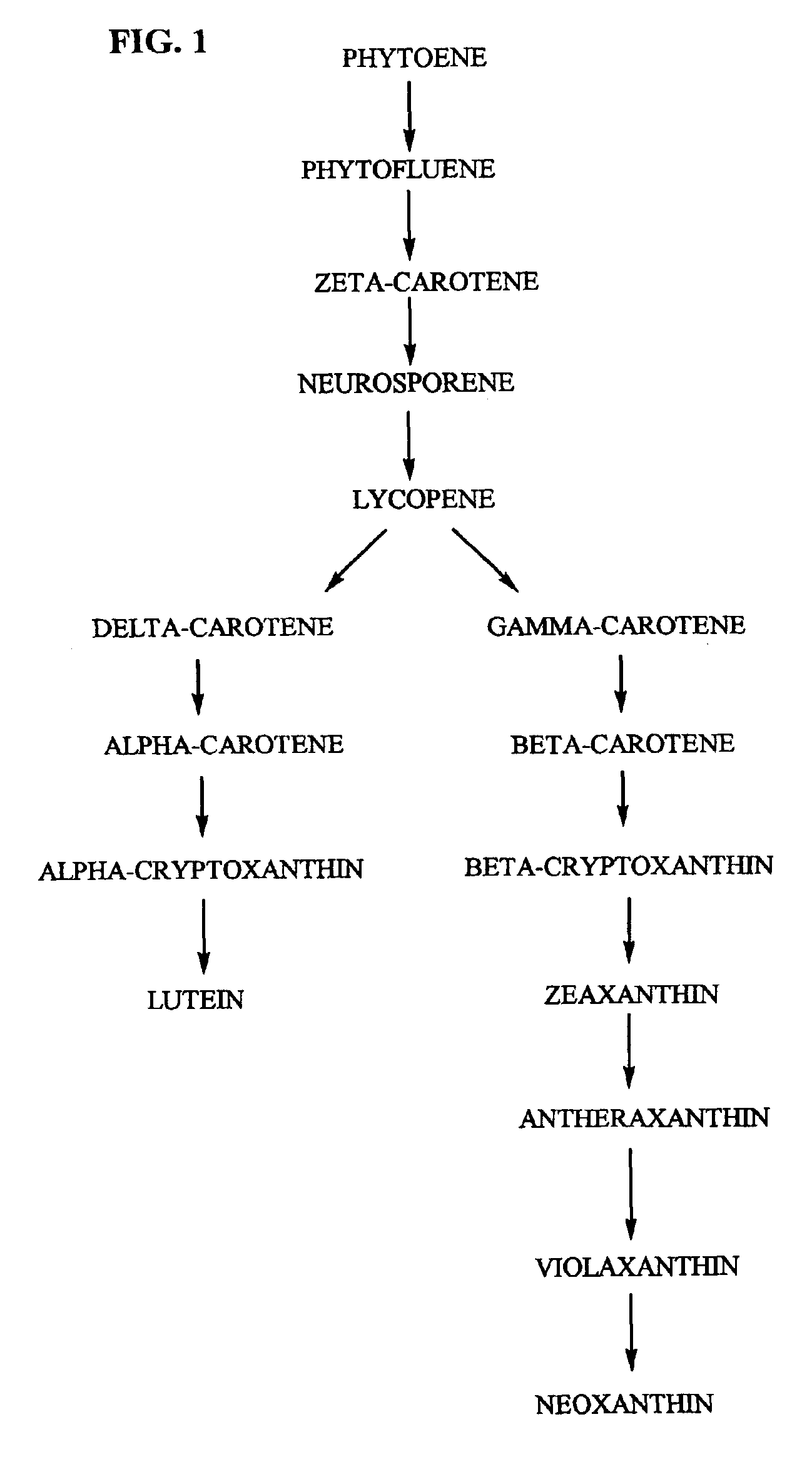
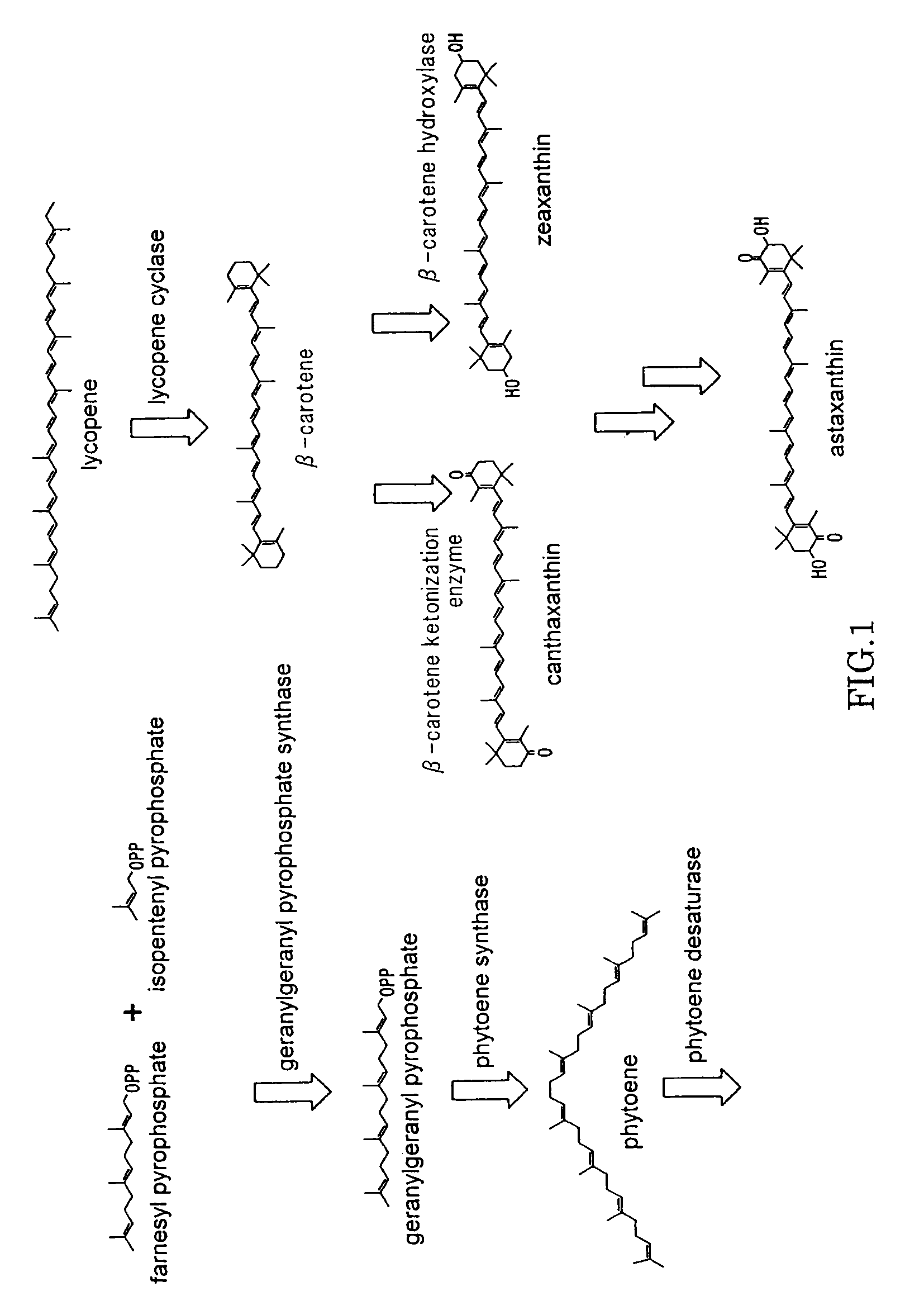
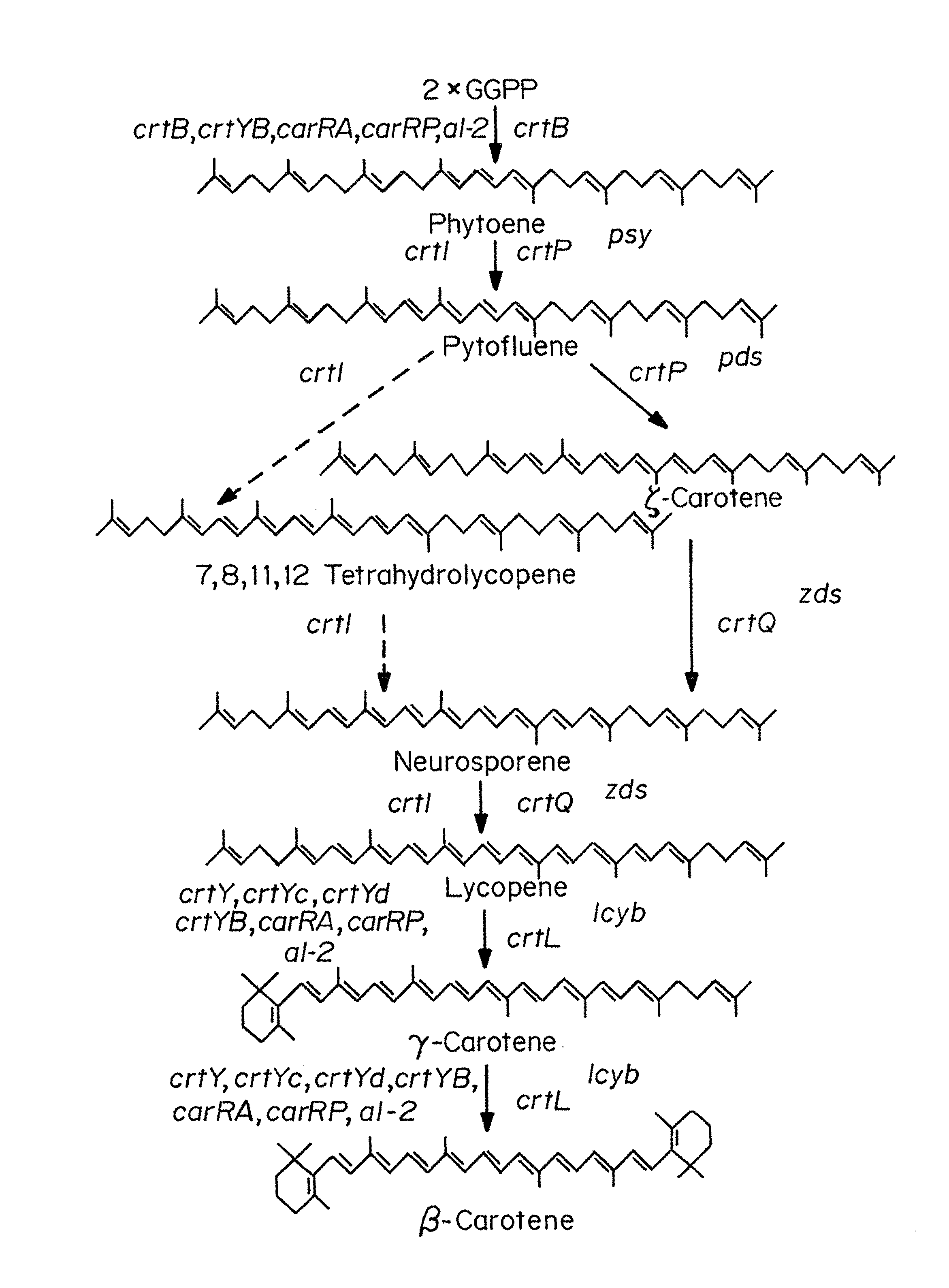
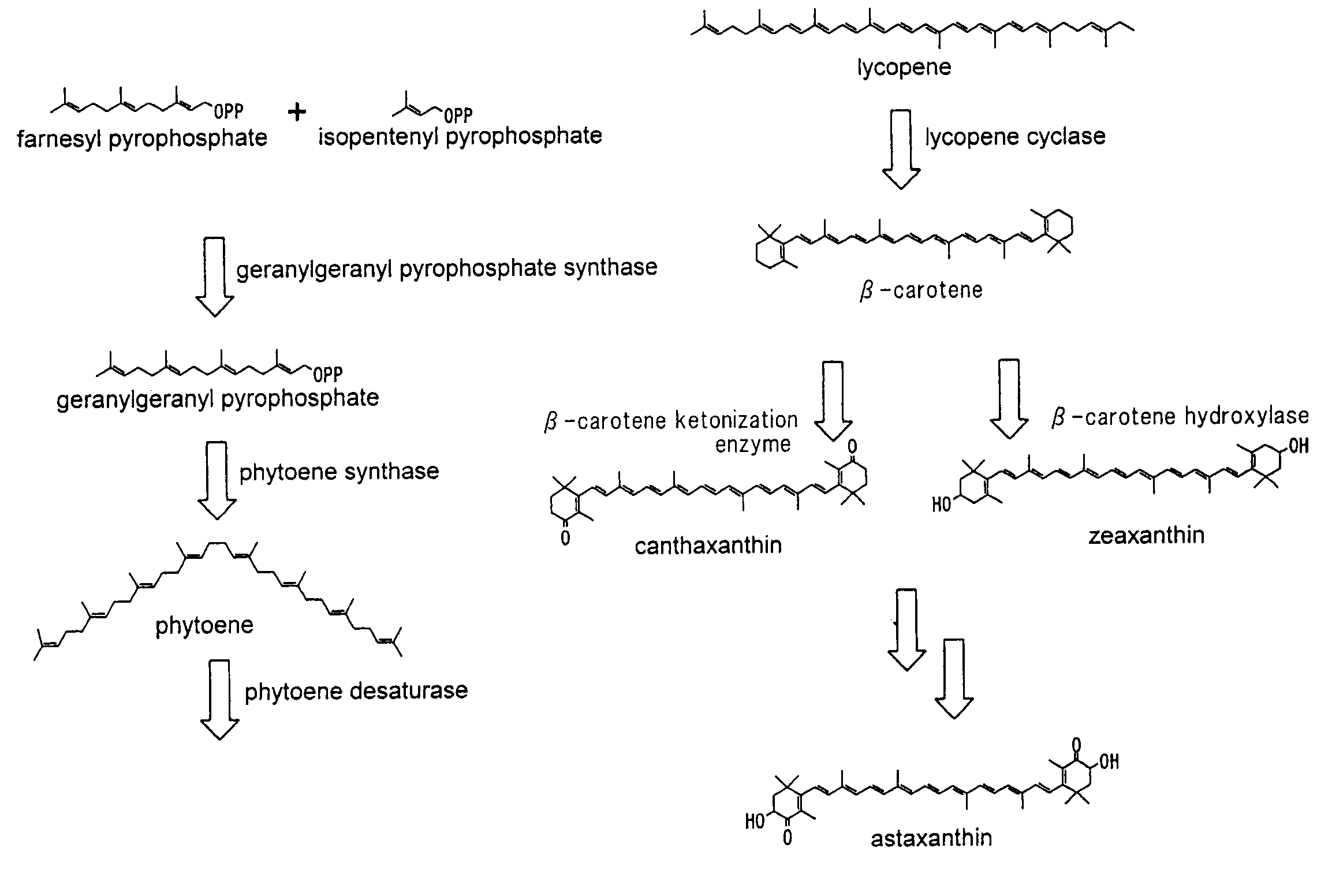
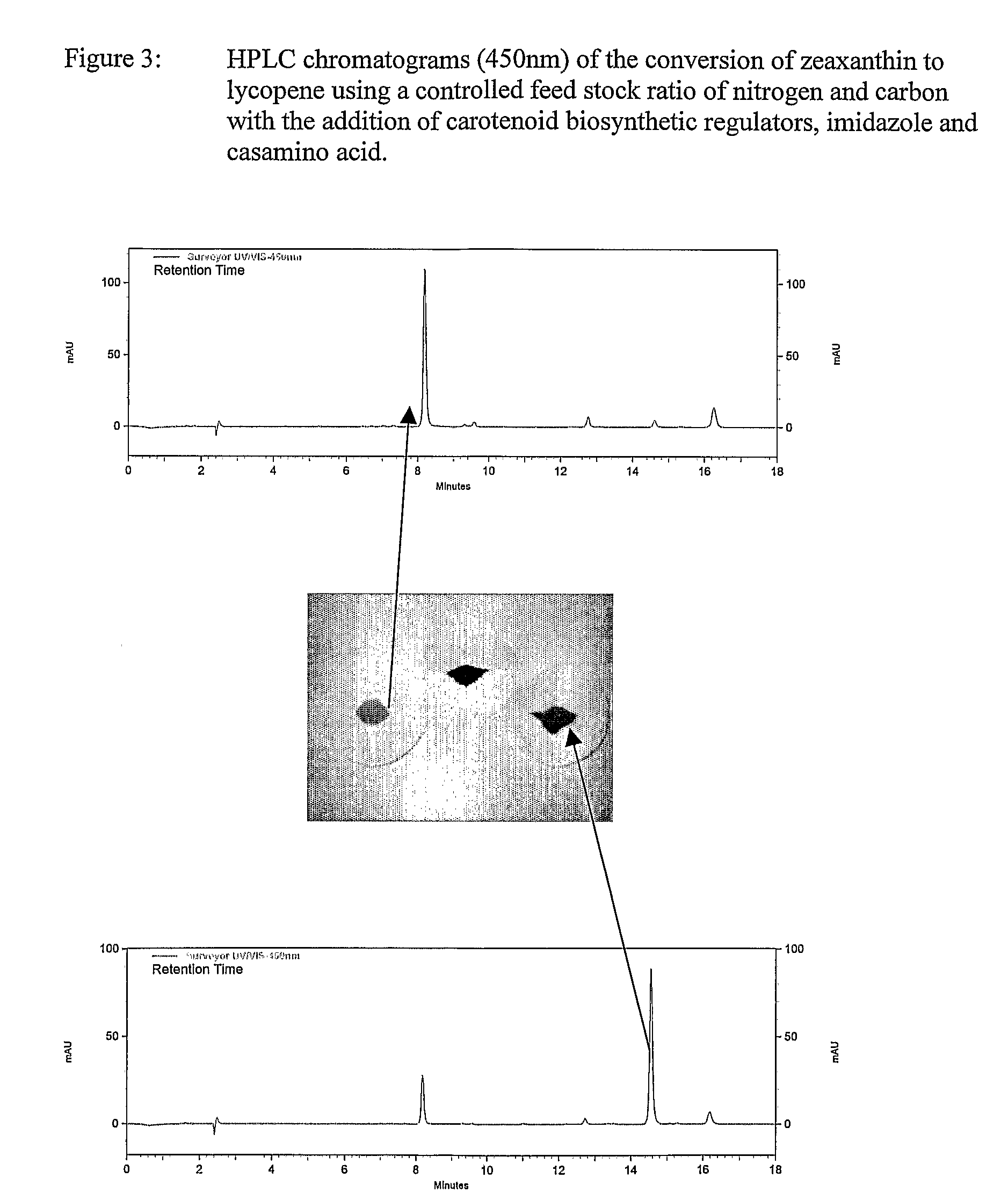
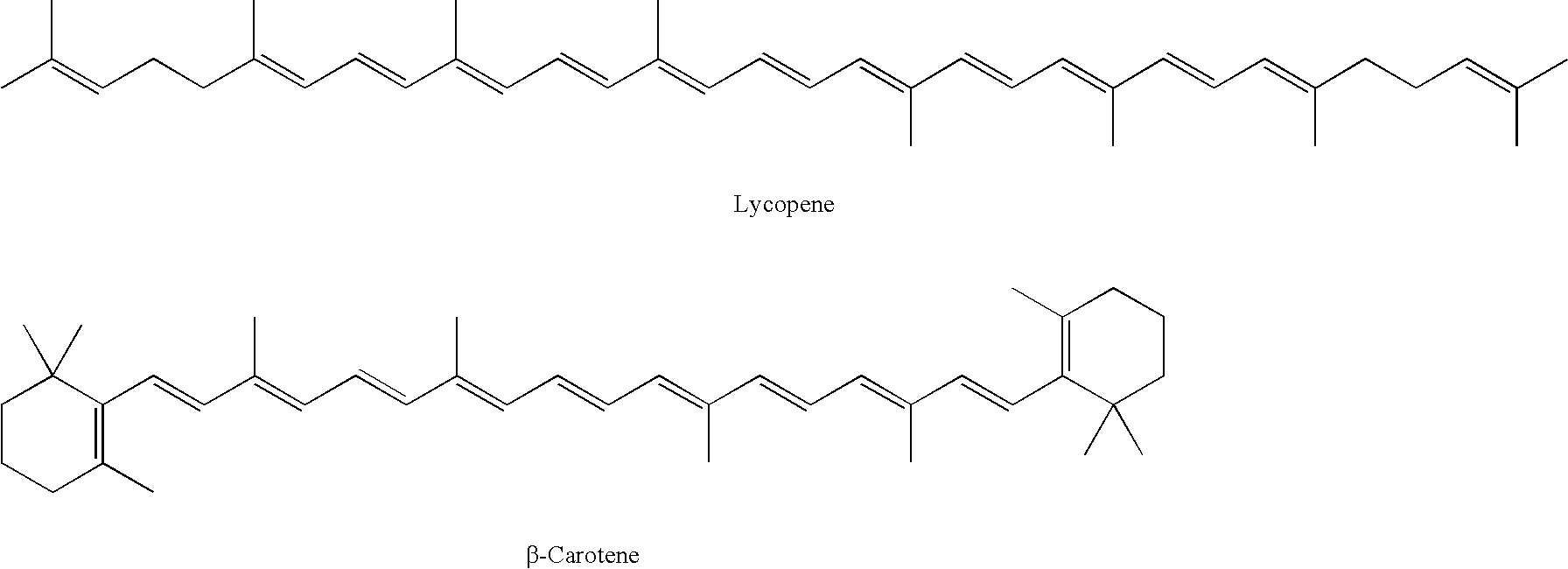
Beta-cryptoxanthin production using a novel lycopene beta-monocyclase gene
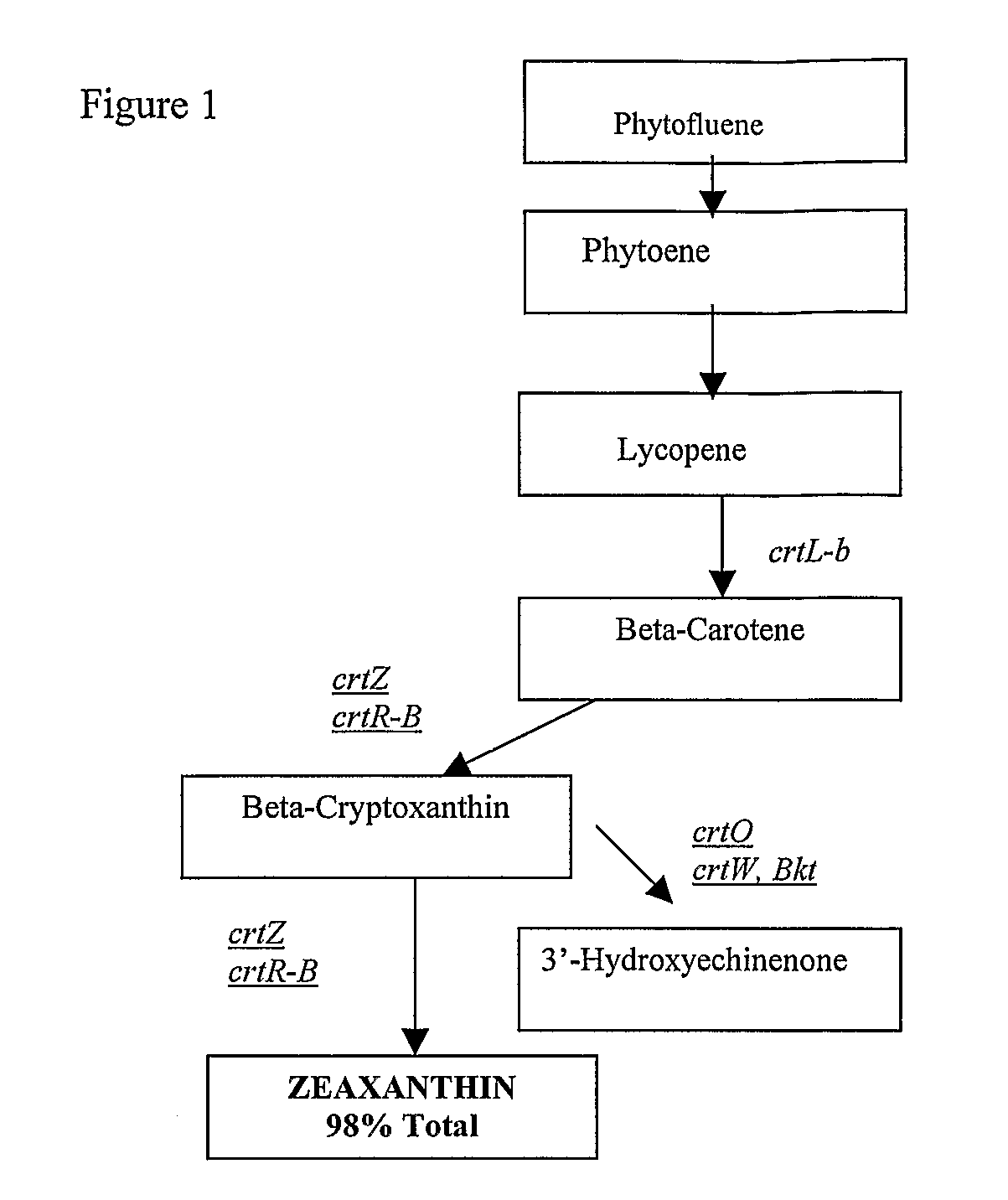
Novel lycopene beta-monocyclase genes were identified and used to transform a host cell to produce β-cryptoxanthin. The host cell produces lycopene and is transformed to express the novel lycopene β-monocyclase that converts lycopene into γ-carotene. The host cell is further transformed to express a lycopene hydroxylase that hydroxylates γ-carotene to 3-hydroxy-γ-carotene and a lycopene β-bicyclase that converts 3-hydroxy-γ-carotene to β-cryptoxanthin. The host cell is grown under conditions whereby γ-carotene is produced which is hydroxylated to 3-hydroxy-γ-carotene and which is converted into β-cryptoxanthin.
Owner:KEMIN FOODS L C
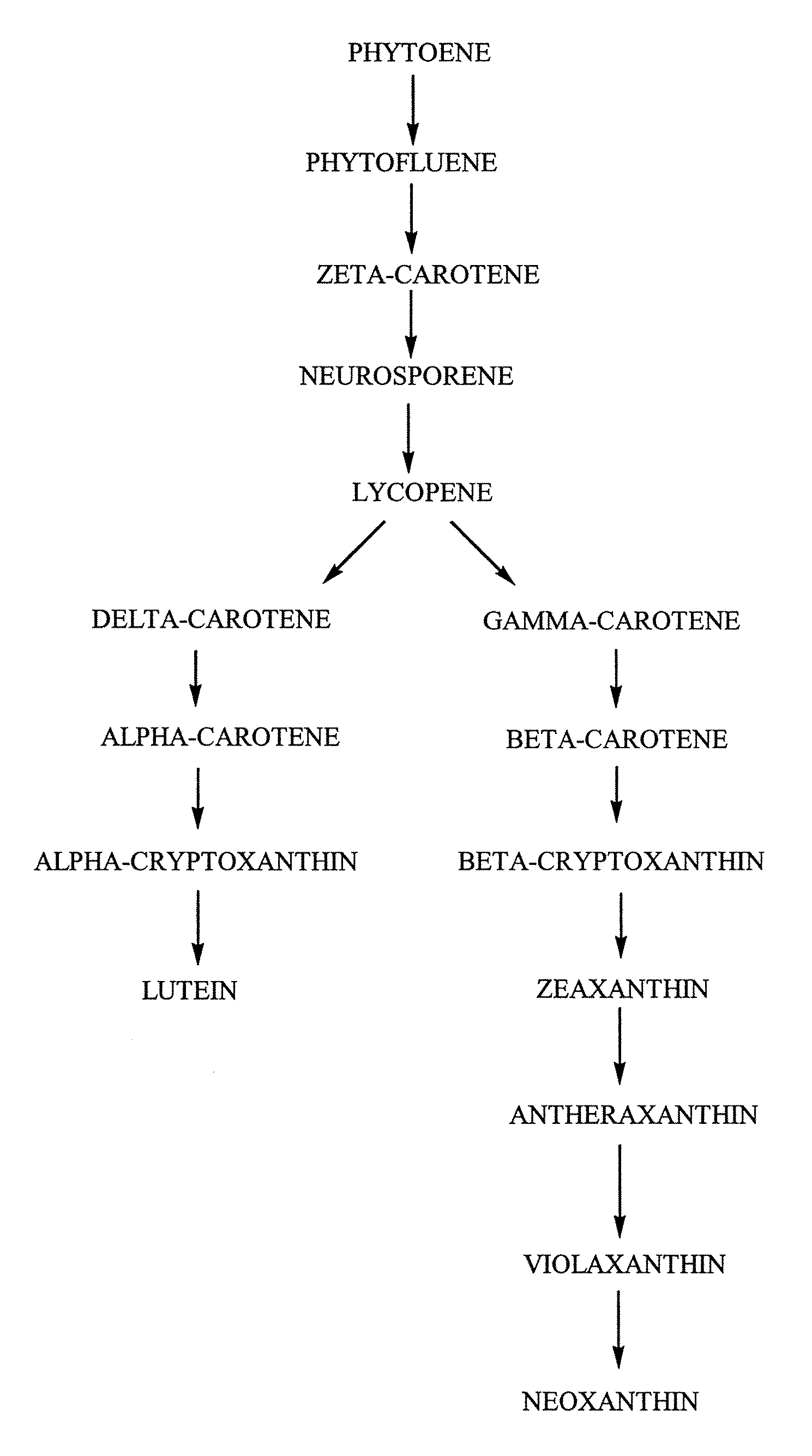
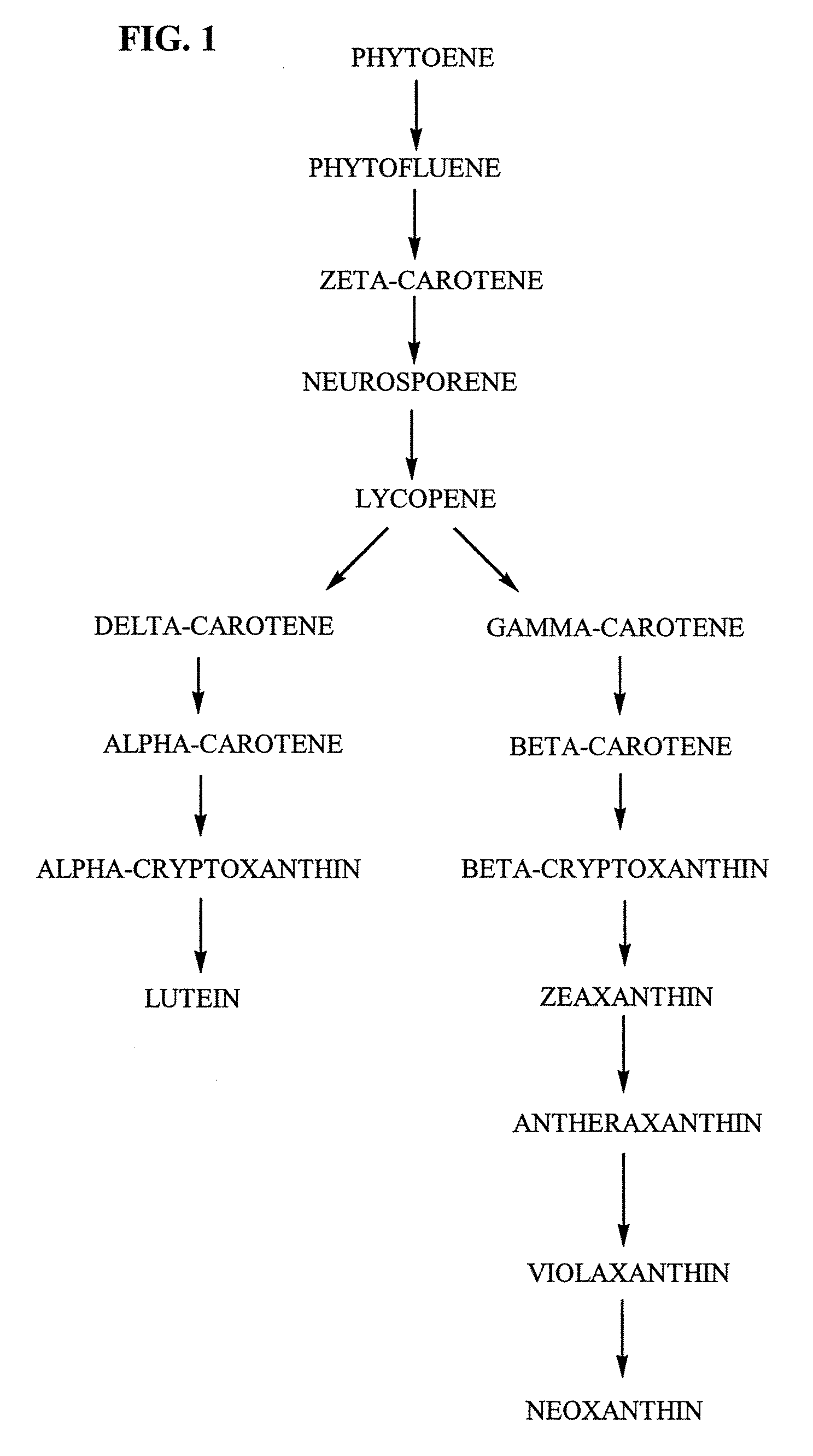
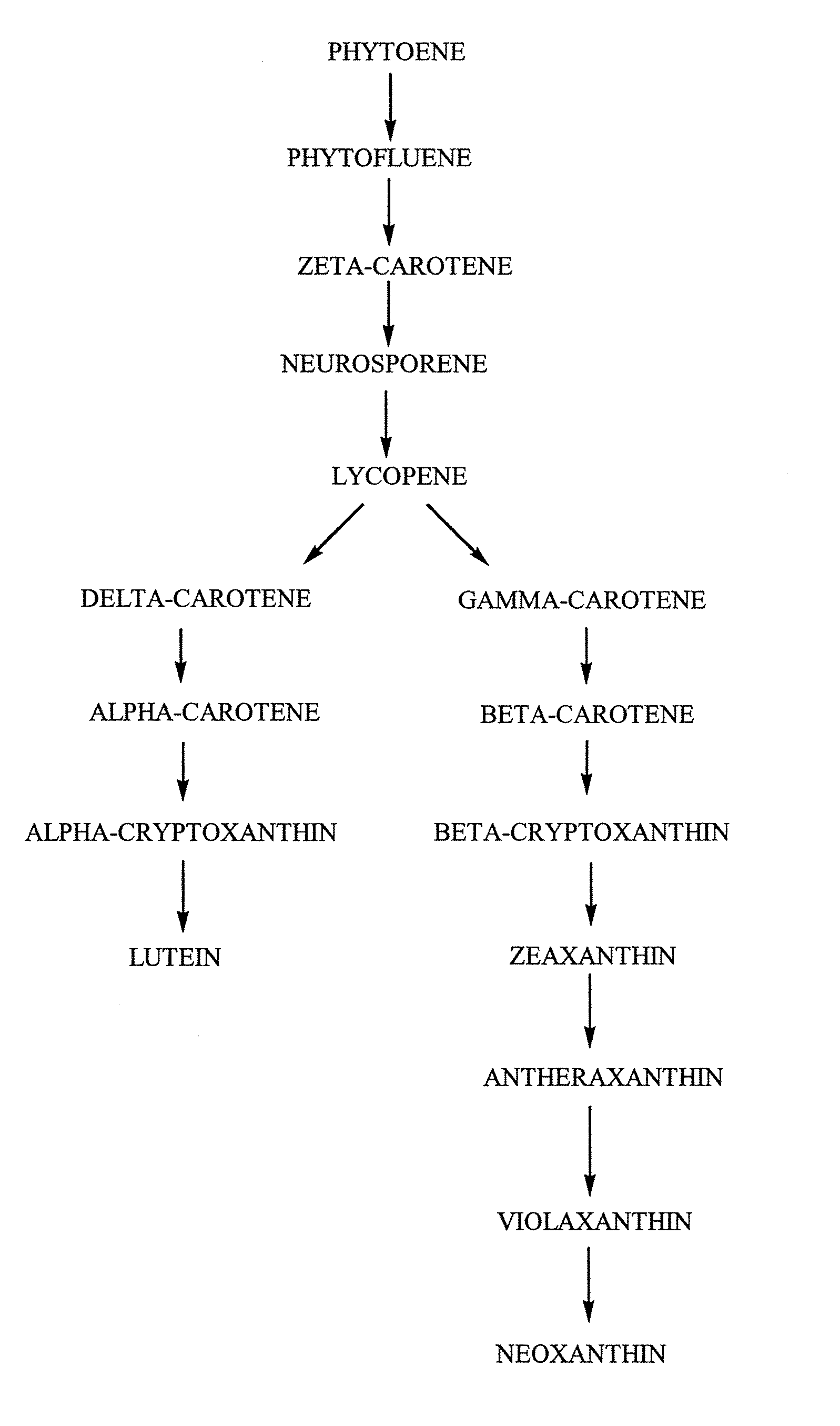
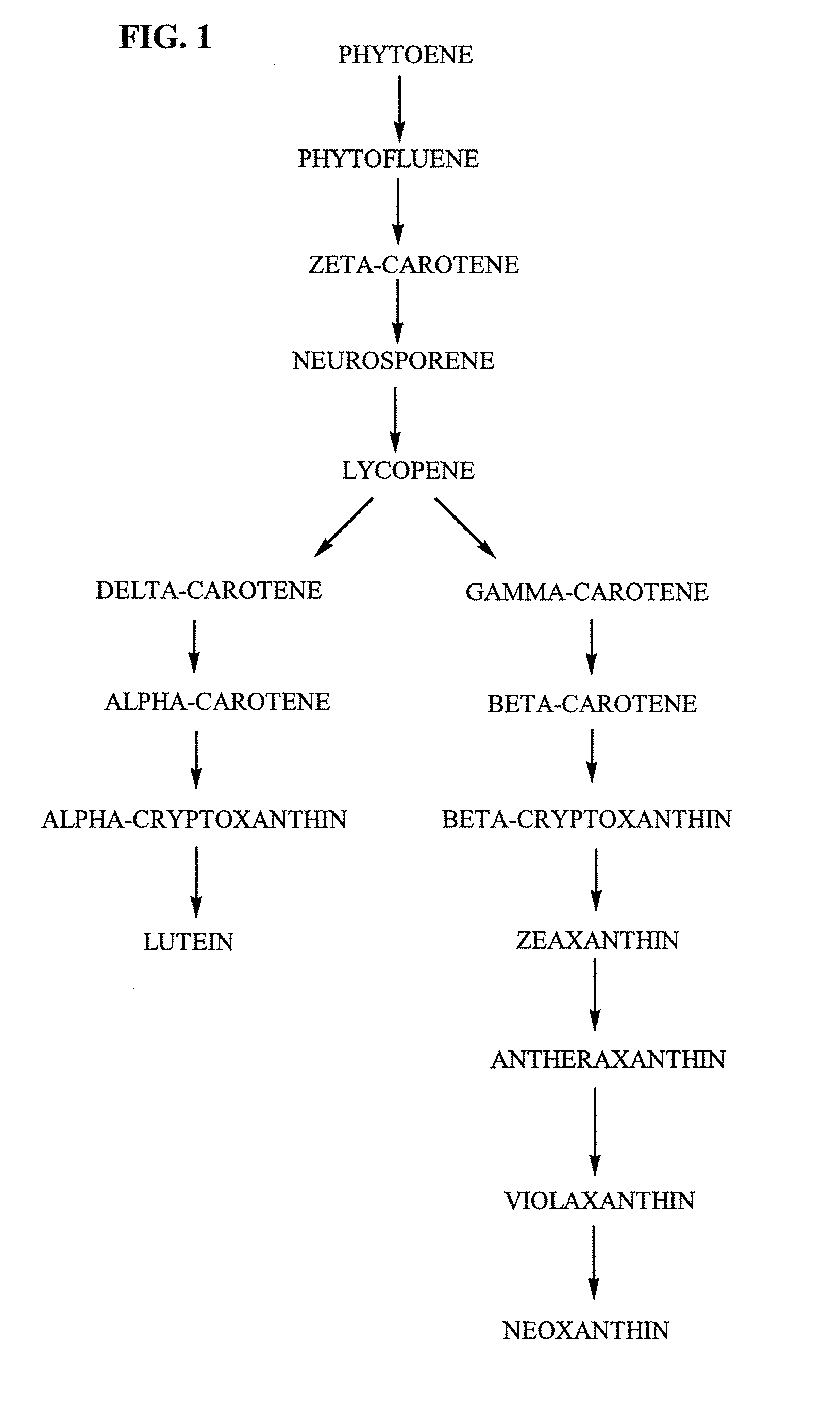
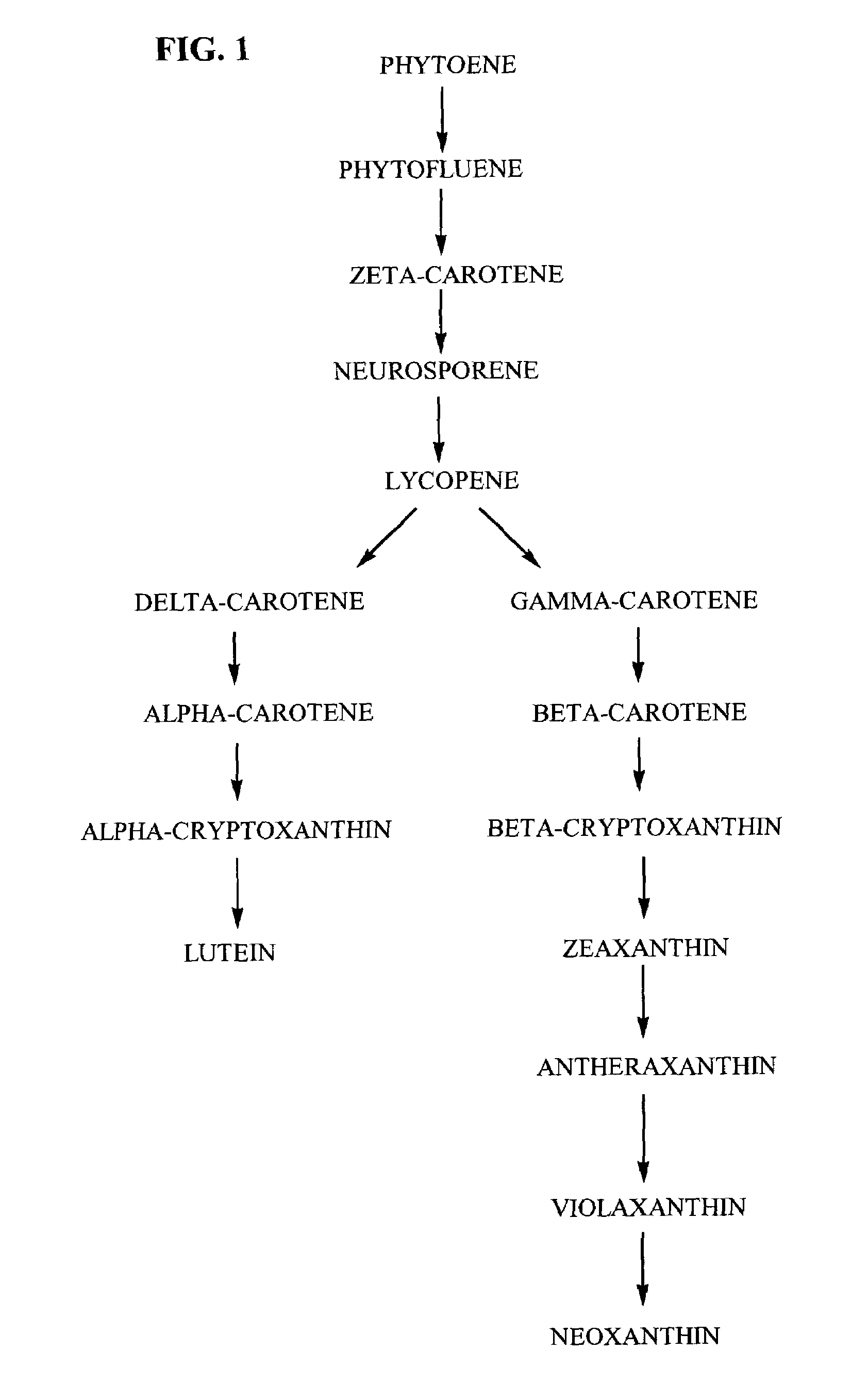
Tagetes erecta MARIGOLDS WITH ALTERED CAROTENOID COMPOSITIONS AND RATIOS
InactiveUS20060265784A1Raise the ratioOther foreign material introduction processesFermentationAlcoholPetal
A marigold plant, a regenerable portion thereof and seed are disclosed whose flower petals, leaves or flower petals and leaves contain one or more of an enhanced neoxanthin plus violaxanthin ratio, an enhanced β-carotene ratio, an enhanced lycopene ratio, an enhanced α-cryptoxanthin ratio, an enhanced phytoene ratio or an enhanced phytofluene ratio relative to that ratio in a non-mutant marigold. A marigold plant, a regenerable portion thereof and seed are also disclosed whose flower petals contain zeaxanthin esters and are substantially free of esters of both neoxanthin and violaxanthin, and wherein zeaxanthin constitutes at least about one-half of the extractable carotenoids when xanthophylls are assayed as alcohols. Also disclosed are methods of preparing such plants, oleoresins and comestible materials that have such carotenoid ratios.
Owner:HAUPTMANN RANDAL +2
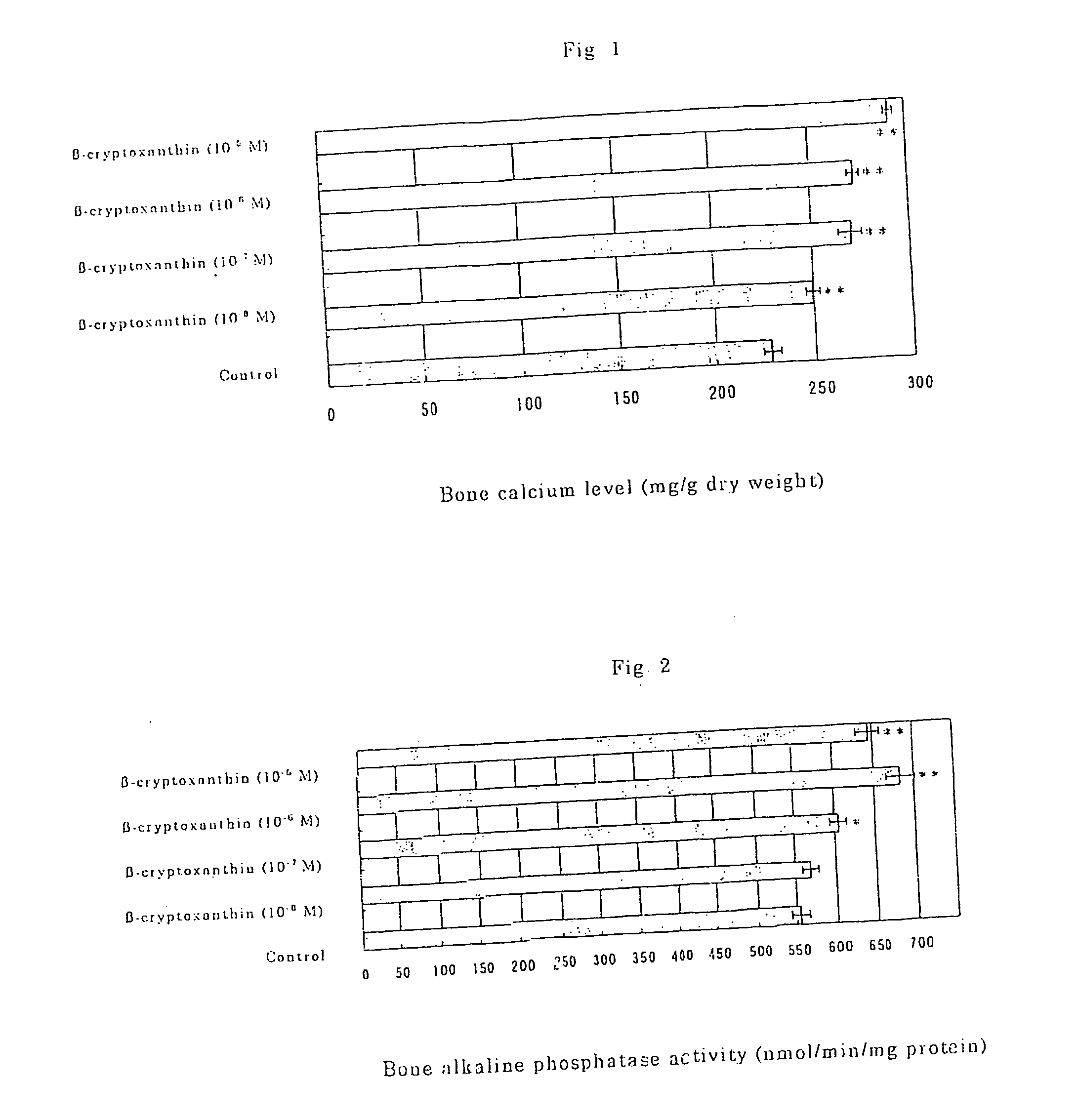
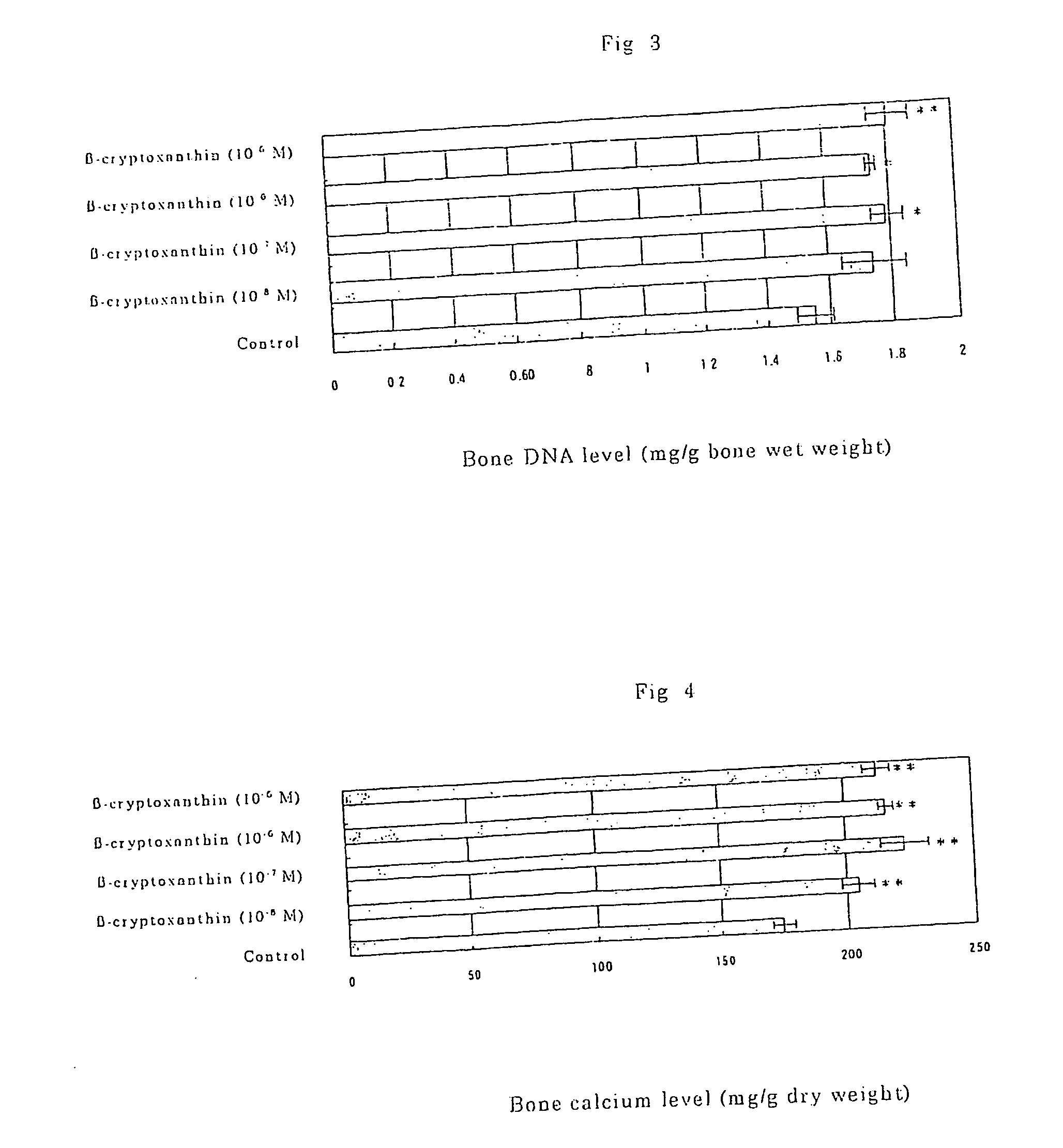
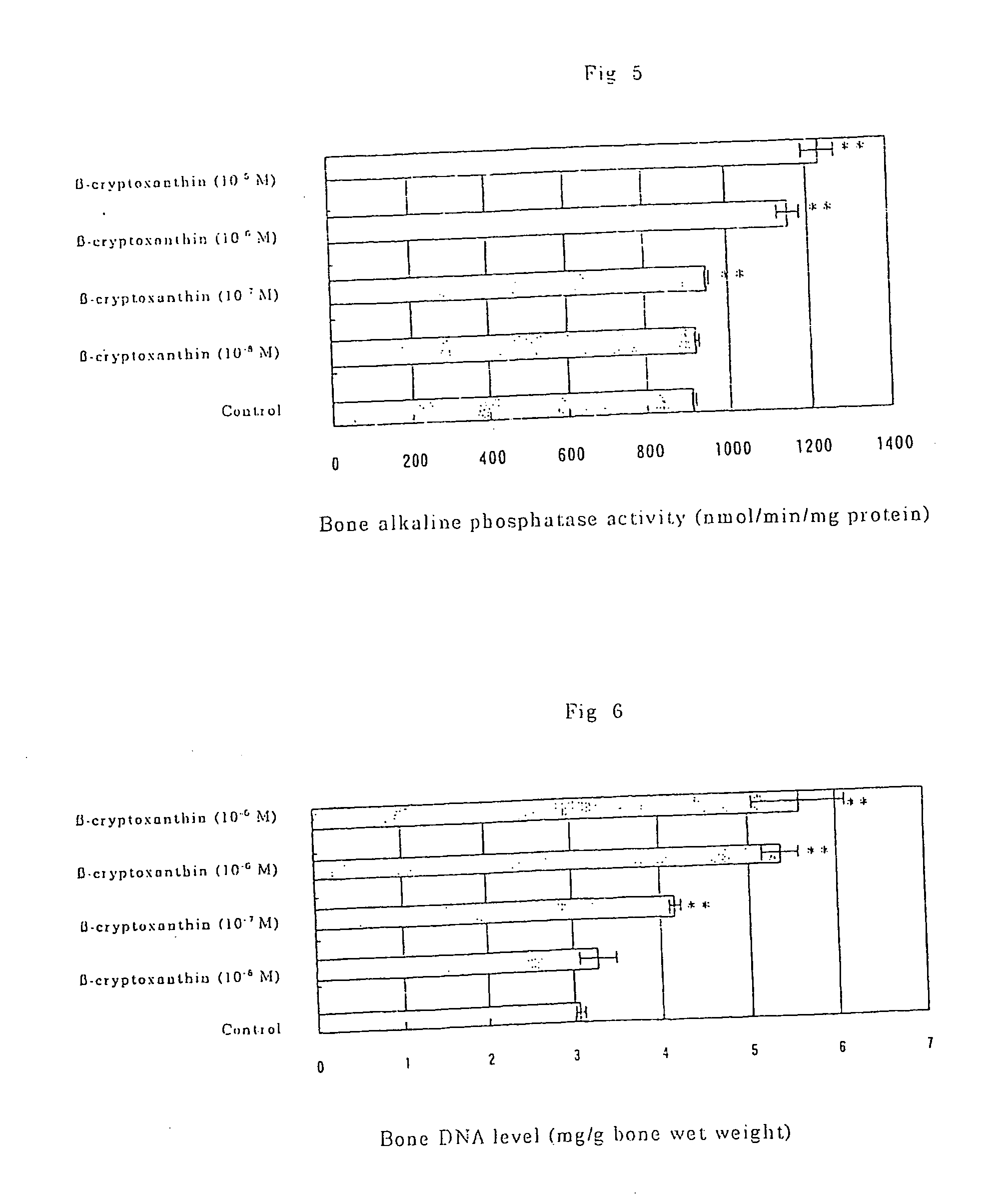
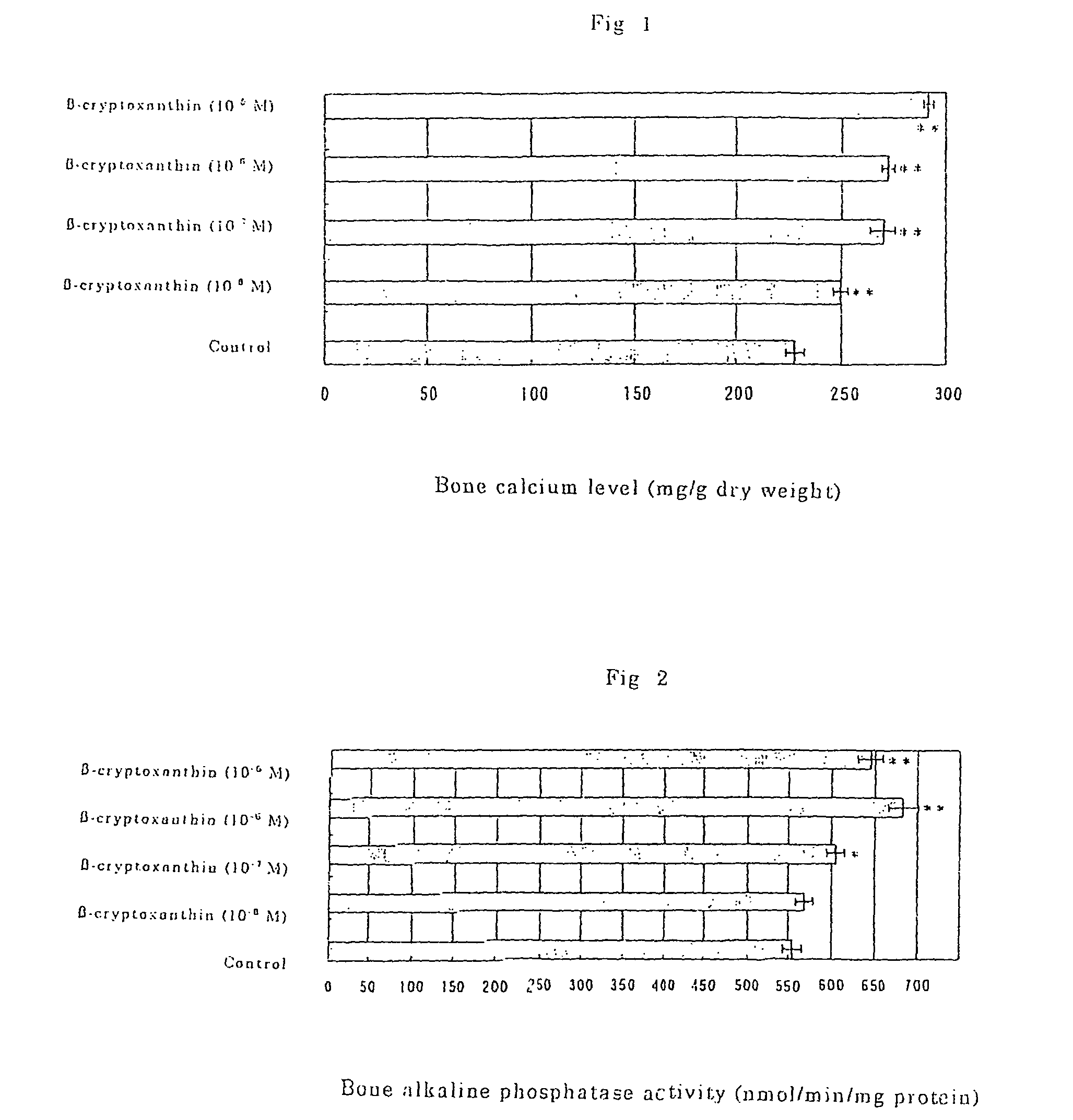
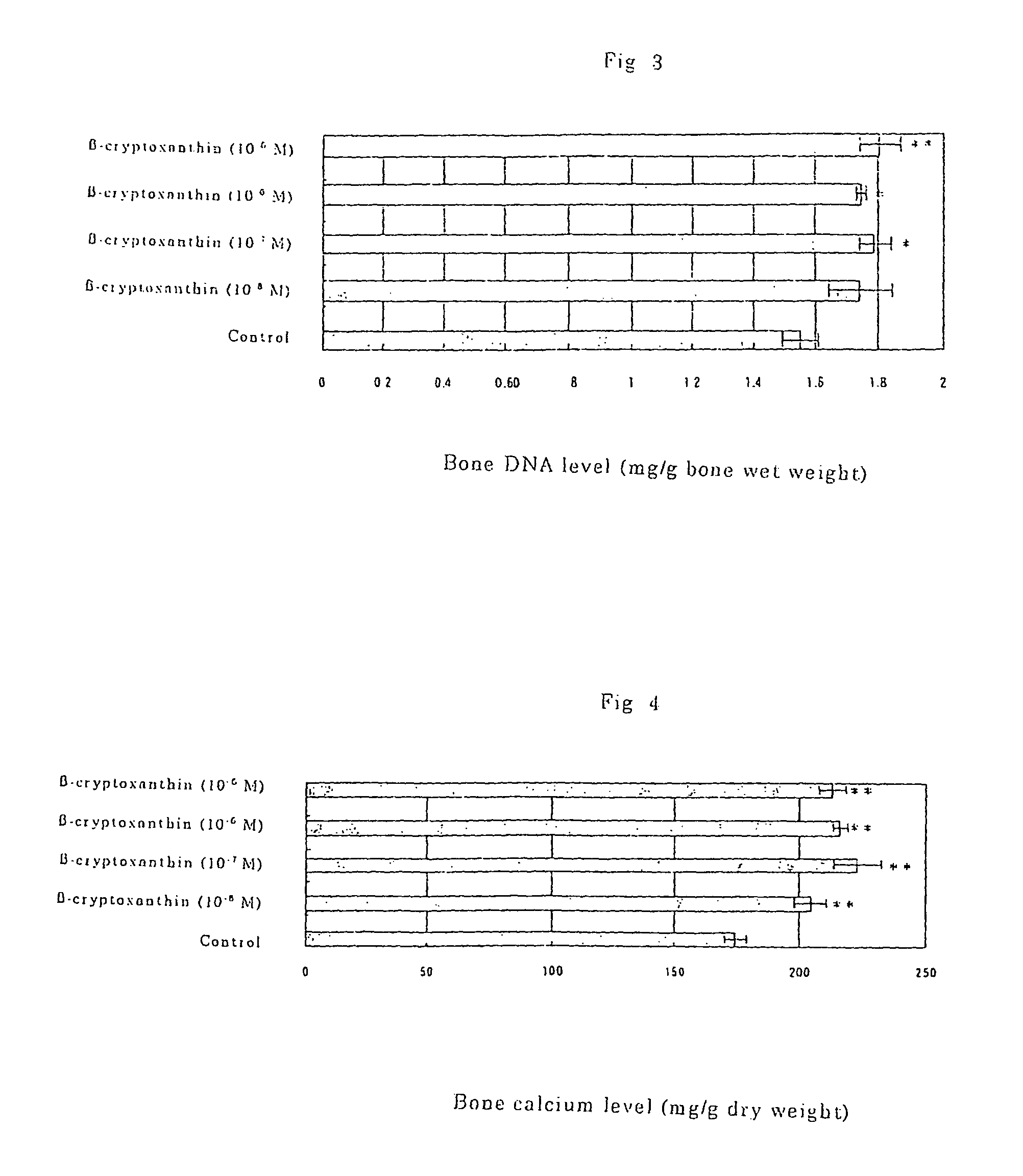
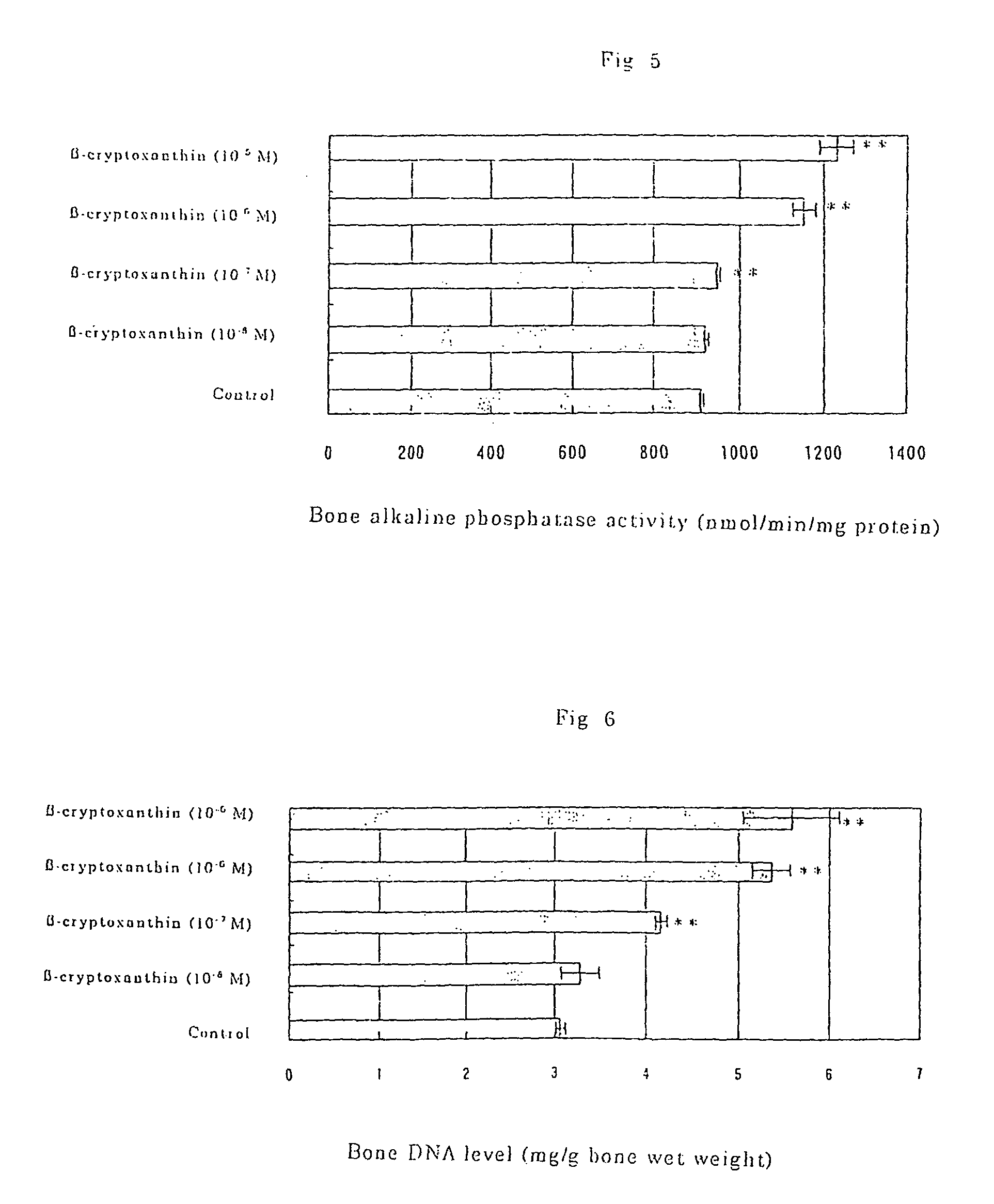
Osteogenesis promoter containing beta-cryptoxanthin as the active ingredient
ActiveUS20060106115A1Preventing/treating bone diseaseIncreasing protein synthesisBiocideHydroxy compound active ingredientsTreatment effectBeta-cryptoxanthin
Owner:BANK OF AMERICA N A
Extract containing beta-cryptoxanthin component from persimmon fruit
The present invention provides an extract containing a β-cryptoxanthin component from a persimmon fruit, a functional food and / or drink supplemented with the extract, and a process for producing the extract. The present invention produces the extract containing a β-cryptoxanthin component from a persimmon fruit by cutting an inexpensive raw material persimmon fruit, especially a persimmon skin, into fine pieces and extracting a β-cryptoxanthin component with an organic solvent such as ethanol. After the β-cryptoxanthin component is extracted with the organic solvent, it is preferred to eliminate the smell of dried persimmon by removing the organic solvent under reduced pressure. According to the present invention, an extract containing a β-cryptoxanthin component that does not raise consumers' anxiety even when added to foods, drinks, and the like can be produced at low cost.
Owner:TOYO SEIKAN KAISHA LTD
Isolation and Purification of Carotenoids from Marigold Flowers
ActiveUS20080051591A1Simple procedureLow amountOrganic chemistryFatty-oils/fats productionHigh concentrationFiltration
The present invention explains a realistic and effective process for isolating and purifying carotenoids containing higher concentrations of carotenoids such as trans-lutein, trans-zeaxanthin, Cis-lutein, β-carotene and Cryptoxanthin from Marigold flower petals under controlled conditions leaving no traces of any organic hazardous solvents. The process involves ensilaging Marigold flowers, dehydration, solvent extraction, alkali hydrolysis of carotenoid esters with absolute alcohol, crystallization / purification using water, absolute alcohol mixture followed by filtration and drying until the crystals are considerably free from moisture and absolutely free from residual hazardous solvents. These crystals are suitable for nutraceutical and food products as supplements.
Owner:KATRA PHYTOCHEM PRIVATE
Tagetes erecta marigolds with altered carotenoid compositions and ratios
A marigold plant, a regenerable portion thereof and seed are disclosed whose flower petals, leaves or flower petals and leaves contain one or more of an enhanced neoxanthin plus violaxanthin ratio, an enhanced β-carotene ratio, an enhanced lycopene ratio, an enhanced α-cryptoxanthin ratio, an enhanced phytoene ratio or an enhanced phytofluene ratio relative to that ratio in a non-mutant marigold. Also disclosed are methods of preparing such plants, oleoresins and comestible materials that have such carotenoid ratios.
Owner:BALL HORTICULTURAL
Extraction method for concentrate containing beta-cryptoxanthin, concentrate obtained by the method, and usage of the obtained concentrate
ActiveCN102219721AAbundant resourcesSolve the technical defect of low extraction efficiency of β-cryptoxanthinOrganic chemistryAnimal feeding stuffBeta-cryptoxanthinPre treatment
The invention relates to a method for extracting concentrate containing beta-cryptoxanthin from persistent calyx of Physalis alkekengi L. var. franchetii (Masters)Makino, the concentrate containing beta-cryptoxanthin obtained by the method, and the usage of the concentrate. The method comprises the steps of pretreatment, enzyme treatment, extraction, saponification, condensation and purification of raw materials. According to the method provided in the invention, the technical defect in the prior art that extraction efficiency of beta-cryptoxanthin is low is overcome; a beta-cryptoxanthin product with great value is obtained; and the content of beta-cryptoxanthin in the product provided in the invention is far more than the content of beta-cryptoxanthin in the product obtained in the prior art, thereby enabling a good prospect for the application of beta-cryptoxanthin products. The raw materials used in the method are easily available, the extraction process is simple, the content of beta-cryptoxanthin in the obtained product is high, and comprehensive cost for the extraction is controllable; therefore, the method is suitable for industrial production and can be used for lengthening manufacturing chain.
Owner:QINHUANGDAO DAHUI BIOLOGICAL TECH
Vision care drug
InactiveCN103070877APromote blood circulationFree from harmSenses disorderHydroxy compound active ingredientsLuteinUltraviolet lights
The invention discloses a vision care drug that can improve ocular region blood circulation, protects retina from the harm of visible light and ultraviolet light, can effectively protect the eyes, prevents and reduces the generating of myopia, hyperopia and amblyopia, and is a vision care drug used conveniently. The drug comprises the following components in weigh: 1-75 mg of crocin, 1-50 mg of lutein, 1-50 mg of resveratrol, 1-50 mg of cryptoxanthin and 1-50 mg of Beta xanthophyll. The vision care drug can be applied to the field of vision care.
Owner:陈阳
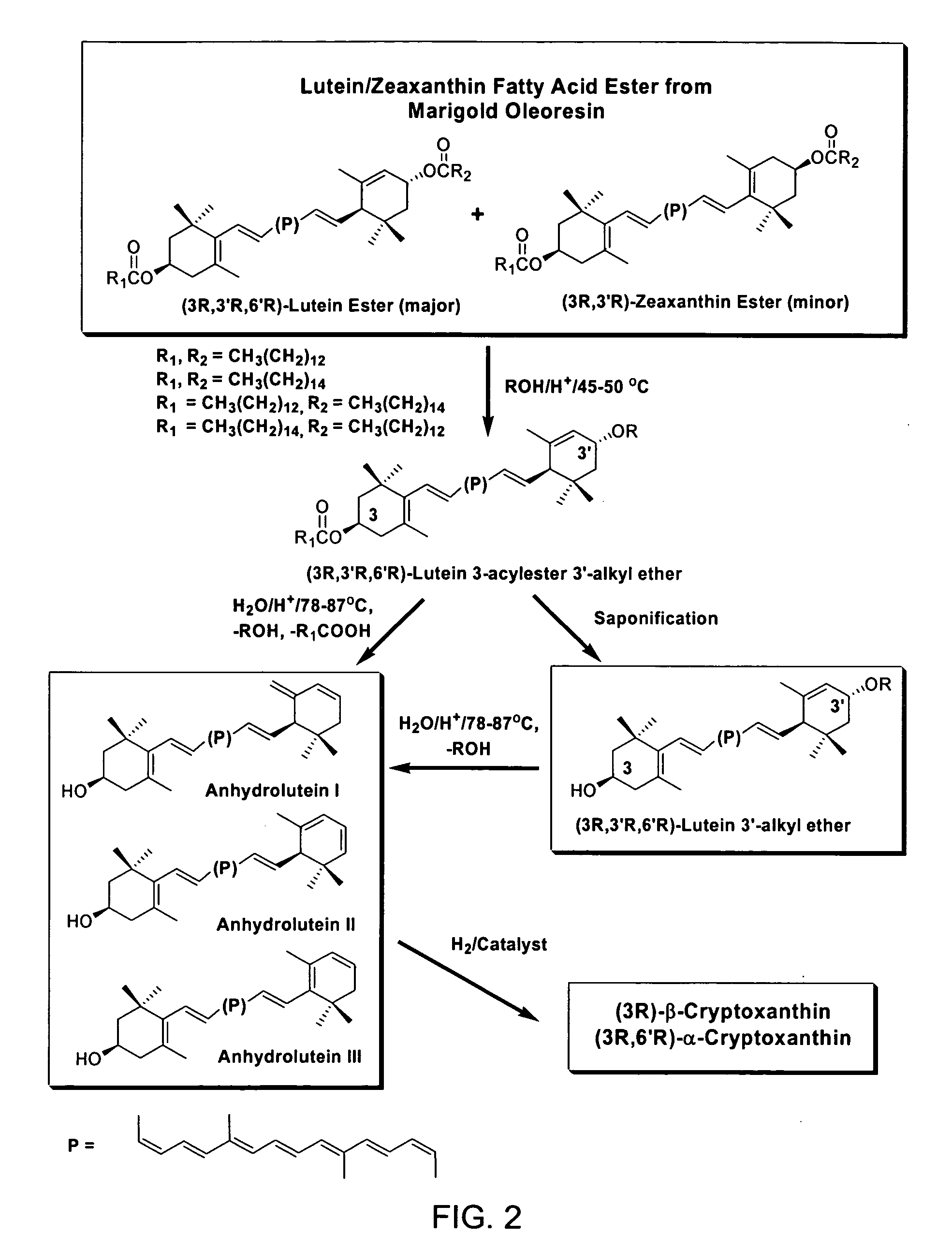
Process for the preparation of alpha- and beta-cryptoxanthin
InactiveUS20060088631A1Easy to useHigh selectivityBeer brewingFood preparationDietary supplementCryptoxanthin
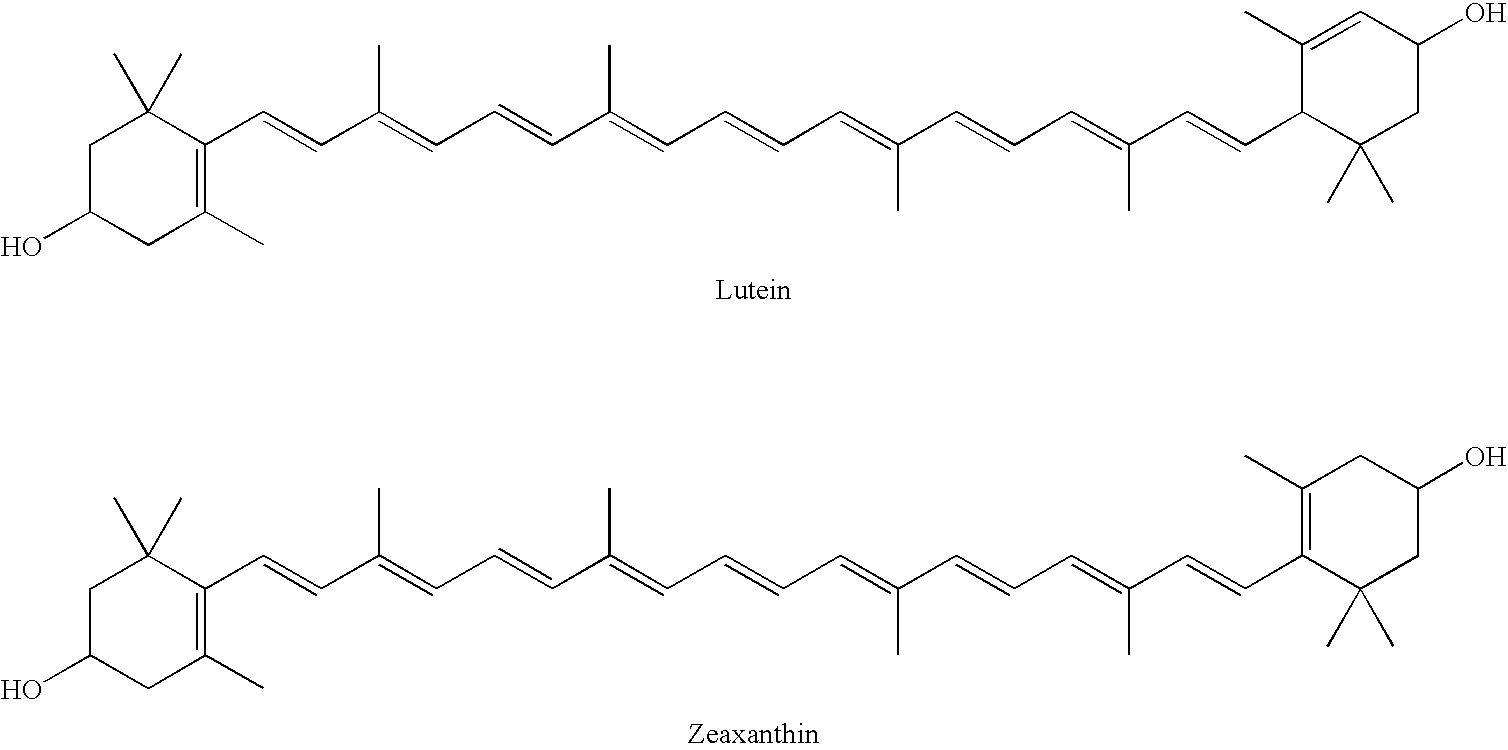
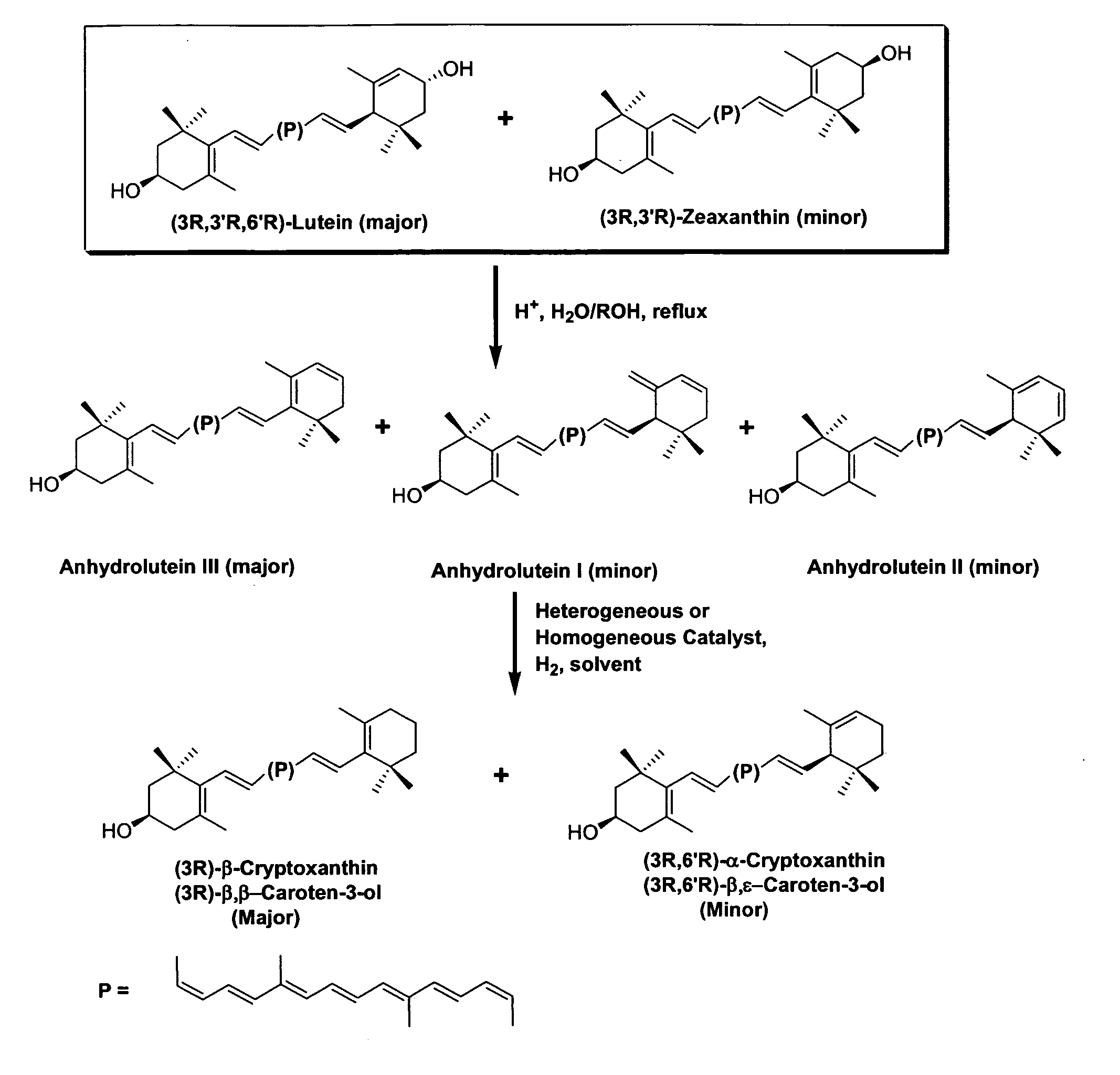
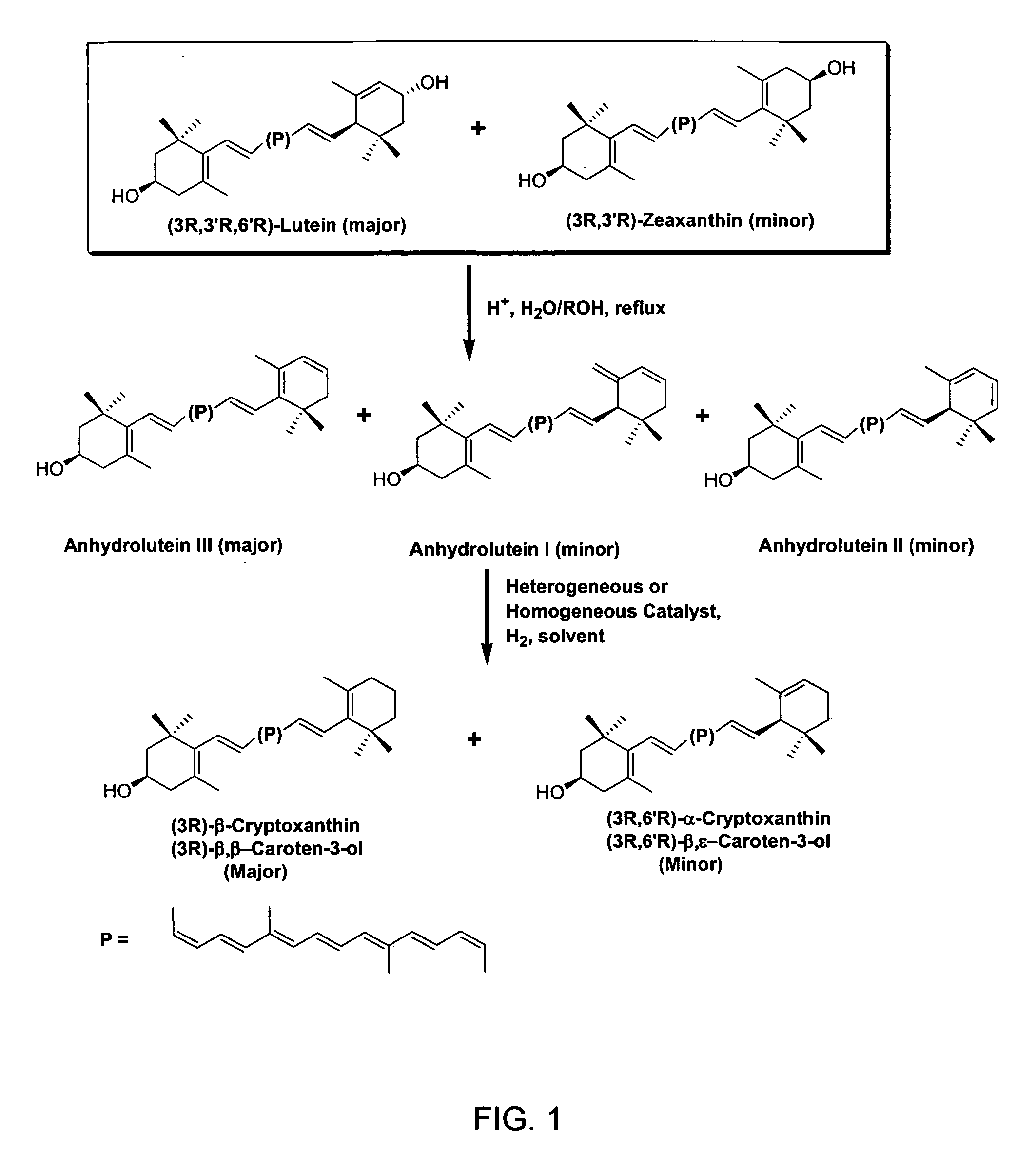
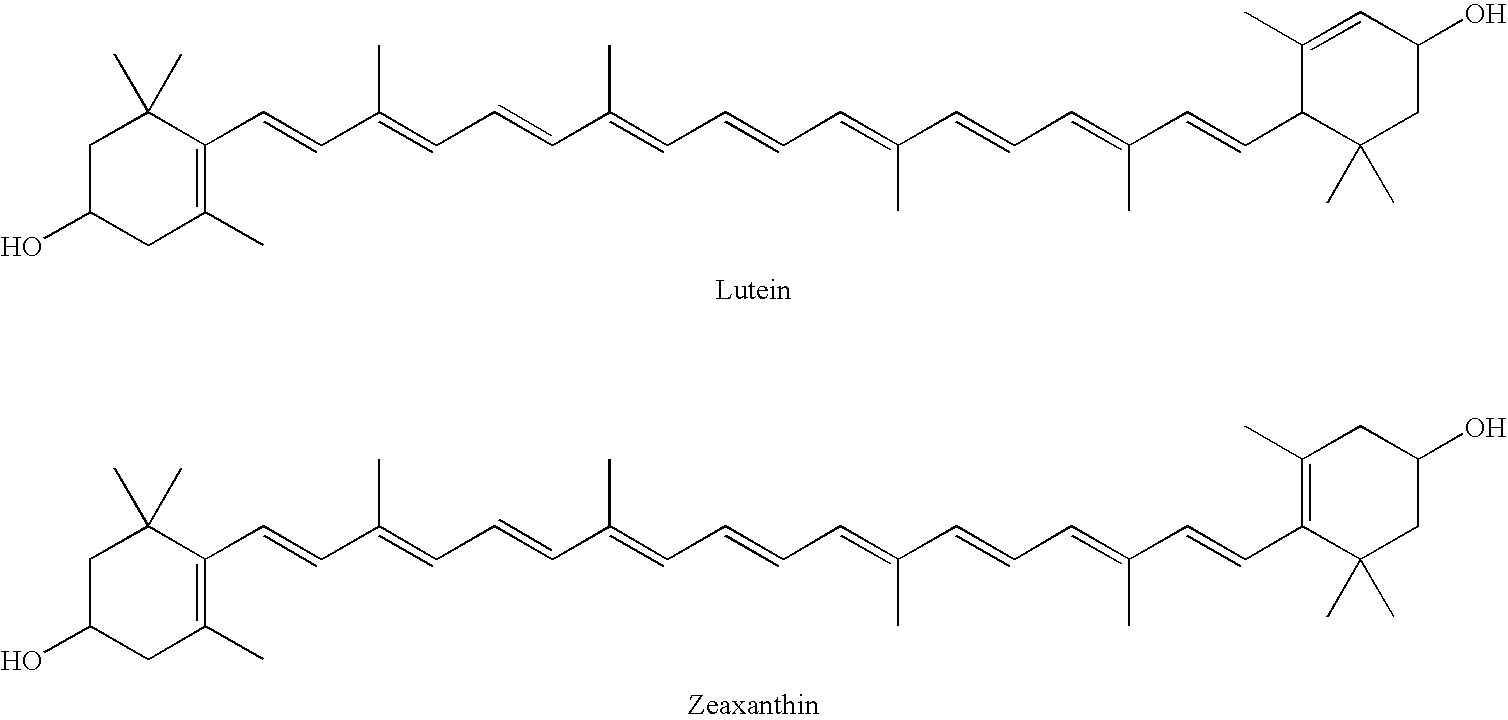
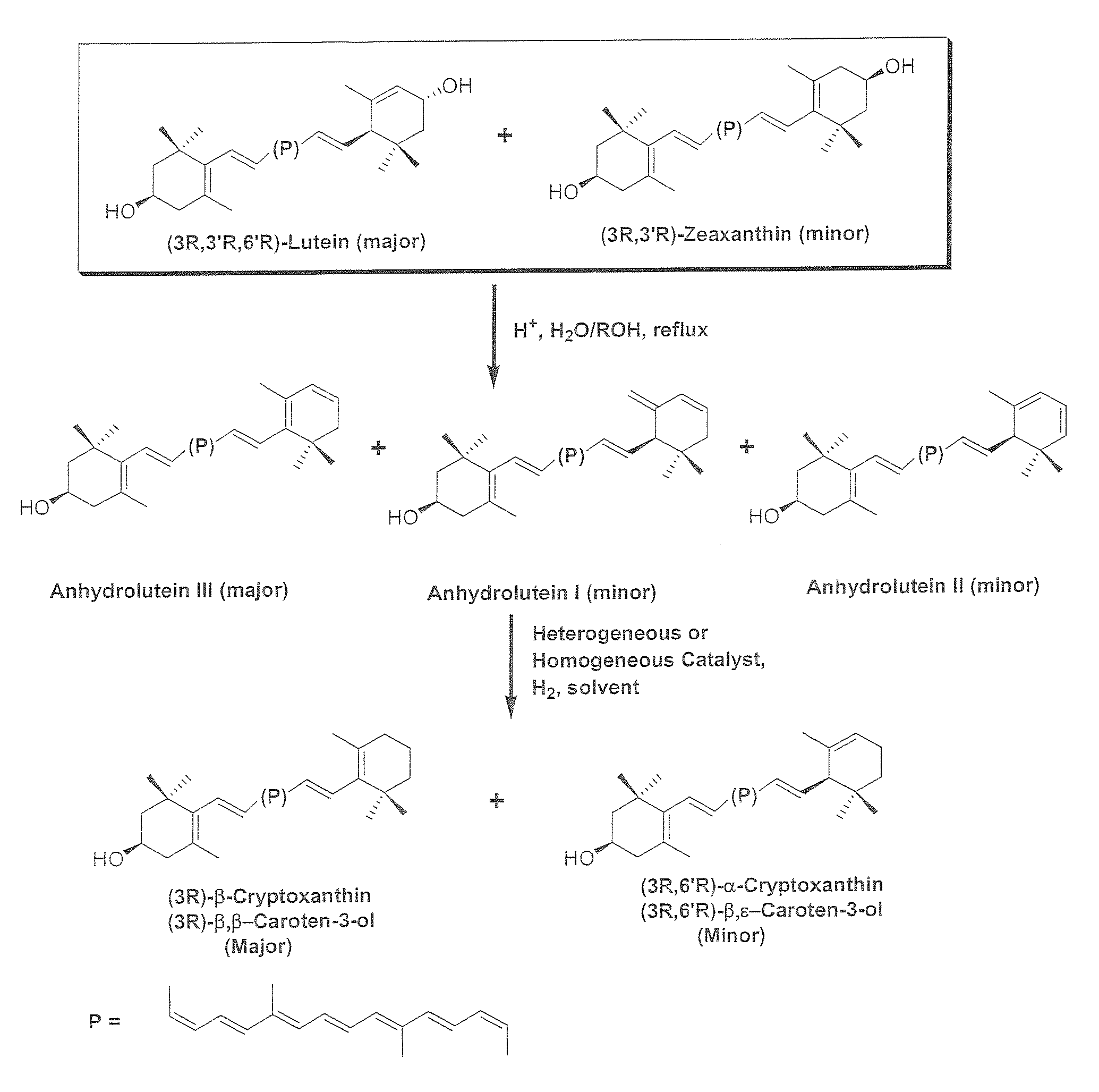
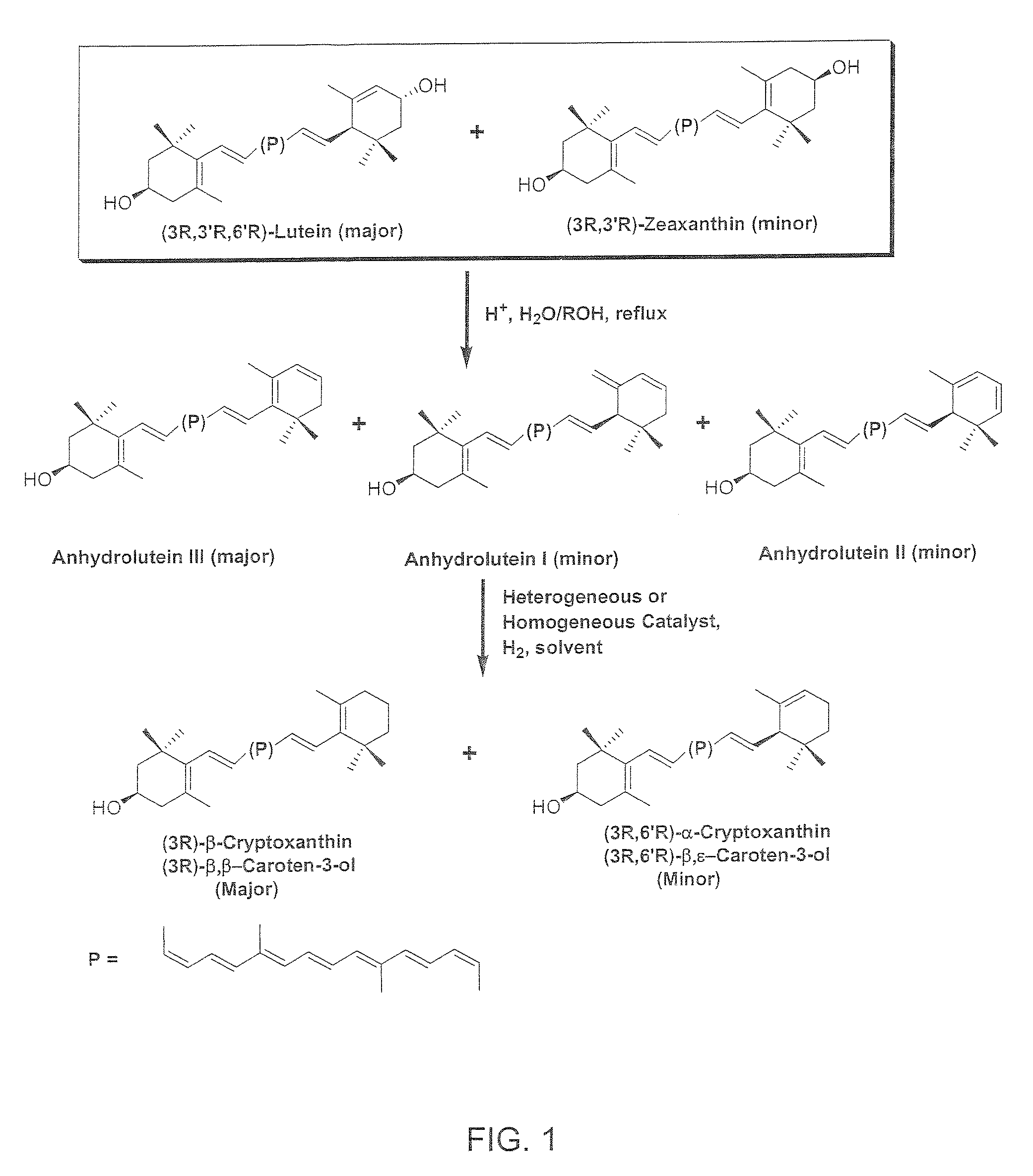
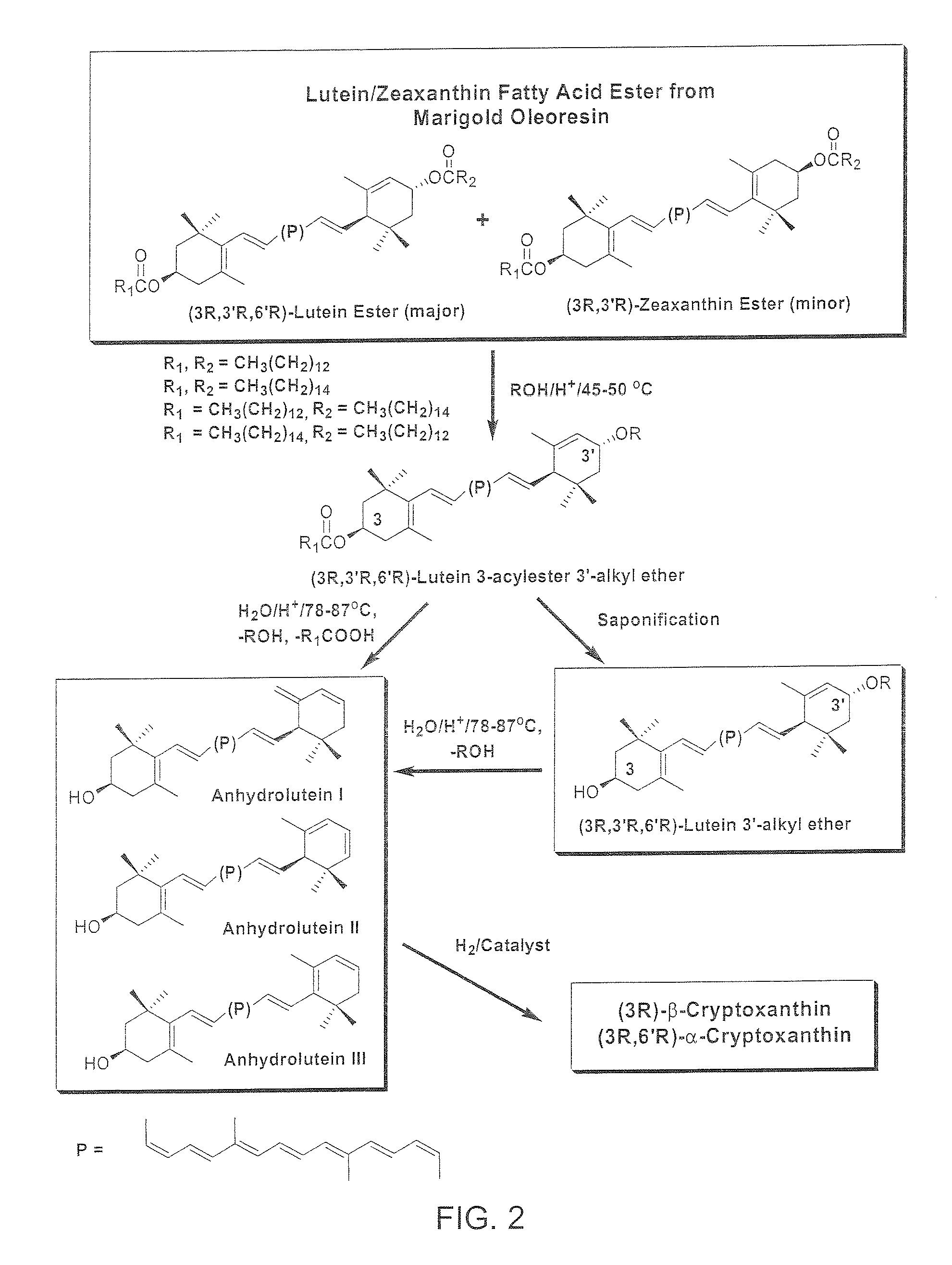
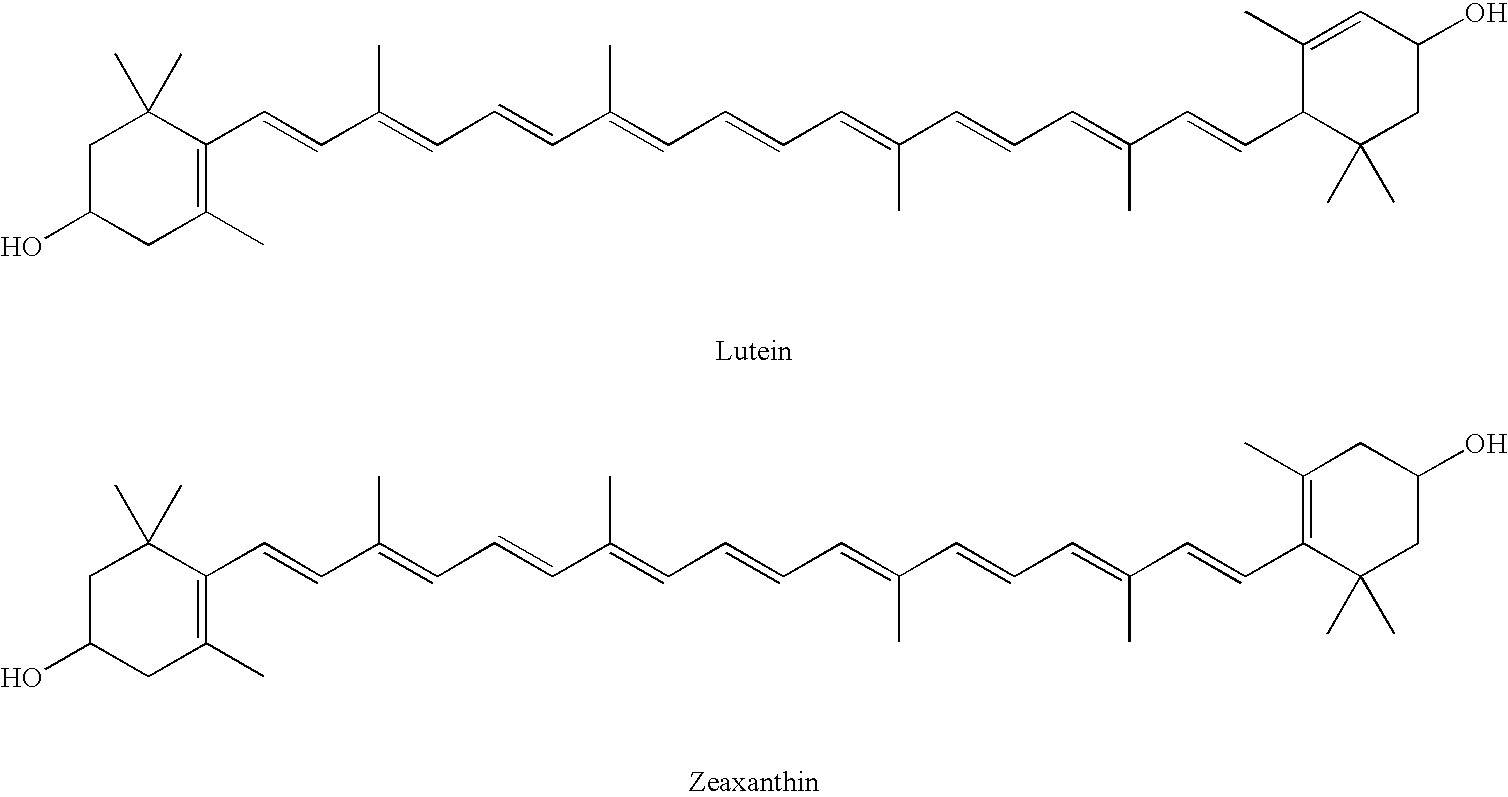
The present invention relates to a process for converting lutein and / or lutein esters to (3R)-β-cryptoxanthin and (3R,6′R)-α-cryptoxanthin, suitable for human consumption as dietary supplements, by employing safe and environmentally friendly reagents. (3R)-β-Cryptoxanthin and (3R,6′R)-α-cryptoxanthin are two rare food carotenoids that are not commercially available and the former exhibits vitamin A activity. In the first synthetic step, commercially available lutein and / or lutein esters are transformed into a mixture of dehydration products of lutein (anhydroluteins) in the presence of a catalytic amount of an acid. The resulting anhydroluteins are then converted to (3R)-β-cryptoxanthin (major product) and (3R,6′R)-α-cryptoxanthin (minor product) by heterogeneous catalytic hydrogenation employing transition elements of group VIII (Pt, Pd, Rh supported on alumina or carbon) in a variety of organic solvents under atmospheric pressure of hydrogen and at temperatures ranging from −15° C. to 40° C. Among these catalysts, Pt supported on alumina at 40° C. in ethyl acetate provides the best yield of (3R)-β-cryptoxanthin and (3R,6′R)-α-cryptoxanthin. Several homogeneous catalysts can also promote the regioselective hydrogenation of anhydroluteins to a mixture of (3R)-β-cryptoxanthin and (3R,6′R)-α-cryptoxanthin in low to moderate yields. The catalysts may be transition metal complexes such as palladium acetylacetonate, Rh(Ph3P)3Cl (Wilkinson's catalyst), [(C6H11)3P[C8H12][C5H5N]Ir+PF6− (Crabtree catalyst), or [C8H12][(MePh2P)2]Ir+PF6−. Among these, Wilkinson catalyst converts anhydroluteins to (3R)-β-cryptoxanthin and (3R,6′R)-α-cryptoxanthin in nearly quantitative yield. A novel feature of this invention is the regioselective hydrogenation of anhydroluteins while the highly conjugated polyene chain of these carotenoids remains intact.
Owner:UNIV OF MARYLAND
Oral administration composition
InactiveUS20100273727A1Reduce actionSimple designBiocideSugar food ingredientsCryptoxanthinSphingolipid metabolism
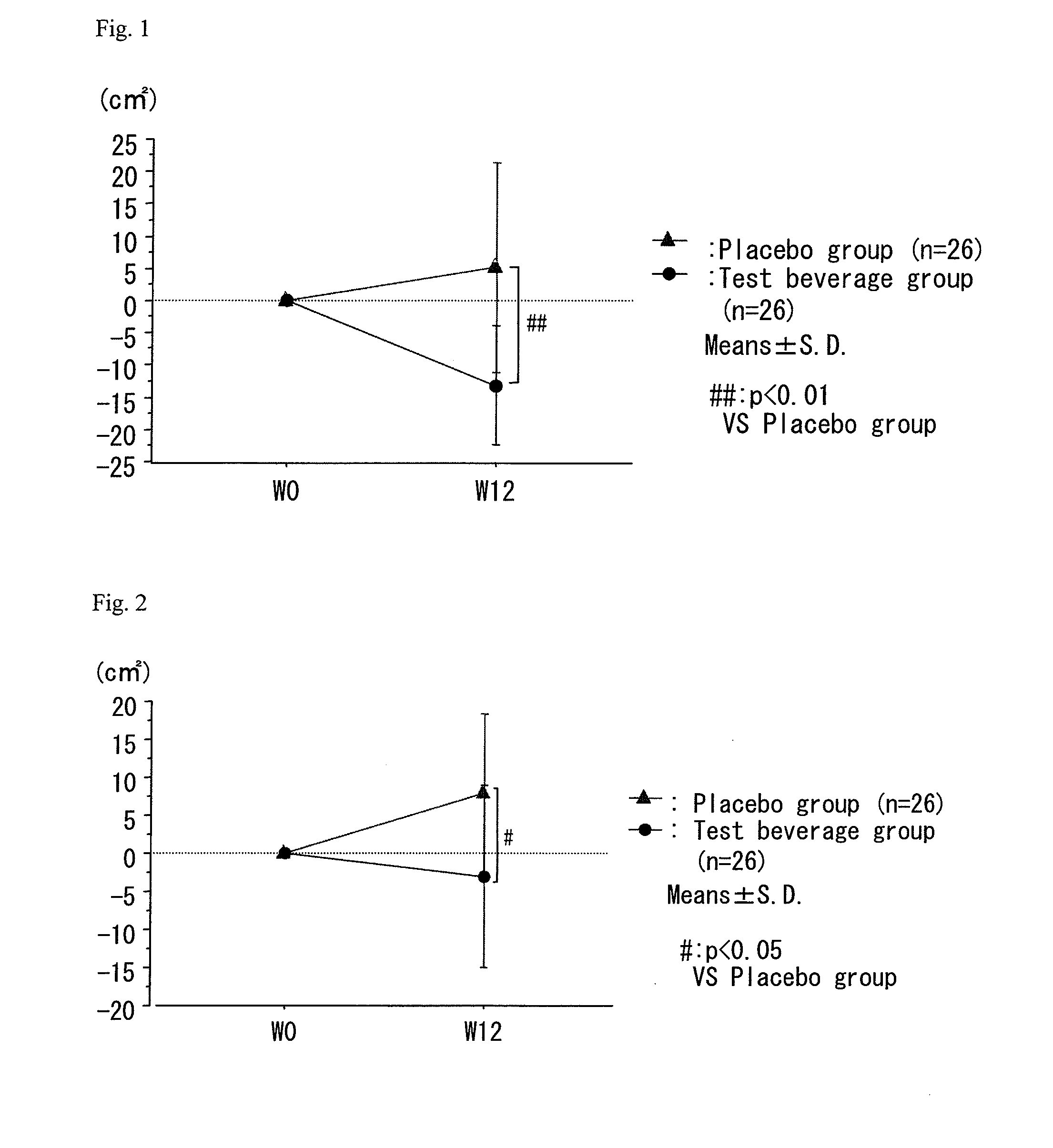
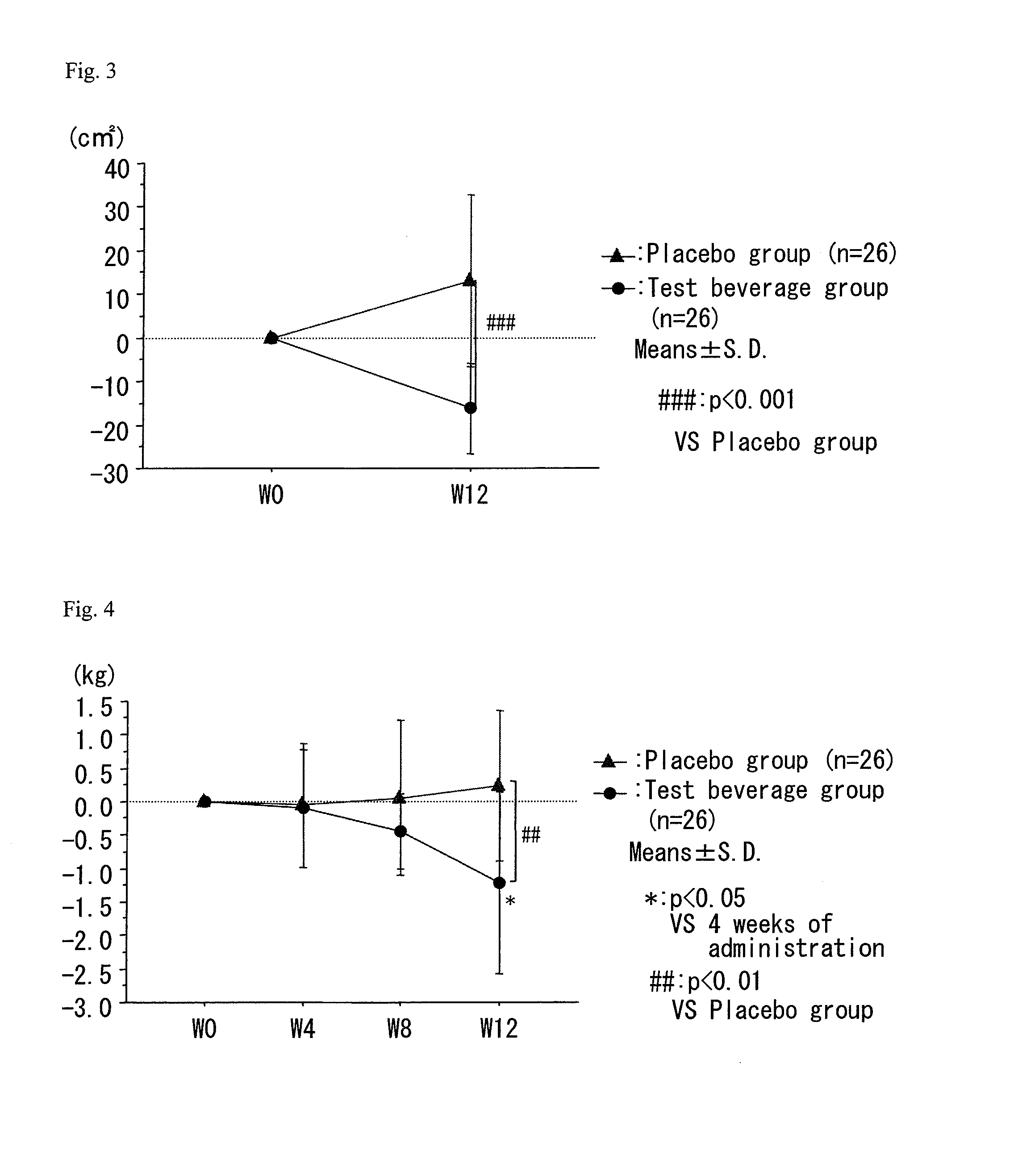
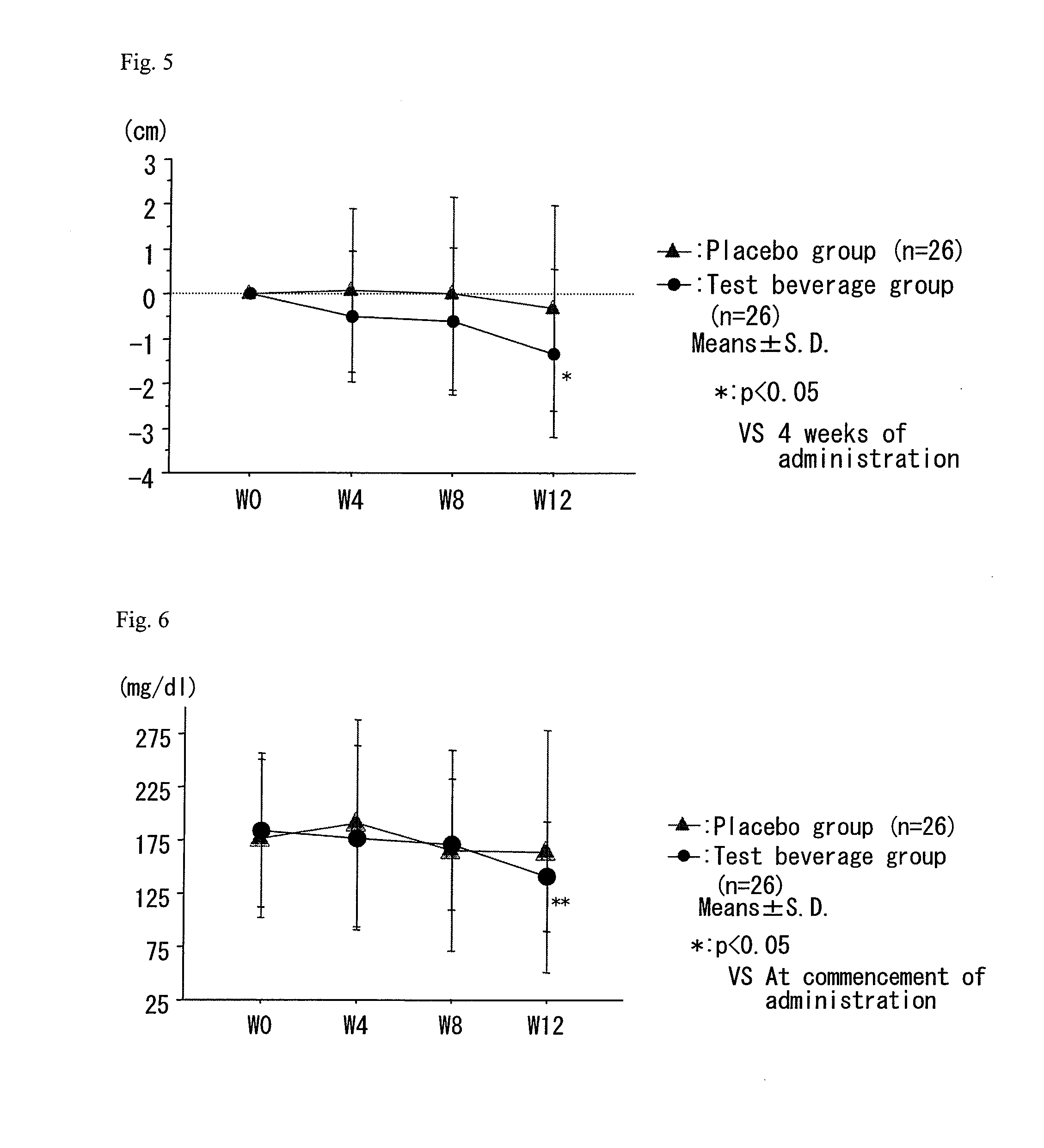
A composition which has high safety and is excellent in the effect of reducing body fat, such as visceral fat, subcutaneous fat and the like, and neutral fat, and a food, a pharmaceutical and a feed comprising the same, are provided.An oral administration composition having a body fat reducing action and / or a neutral fat reducing action, which is a composition comprising a carotenoid and a sphingolipid or a carotenoid and a flavonoid and / or a derivative thereof, preferably a composition comprising a cryptoxanthin and a sphingolipid or a cryptoxanthin and a flavonoid and / or a derivative thereof, wherein the cryptoxanthin and / or the sphingolipid and / or the flavonoid are derived from a citrus fruit and the citrus fruit is preferably Citrus unshiu.
Owner:UNITIKA LTD
Process for Synthesis of (3S)- and (3R)-3-Hydroxy-Beta-Ionone, and Their Transformation to Zeaxanthin and Beta-Cryptoxanthin
InactiveUS20090311761A1High yieldOrganic compound preparationCarbonyl compound preparationMetaboliteIsomerization
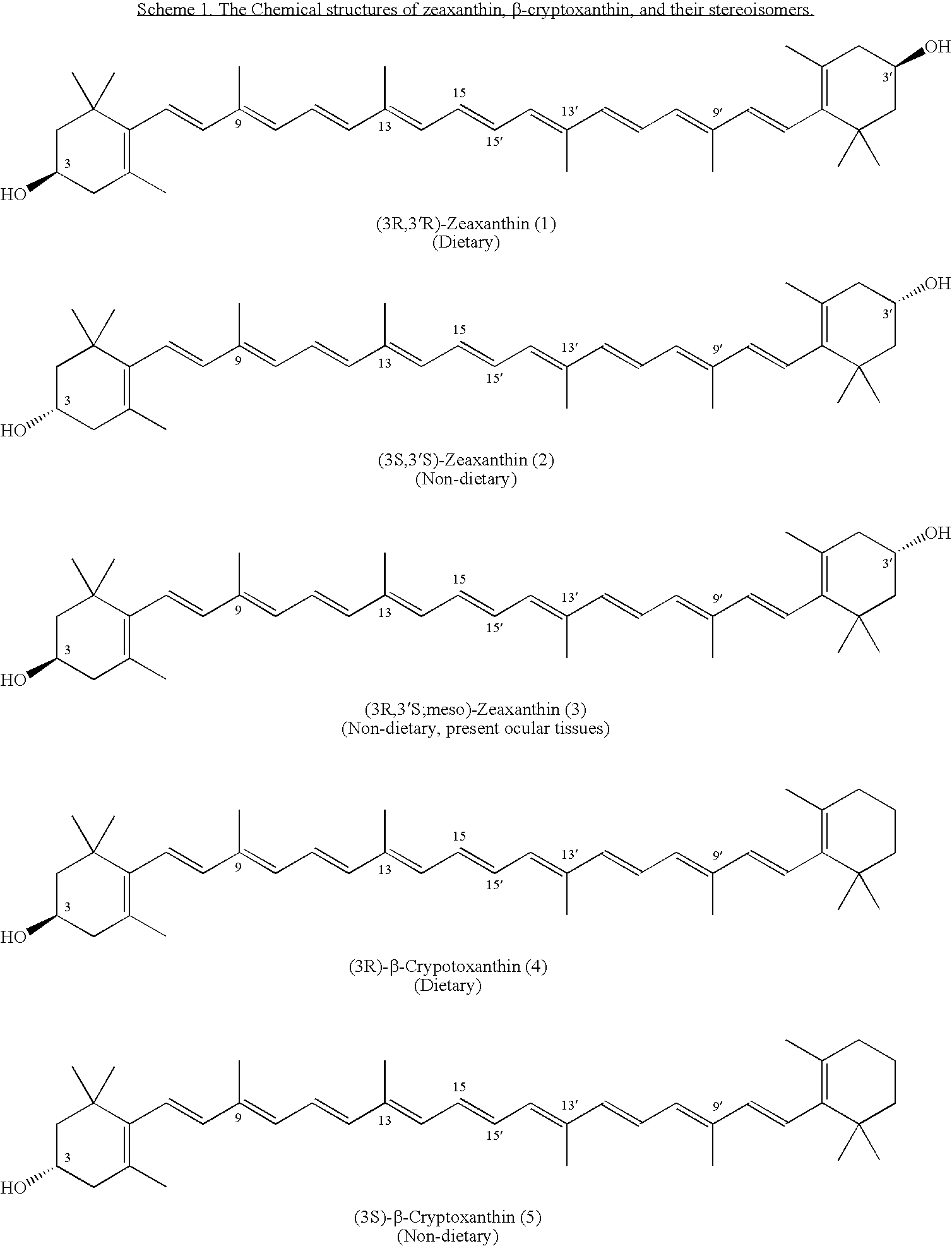
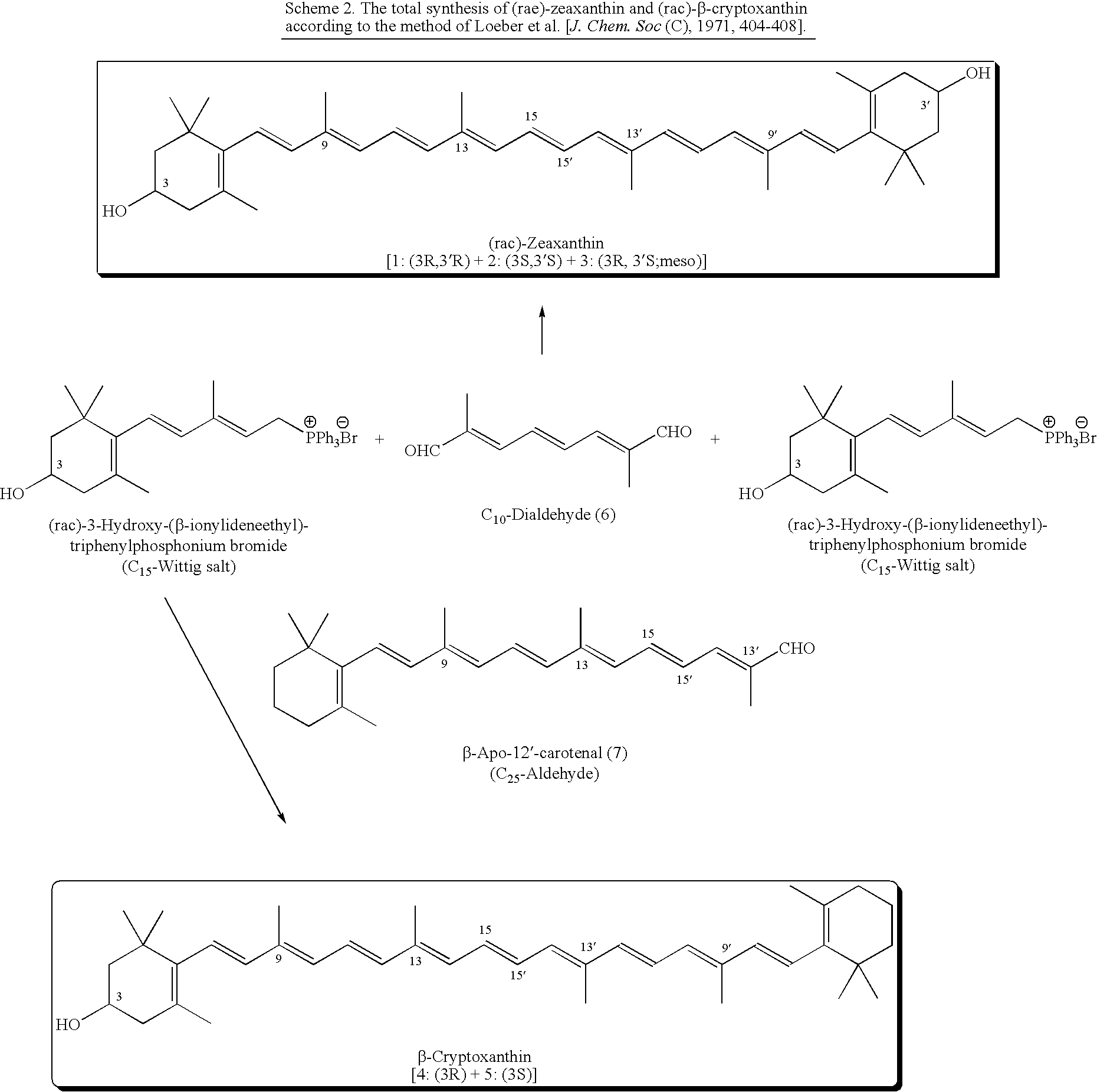
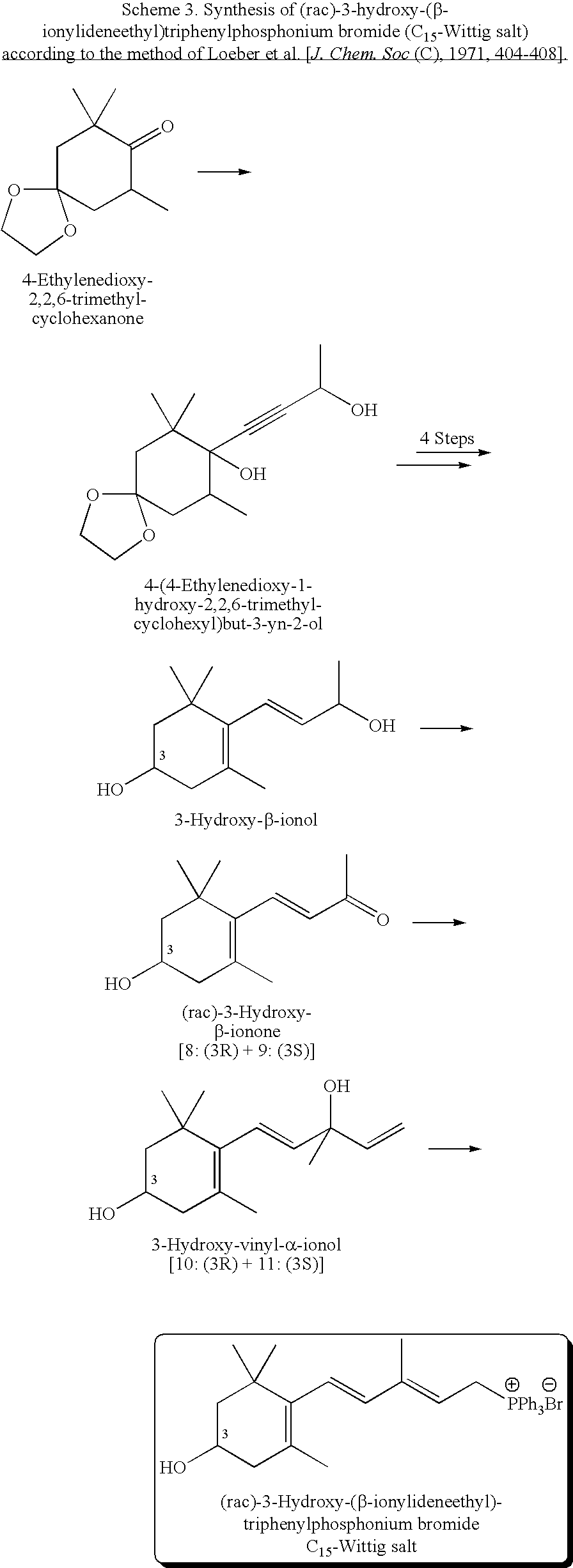
(3R)-3-Hydroxy-β-ionone and (3S)-3-hydroxy-β-ionone are two important intermediates in the synthesis of carotenoids with β-end group such as lutein, zeaxanthin, β-cryptoxanthin, and their stereoisomers. Among the various stereoisomers of these carotenoids, only (3R,3′R,6′R)-lutein, (3R,3′R)-zeaxanthin, and (3R)-β-cryptoxanthin are present in commonly consumed fruits and vegetables. There are 3 possible stereoisomers for zeaxanthin, these are: dietary (3R,3′R)-zeaxanthin (1), non-dietary (3S,3′S)-zeaxanthin (2), and non-dietary (3R,3′S;meso)-zeaxanthin (3) which is a presumed metabolite of dietary lutein. Dietary lutein as well as 1 and 3 are accumulated in the human macula and have been implicated in the prevention of age-related macular degeneration. (3R)-β-Cryptoxanthin (4) is also present in selected ocular tissues at a very low concentration whereas its enantiomer (3S)-β-cryptoxanthin (5) is absent in foods and human plasma.The present invention relates to a process for the synthesis of (3R)-3-hydroxy-β-ionone and its (3S)-enantiomer in high optical purity from commercially available (rac)-α-ionone. The key intermediate for the synthesis of these hydroxyionones is 3-keto-α-ionone ketal that was prepared from (rac)-α-ionone after protection of this ketone as a 1,3-dioxolane. Reduction of 3-keto-α-ionone ketal followed by deprotection, lead to 3-hydroxy-α-ionone that was transformed into (rac)-3-hydroxy-β-ionone by base-catalyzed double bond isomerization in 46% overall yield from (rac)-α-ionone. The racemic mixture of these hydroxyionones was then resolved by enzyme-mediated acylation in 96% ee. (3R)-3-Hydroxy-β-ionone and its (3S)-enantiomer were respectively transformed to (3R)-3-hydroxy-(β-ionylideneethyl)triphenylphosphonium chloride [(3R)—C15-Wittig salt] and its (3S)-enantiomer [(3S)—C15-Wittig salt] according to known procedures. Double Wittig condensation of these Wittig salts with commercially available 2,5-dimethylocta-2,4,6-triene-1,8-dial provided all 3 stereoisomers of zeaxanthin (1-3). Similarly, (3R)—C15-Wittig and its (3S)-enantiomer were each coupled with β-apo-12′-carotenal to yield 4 and 5.
Owner:UNIV OF MARYLAND
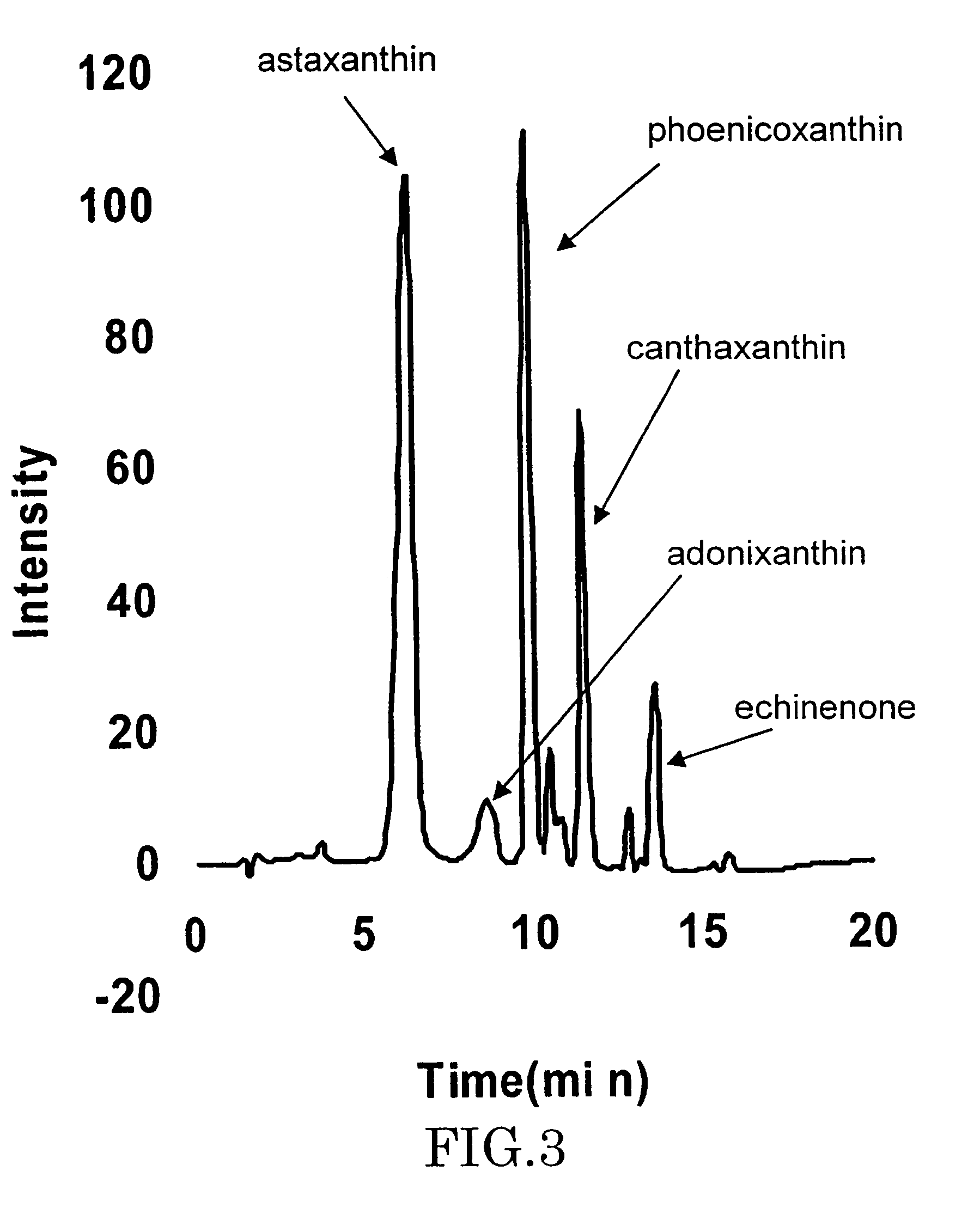
Astaxanthin production using a recombinant microbial host cell
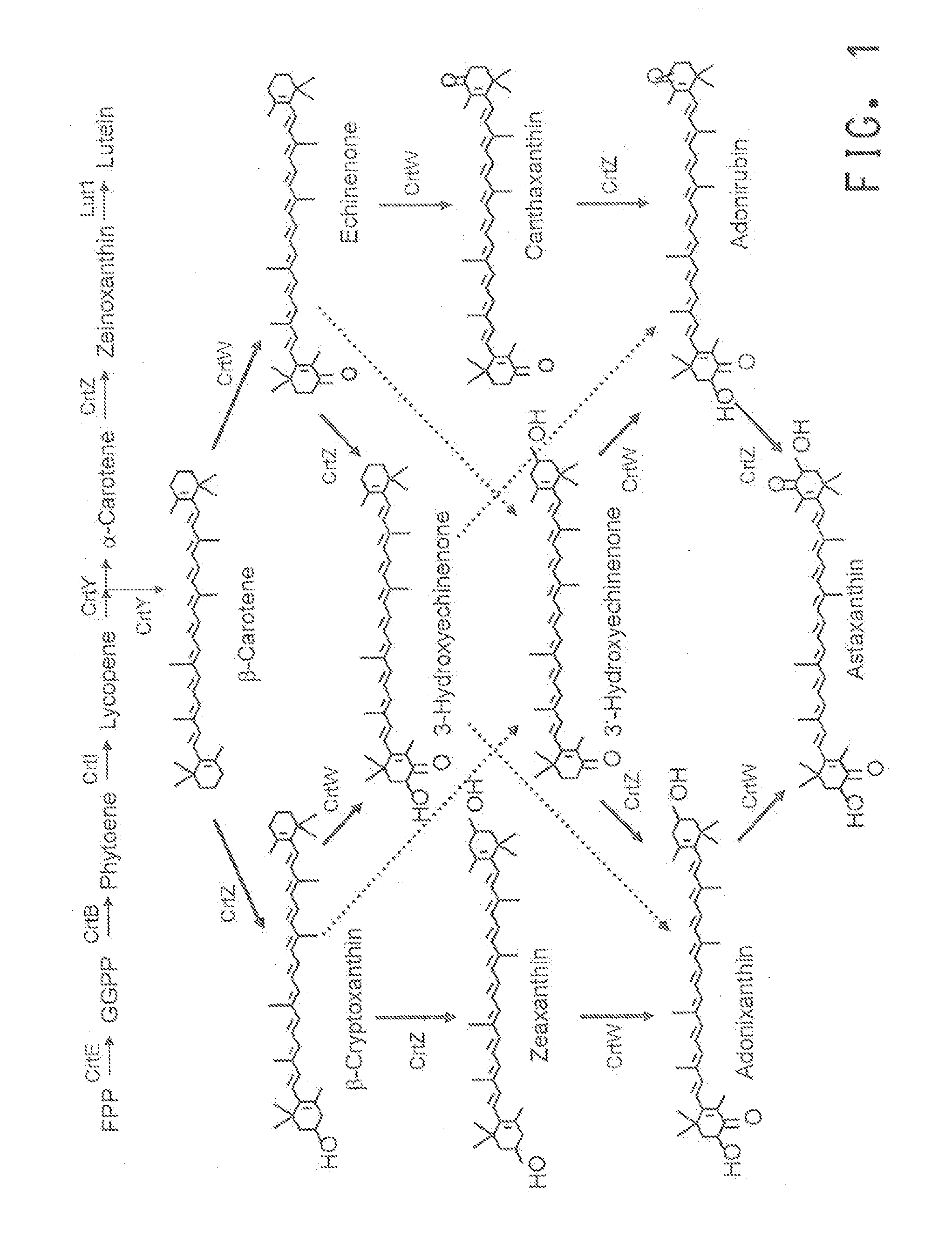
A recombinant microbial host cell is provided capable of producing astaxanthin from β-carotene without a measurable concomitant accumulation of ketolated or hydroxylated intermediates such as adonixanthin, zeaxanthin, adonirubin, echinenone, 3-hydroxyechinenone, 3′-hydroxyechinenone, canthaxanthin, and β-cryptoxanthin. Specifically, a β-carotene producing microbial host cell was engineered to express two heterologous genes, a β-carotene ketolase from Chlamydomonas reinhardtii in combination with a carotenoid hydroxylase from Brevundimonas vesicularis or Arabidopsis thaliana.
Owner:EI DU PONT DE NEMOURS & CO
Highly bioavailable oral administration composition of cryptoxanthin
InactiveUS20090258111A1Safely ingestedImprove antioxidant capacityHydroxy compound active ingredientsTea extractionNatural productOral medication
A composition having improved bioavailability, of cryptoxanthin derived from a natural product, particularly that derived from Citrus unshiu Marc known to be rich in cryptoxanthin, and a method for economically and conveniently producing the same are provided. As a cryptoxanthin highly bioavailable oral administration composition, which is a composition comprising natural product-derived cryptoxanthin, wherein the amount of dietary fibers contained in said composition is 400 times by weight or less based on cryptoxanthin, this composition is a composition wherein the bioavailability of cryptoxanthin is improved 2 times or more in comparison with the case of directly ingesting a natural product containing said cryptoxanthin.
Owner:UNITIKA LTD
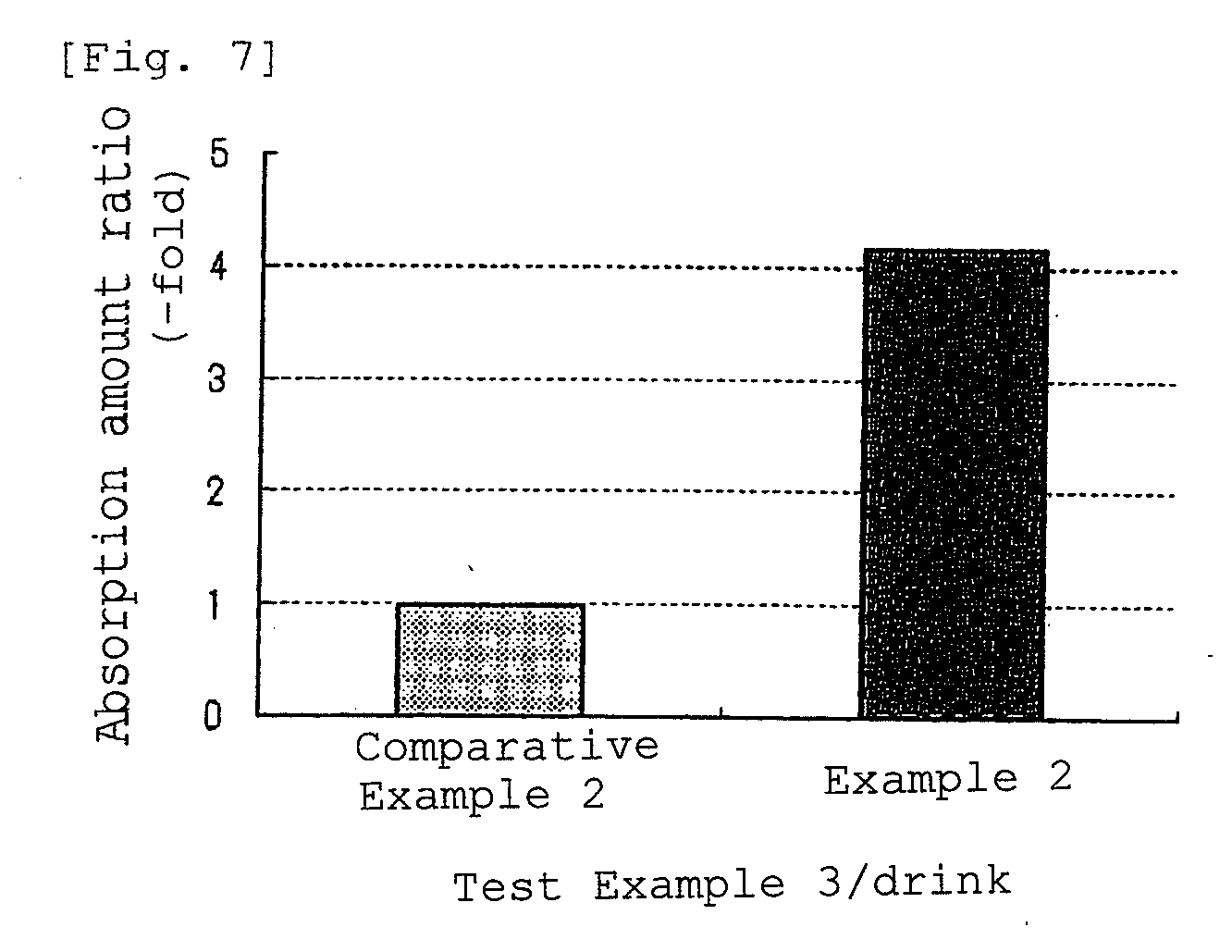
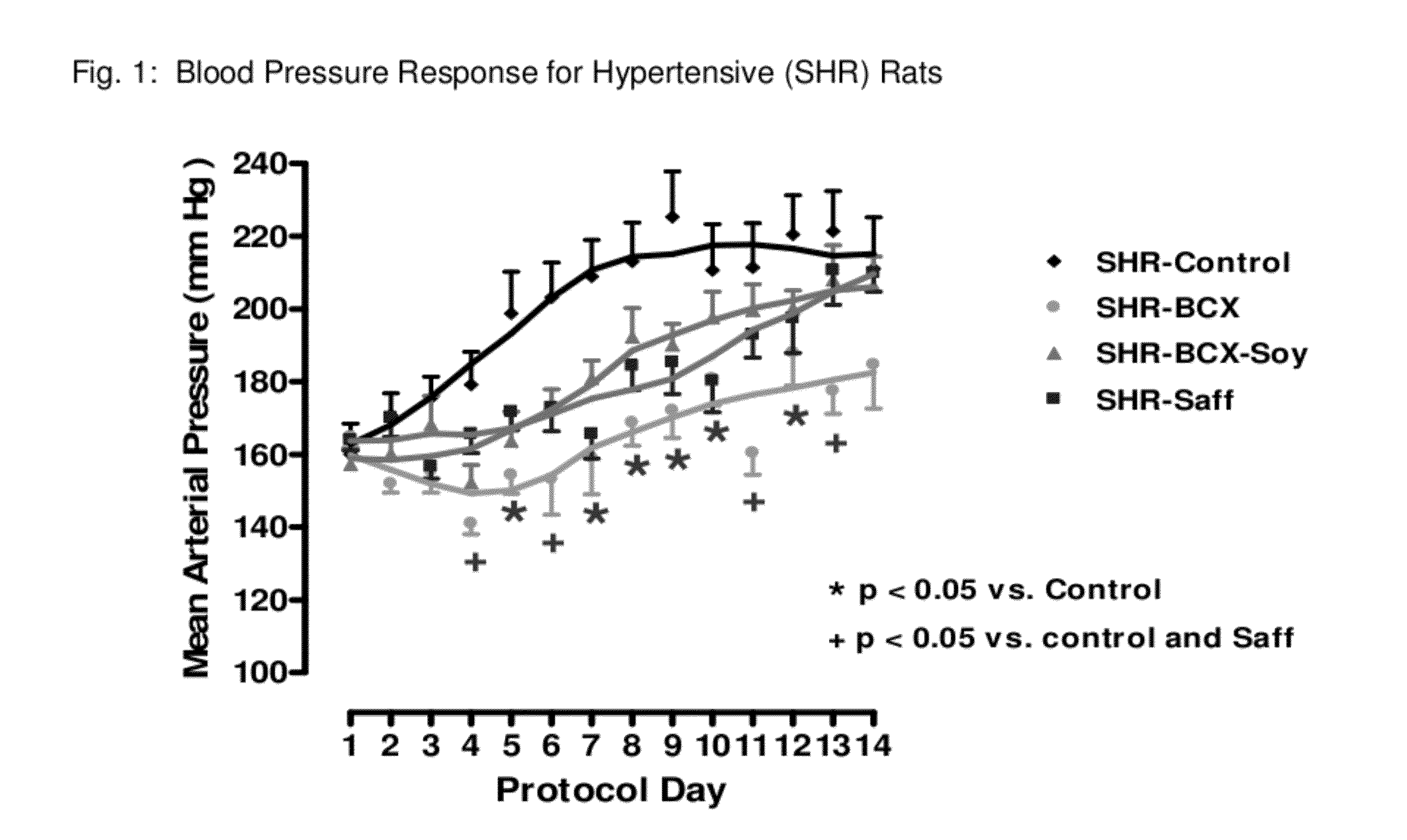
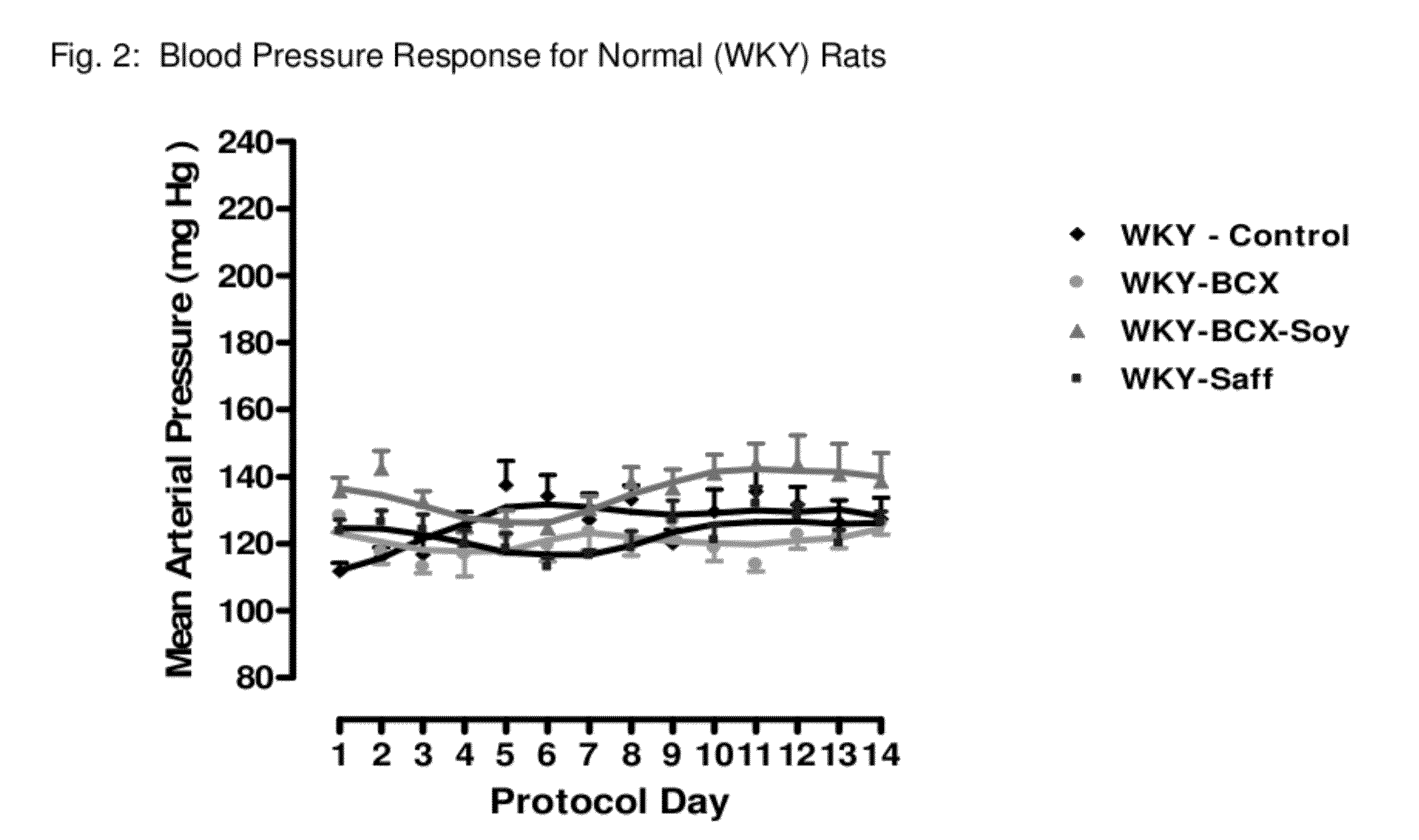
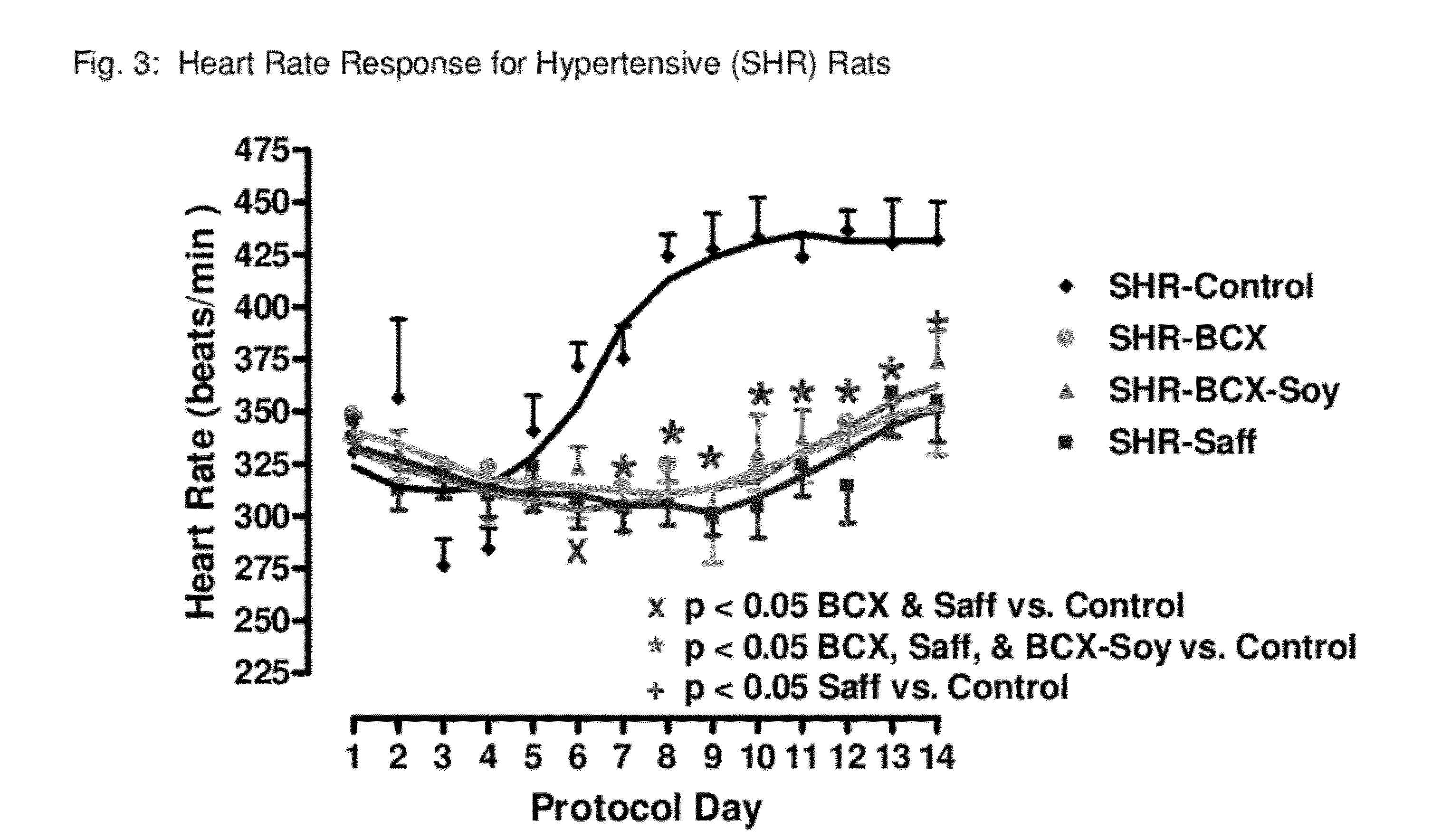
Method of improving cardiovascular health
ActiveUS20120053247A1Promotes cardiovascular healthLower blood pressureBiocideHydroxy compound active ingredientsΒ cryptoxanthinBeta-cryptoxanthin
A nutritional supplement including β-cryptoxanthin is found to be effective at lowering blood pressure in mammals. β-cryptoxanthin therefore may be used to maintain cardiovascular health by lowering blood pressure, preventing high, elevated blood pressure and / or maintaining healthy blood pressure. Administration of β-cryptoxanthin in combination with safflower oil is particularly effective.
Owner:BANK OF AMERICA NAT TRUST & SAVINGS ASSOC
Oral administration composition
InactiveUS20120282358A1Reduce actionImprove securityBiocideSugar food ingredientsCryptoxanthinSphingolipid metabolism
A composition which has high safety and is excellent in the effect of reducing body fat, such as visceral fat, subcutaneous fat and the like, and neutral fat, and a food, a pharmaceutical and a feed comprising the same, are provided. An oral administration composition having a body fat reducing action and / or a neutral fat reducing action, which is a composition comprising a carotenoid and a sphingolipid or a carotenoid and a flavonoid and / or a derivative thereof, preferably a composition comprising a cryptoxanthin and a sphingolipid or a cryptoxanthin and a flavonoid and / or a derivative thereof, wherein the cryptoxanthin and / or the sphingolipid and / or the flavonoid are derived from a citrus fruit and the citrus fruit is preferably Citrus unshiu.
Owner:UNITIKA LTD
Extract liquid containing -cryptoxanthin ingredient, and food or beverage and soap or cosmetic each containing the extract liquid
There are provided an extract solution to be added to food and drink containing a Beta-cryptoxanthin component consisting of an extract containing a Beta-cryptoxanthin component dissolved or dispersed in fat or oil or in a solution comprising an emulsifier and an extract solution to be added to soaps or cosmetics containing a Beta-cryptoxanthin component consisting of an extract containing a Beta-cryptoxanthin component dissolved or dispersed in fat or oil or in a solution comprising an emulsifier. There are also provided food and drink or soaps or cosmetics added with such extract solution containing a Beta-cryptoxanthin component. An extract containing a Beta-cryptoxanthin component derived from persimmon peel is preferable.
Owner:TOYO SEIKAN KAISHA LTD
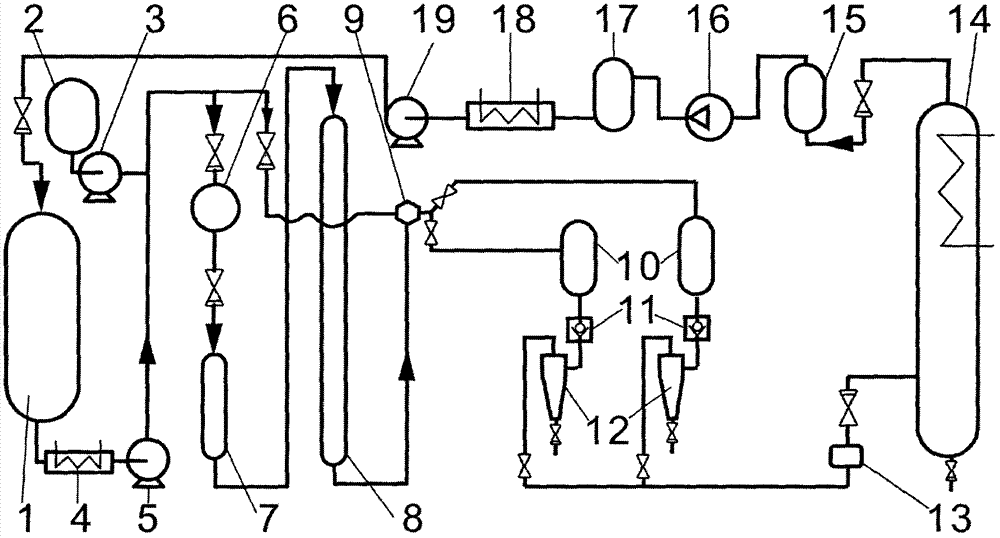
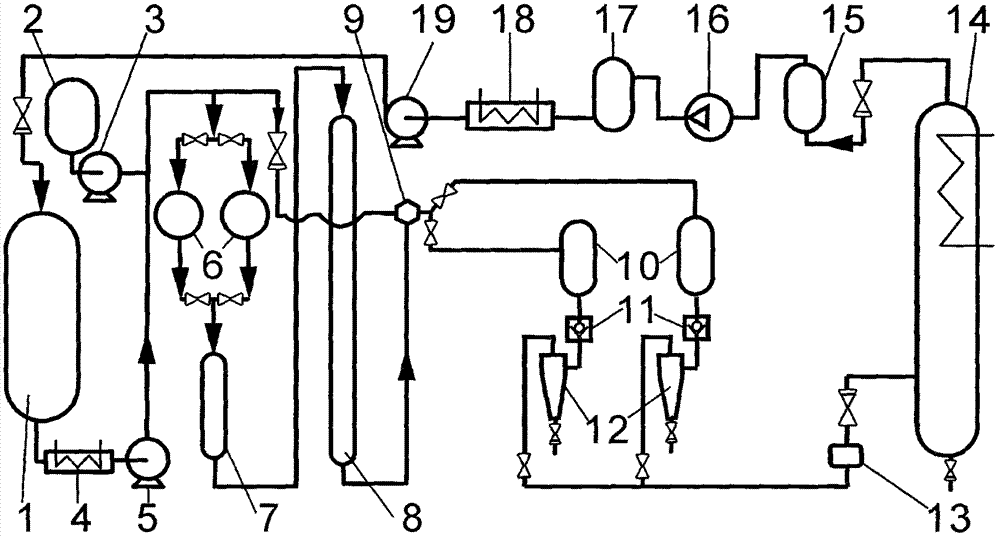
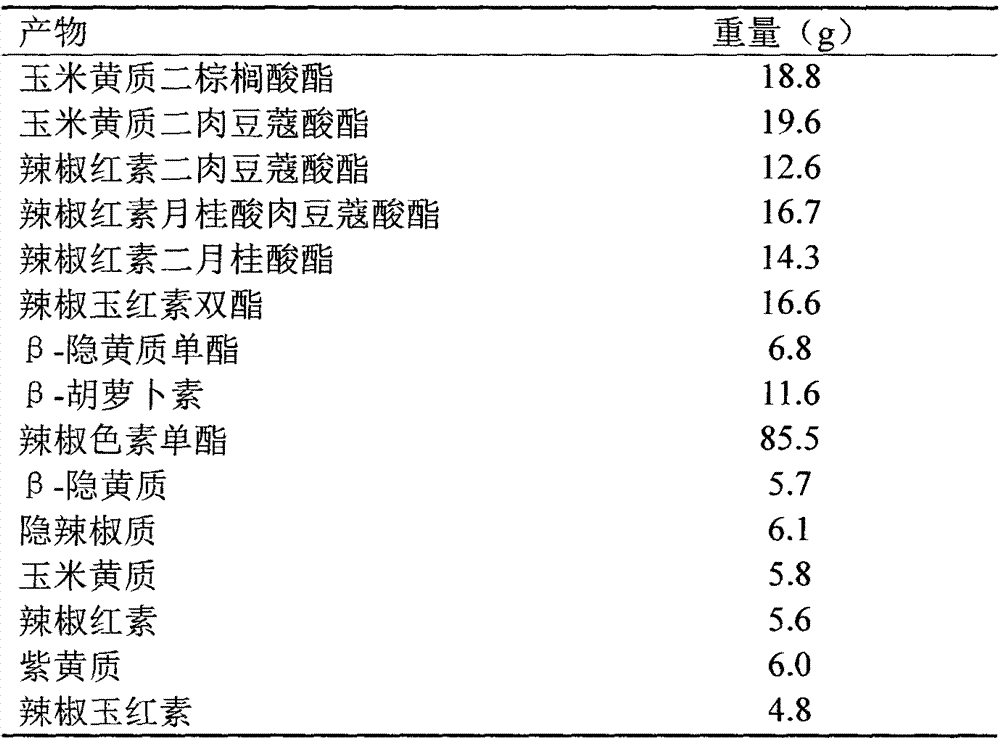
Method and device for preparing highly-pure capsicum pigment capsaicin through supercritical fluid column chromatography
InactiveCN107163618ANo pollution in the processSafe and efficient processSolid sorbent liquid separationCarboxylic acid amide separation/purificationChromatographic separationBeta-cryptoxanthin
The invention relates to a method and a device for preparing a highly-pure capsicum pigment capsaicin through supercritical fluid column chromatography. The method comprises the following steps: mixing and dissolving supercritical carbon dioxide or modified supercritical carbon dioxide and capsicum oleoresin or saponification products thereof in a feeding tank, filtering the obtained solution, carrying out adsorption and desorption separation through a preparative chromatography column filled with solid adsorbent particles, collecting an eluate containing different solutes to corresponding collectors according to the signal of a detector, separating the solute components and carbon dioxide in a separator, carrying out filtering dedusting, condensation liquid removal, adsorption column purification and compression on tail gas to form a liquid, and returning the liquid to a storage tank in order to carry out cycle use. Extremely low solvent residual and even no solvent residual products with a high purity, such as capsanthin, capsorubin, beta-carotene, beta-cryptoxanthin, zeaxanthine, capsanthin ester, capsorubin ester and cryptoxanthin ester, and highly pure products, such as nordihydrocapsaicin, capsaicin and dihydrocapsaicin can be prepared through the method.
Owner:GUANGZHOU LEADER BIO TECH
Nutritive health product for protecting eyes and improving eyesight
InactiveCN102772572AAvoid attackDelay agingSenses disorderHydroxy compound active ingredientsDiseaseSide effect
The invention relates to the technical field of health care medicine and foods, in particular to a nutritive health product for protecting eyes and improving eyesight. The product comprises an oplismenus undulatifolius extract, a black bean peel extract, a fuctus Lycii extract, xanthophyll, cryptoxanthin, vaccinumvitis-idaeallnn, a vitamin B2 and a vitamin E according to certain match ratio; multiple natural plant extracts in the health product are scientifically prescribed and supplemented with various vitamins for nourishing eyes, wherein the xanthophyll has the effects of effectively absorbing ultraviolet rays and blue light, preventing free radicals from attacking the eyes so as to protect the tissues in the eyes; and a potent antioxidant is capable of inhibiting the oxidation of crystalline lenses and retinas. The nutritive health product provided by the invention is conducive to delaying the occurrence rate of aging, degeneration and pathological changes of the eyes and reducing eye diseases, and protecting the retinas from being damaged by light, especially effectively preventing cataract and macular degeneration of old peoples. The nutritive health product for protecting eyes and improving eyesight provided by the invention is highly efficient and reliable, scientific in prescription, and free from toxic and side effects, therefore, the nutritive health product has the health care and preventive and therapeutic effects for various eye degeneration, aging and eye diseases after being taken for a long period of time.
Owner:东莞市光华生物科技有限公司
Tagetes erecta MARIGOLDS WITH ALTERED CAROTENOID COMPOSITIONS AND RATIOS
A marigold plant, a regenerable portion thereof and seed are disclosed whose flower petals, leaves or flower petals and leaves contain one or more of an enhanced neoxanthin plus violaxanthin ratio, an enhanced β-carotene ratio, an enhanced lycopene ratio, an enhanced α-cryptoxanthin ratio, an enhanced phytoene ratio or an enhanced phytofluene ratio relative to that ratio in a non-mutant marigold. A marigold plant, a regenerable portion thereof and seed are also disclosed whose flower petals contain zeaxanthin esters and are substantially free of esters of both neoxanthin and violaxanthin, and wherein zeaxanthin constitutes at least about one-half of the extractable carotenoids when xanthophylls are assayed as alcohols. Also disclosed are methods of preparing such plants, oleoresins and comestible materials that have such carotenoid ratios.
Owner:HAUPTMANN RANDAL +2
Process For The Preparation of Beta and Alpha Cryptoxanthin
ActiveUS20100280286A1Easy to useGood choiceOrganic compound preparationCarbonyl compound preparationDietary supplementCryptoxanthin
The present invention relates to a process for converting lutein and / or lutein esters to (3R)-β-cryptoxanthin and (3R,6′R)-α-cryptoxanthin, suitable for human consumption as dietary supplements, by employing safe and environmentally friendly reagents. (3R)-β-Cryptoxanthin and (3R,6′R)-α-cryptoxanthin are two rare food carotenoids that are not commercially available and the former exhibits vitamin A activity. In the first synthetic step, commercially available lutein and / or lutein esters are transformed into a mixture of dehydration products of lutein (anhydroluteins) in the presence of a catalytic amount of an acid. The resulting anhydroluteins are then converted to (3R)-β-cryptoxanthin (major product) and (3R,6′R)-α-cryptoxanthin (minor product) by heterogeneous catalytic hydrogenation employing transition elements of group VIII (Pt, Pd, Rh supported on alumina or carbon) in a variety of organic solvents under atmospheric pressure of hydrogen and at temperatures ranging from −15° C. to 40° C. Among these catalysts, Pt supported on alumina at 40° C. in ethyl acetate provides the best yield of (3R)-β-cryptoxanthin and (3R,6′R)-α-cryptoxanthin. Several homogeneous catalysts can also promote the regioselective hydrogenation of anhydroluteins to a mixture of (3R)-β-cryptoxanthin and (3R,6′R)-α-cryptoxanthin in low to moderate yields. The catalysts may be transition metal complexes such as palladium acetylacetonate, Rh(Ph3P)3Cl (Wilkinson's catalyst), [(C6H11)3P[C8H12][C5H5N] Ir+PF6− (Crabtree catalyst), or [C8H12][(MePh2P)2]Ir+PF6−. Among these, Wilkinson catalyst converts anhydroluteins to (3R)-β-cryptoxanthin and (3R,6′R)-α-cryptoxanthin in nearly quantitative yield. A novel feature of this invention is the regioselective hydrogenation of anhydroluteins while the highly conjugated polyene chain of these carotenoids remains intact.
Owner:UNIV OF MARYLAND
Osteogenesis promoter containing β-cryptoxanthin as the active ingredient
It is intended to provide an osteogenesis promoter having a remarkable effect of positively promoting osteogenesis and thus preventing / treating bone diseases; a preventive / a remedy for bone diseases such as osteoporosis having both of an osteogenesis-promoting effect and a bone resorption-inhibiting effect; and a method of screening an active ingredient for preventing / treating bone diseases by using a compound having both of an osteogenesis-promoting effect and a bone resorption-inhibiting effect as a lead compound. It is confirmed that beta-cryptoxanthin, which occurs in a large amount in peel and sarcocarp of satsuma orange, has an osteogenesis-promoting effect and a therapeutic effect on bone disease. Thus, beta-cryptoxanthin or its composition is used as an osteogenesis promoter, a preventive / a remedy for bone diseases, a functional food or a food material for preventing / treating bone diseases and a feed composition.
Owner:BANK OF AMERICA NAT TRUST & SAVINGS ASSOC
Microorganism and method for producing carotenoid using it
A carotenoid producing bacterium belonging to the genus Paracoccus that selectively produces canthaxanthin so that the amount thereof is not less than 90 percent by weight of the total amount of produced carotenoids including β-carotene, β-cryptoxanthin, echinenone, canthaxanthin, 3-hydroxyechinenone, 3′-hydroxyechinenone, zeaxanthin, phoenicoxanthin, adonixanthin, and astaxanthin. A method for producing canthaxanthin by culturing the above bacterium, and then collecting carotenoids from bacterial cells or a culture solution after the culturing.
Owner:TOSOH CORP
Production of provitamin A carotenoids in mushrooms and uses thereof
Owner:MEDICINE & NEED CORP
Novel microorganism and method for producing carotenoid using the same
[Object] To provide a canthaxanthin selective producing bacterium being able to easily extract and purify canthaxanthin, which is one of carotenoids, and a method for producing canthaxanthin by a culture method.[Solving Means] A carotenoid producing bacterium belonging to the genus Paracoccus is provided which selectively produces canthaxanthin so that the amount thereof is not less than 90 percent by weight of the total amount of produced carotenoids including β-carotene, β-cryptoxanthin, echinenone, canthaxanthin, 3-hydroxyechinenone, 3′-hydroxyechinenone, zeaxanthin, phoenicoxanthin, adonixanthin, and astaxanthin. In addition, a method for producing canthaxanthin is provided which has the steps of culturing the above bacterium, and then collecting carotenoids from bacterial cells or a culture solution after the culturing.
Owner:TOSOH CORP
Biological Production of Zeaxanthin and Carotenoid Biosynthesis Control
InactiveUS20080293097A1Promote pharmacological effectPromote physiological effectBacteriaUnicellular algaeBacteroidesLutein / zeaxanthin
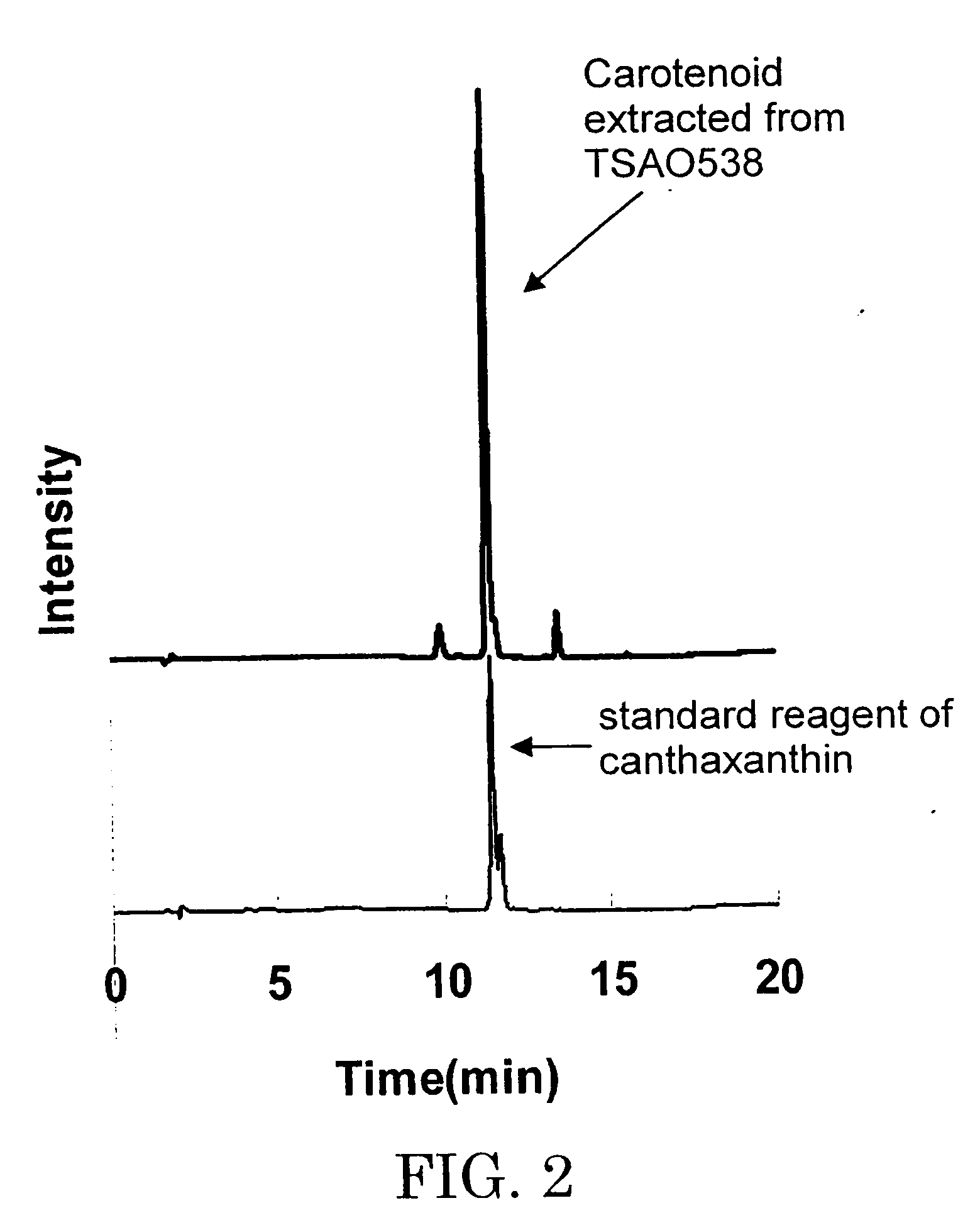
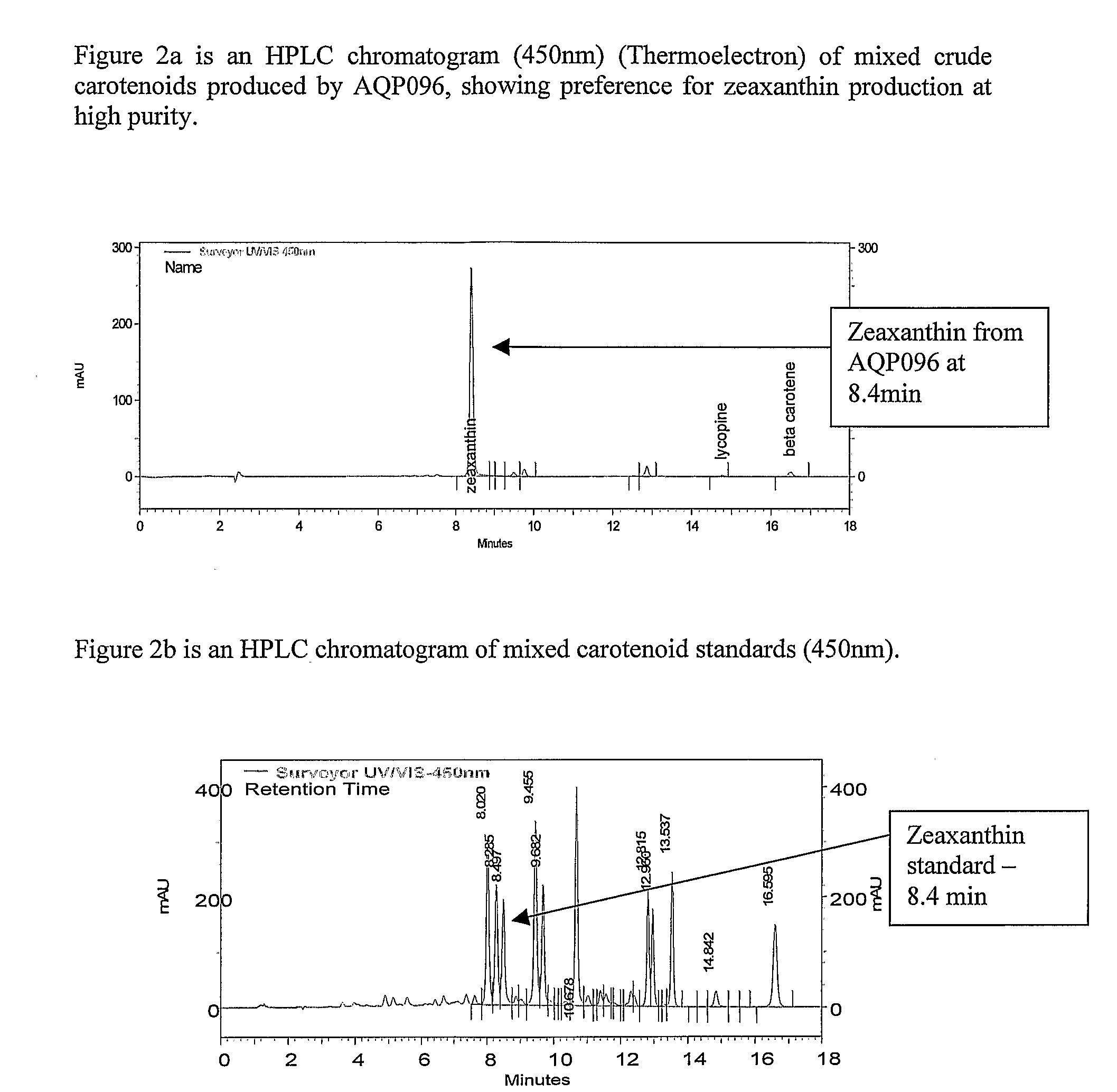
The present invention relates to the isolation of carotenoids and in particular the xanthophyll zeaxanthin (zeaxanthin-β,β-Carotene-3,3′-diol) and optionally other carotenoids such as lycopene, β,β-carotene, 3′-hydroxyechinenone β-cryptoxanthin and the colourless carotenoids, phytoene and phytofluene from a marine bacterium belonging to the genus Algibacter which is capable of producing the aforementioned compounds. The present invention also provides a strain of Algibacter which is capable of producing significant levels of carotenoids, especially zeaxanthin at high purity, as well as methods of using the Algibacter strain and uses of the carotenoids produced.
Owner:AQUAPHARM BIO DISCOVERY
Tagetes erecta with altered carotenoid compositions and ratios
A marigold plant, a regenerable portion thereof and seed are disclosed whose flower petals, leaves or flower petals and leaves contain one or more of an enhanced neoxanthin plus violaxanthin ratio, an enhanced β-carotene ratio, an enhanced lycopene ratio, an enhanced α-cryptoxanthin ratio, an enhanced phytoene ratio or an enhanced phytofluene ratio relative to that ratio in a non-mutant marigold. A marigold plant, a regenerable portion thereof and seed are also disclosed whose flower petals contain zeaxanthin esters and are substantially free of esters of both neoxanthin and violaxanthin, and wherein zeaxanthin constitutes at least about one-half of the extractable carotenoids when xanthophylls are assayed as alcohols. Also disclosed are methods of preparing such plants, oleoresins and comestible materials that have such carotenoid ratios.
Owner:BALL HORTICULTURAL
Anti-free-radical composition and preparation method and application thereof
PendingCN111297840AImprove cleanlinessUse low concentrationTobacco treatmentAntinoxious agentsPolymer scienceVegetable oil
The invention discloses an anti-free-radical composition and a preparation method and application thereof, and belongs to the field of cigarette additives and application thereof. The composition is prepared from the following raw materials in parts by weight: 3 to 10 parts of cryptoxanthin, 4.5 to 20 parts of curcumin, 0.2 to 2 parts of an antioxidant, 0.4 to 1.2 parts of an excipient, 8 to 15 parts of vegetable oil and 72 to 89 parts of a water-soluble polymer. The method comprises the following steps: 1) mixing the cryptoxanthin, the curcumin, the antioxidant, the excipient, the vegetable oil and the water-soluble polymer in parts by weight to obtain a mixture; and (2) adding the mixture into a double-screw extruder, and performing extruding to obtain a powder, namely the anti-free-radical composition. The invention discloses the application of the anti-free-radical composition in the field of preparation of electronic cigarette additives, and the anti-free-radical composition has agood effect of scavenging free radicals, and is easy to atomize, stable in character and free from toxic or side effect.
Owner:山东天音生物科技有限公司
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com