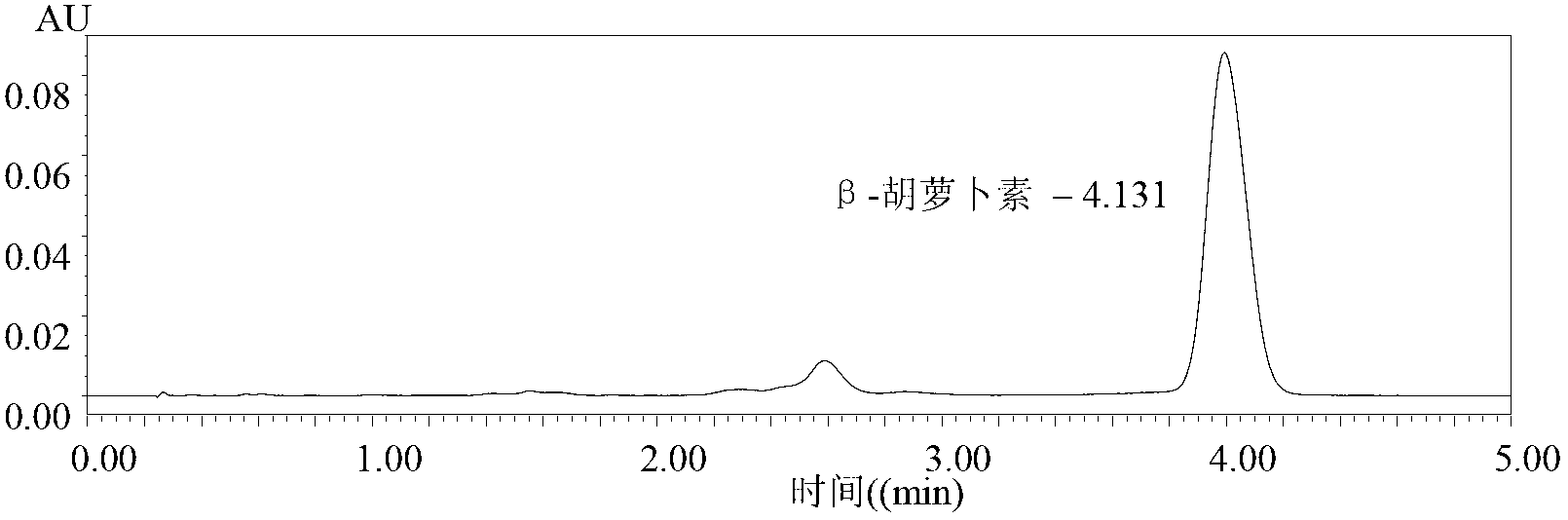
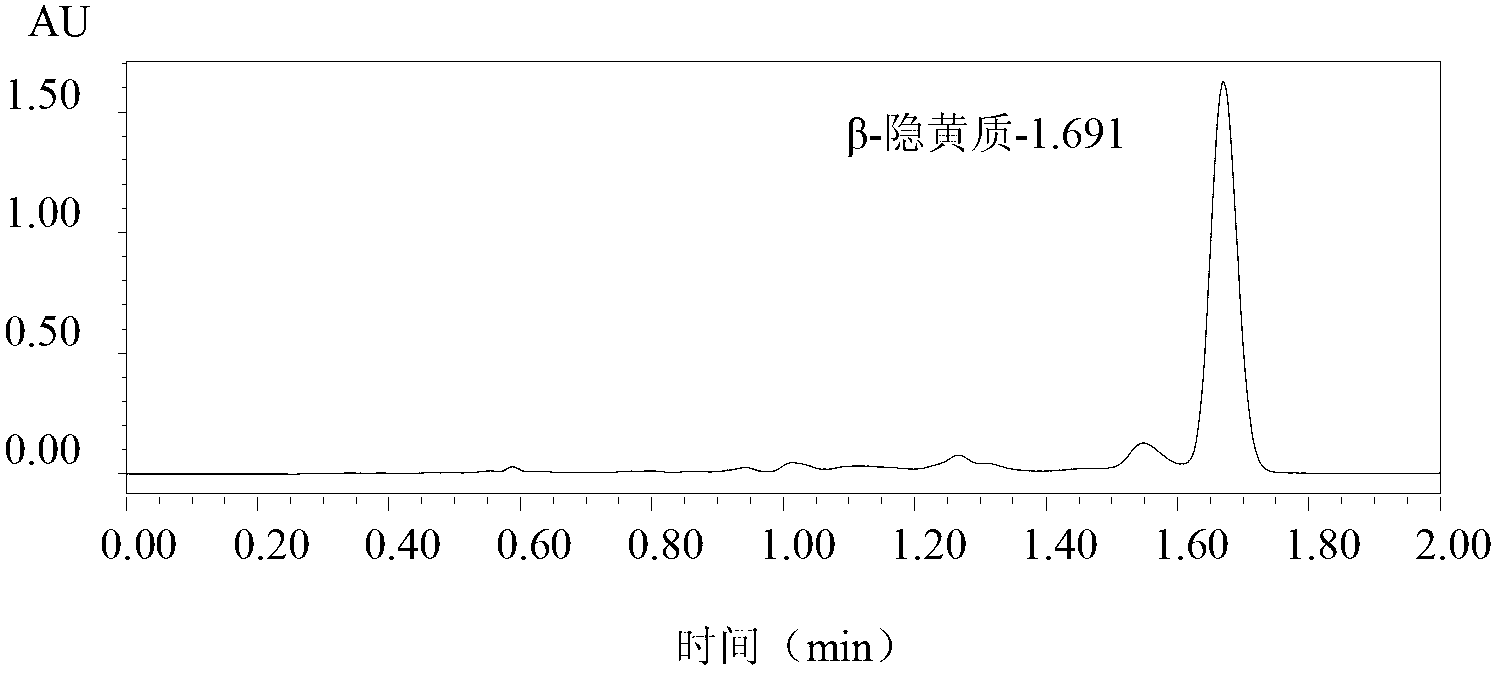
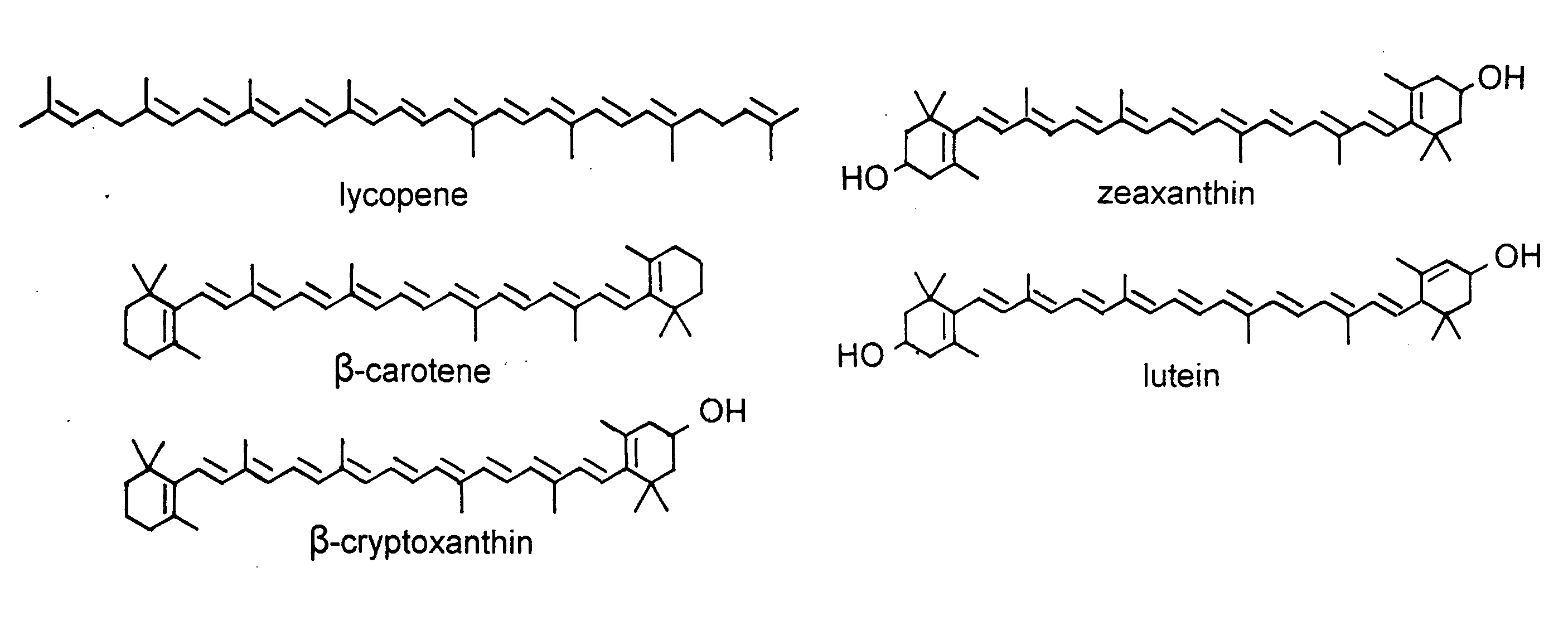
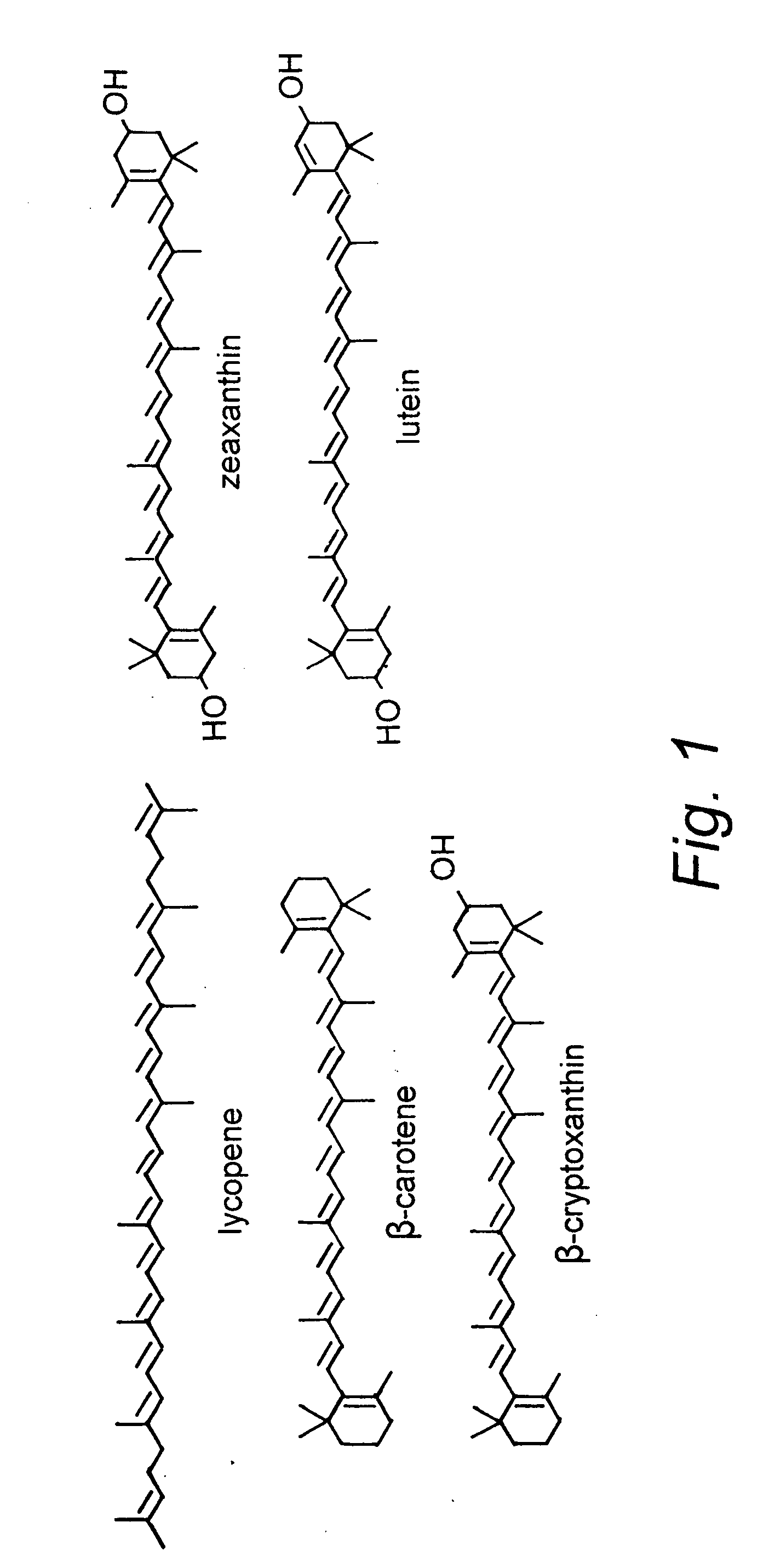
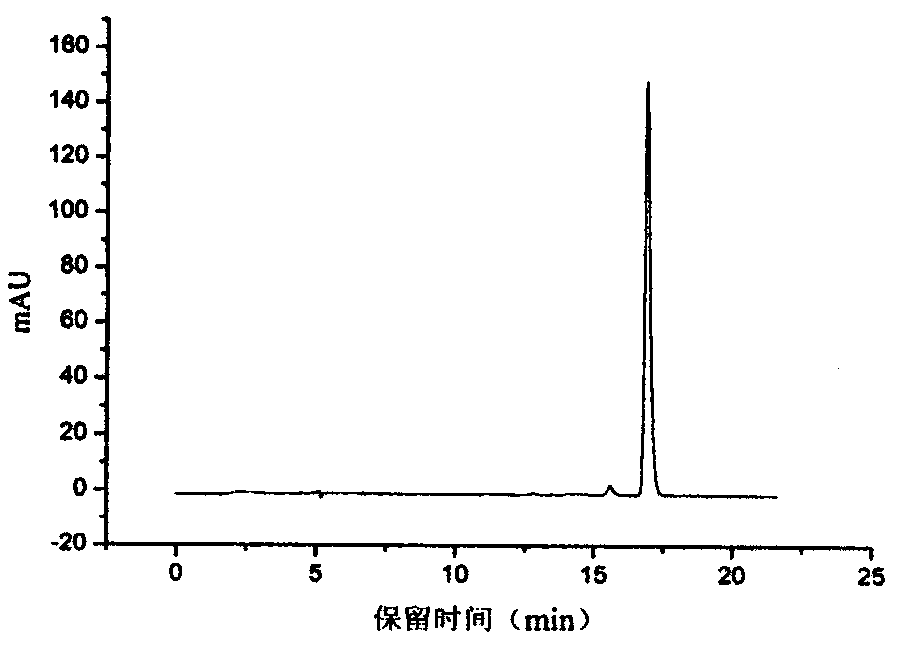
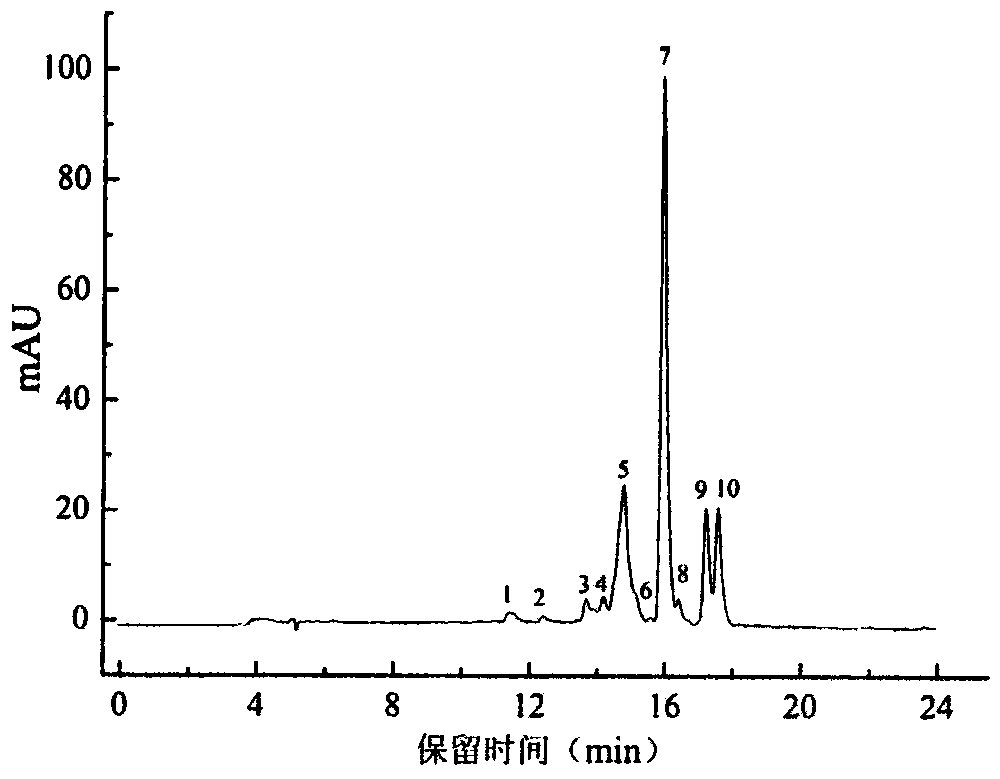
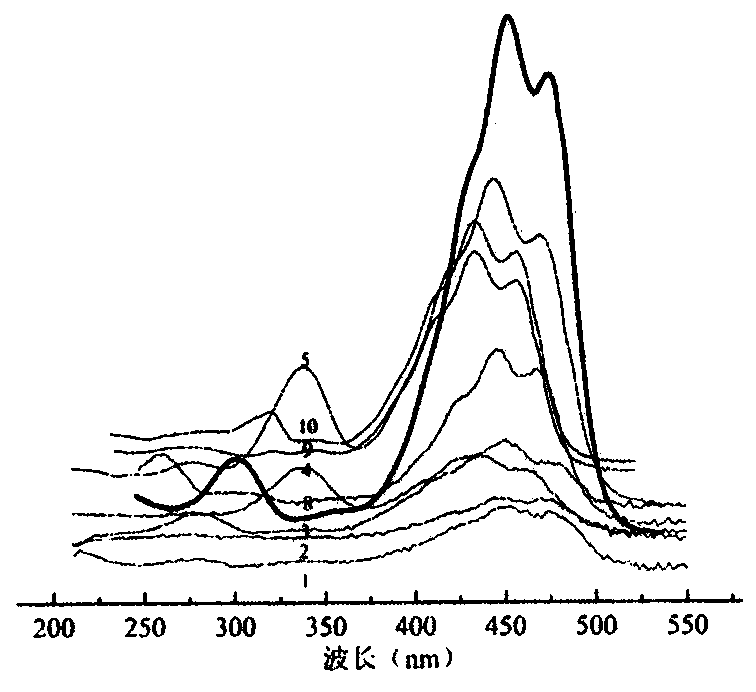
Patents
Literature
44 results about "Beta-cryptoxanthin" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Beta-cryptoxanthin is a carotenoid that can be converted directly into vitamin A. In a British study, intake was associated with a 50 percent reduced risk of polyarthritis (arthritis affecting two or more joint groups).
Process for extraction and purification of lutein, zeaxanthin and rare carotenoids from marigold flowers and plants
InactiveUS6262284B1Oxygen-containing compound preparationOrganic compound preparationBeta-CaroteneBeta-cryptoxanthin
A process for simultaneously extracting, saponifying, and isolating lutein and zeaxanthin, and a mixture of several rare carotenoids in high purity from plants without the use of harmful organic solvents. Lutein crystals containing 5% zeaxanthin were obtained from the dried petals of Marigold flowers Tagete erecla while zeaxanthin was isolated and purified from the berries of Lycium Chinese Mill (LCM berries). Similarly, this process has been employed to isolate and purify a mixture of lutein, beta-carotene, neoxanthin, violaxanthin, and lutein epoxide from green plants, preferably, kale, collard green, and spinach. These plants, to a lesser extent, also serve as a source of several rare carotenoids such as alpha-cryptoxanthin (Marigolds) and beta-cryptoxanthin (LCM berries). The purified carotenoids isolated by this process are free from impurities and serve as a safe source of nutritional supplement for human consumption as well as providing a suitable and effective color additive for human foods.
Owner:UNIV OF MARYLAND
Carotenoid-containing compositions and methods
InactiveUS20090118229A1Good for bone healthImprove respiratory healthBiocideHydroxy compound active ingredientsBeta-CaroteneBeta-cryptoxanthin
The present invention is directed to carotenoid-containing compositions and methods for improving bone or respiratory health in a subject comprising administering to the subject a combination of lycopene, beta-carotene, and beta-cryptoxanthin.
Owner:MEAD JOHNSON NUTRITION
Strawberry carotenoid component content determination method
InactiveCN103175934AImprove the extraction effectReduce dosageComponent separationBeta-CaroteneBeta-cryptoxanthin
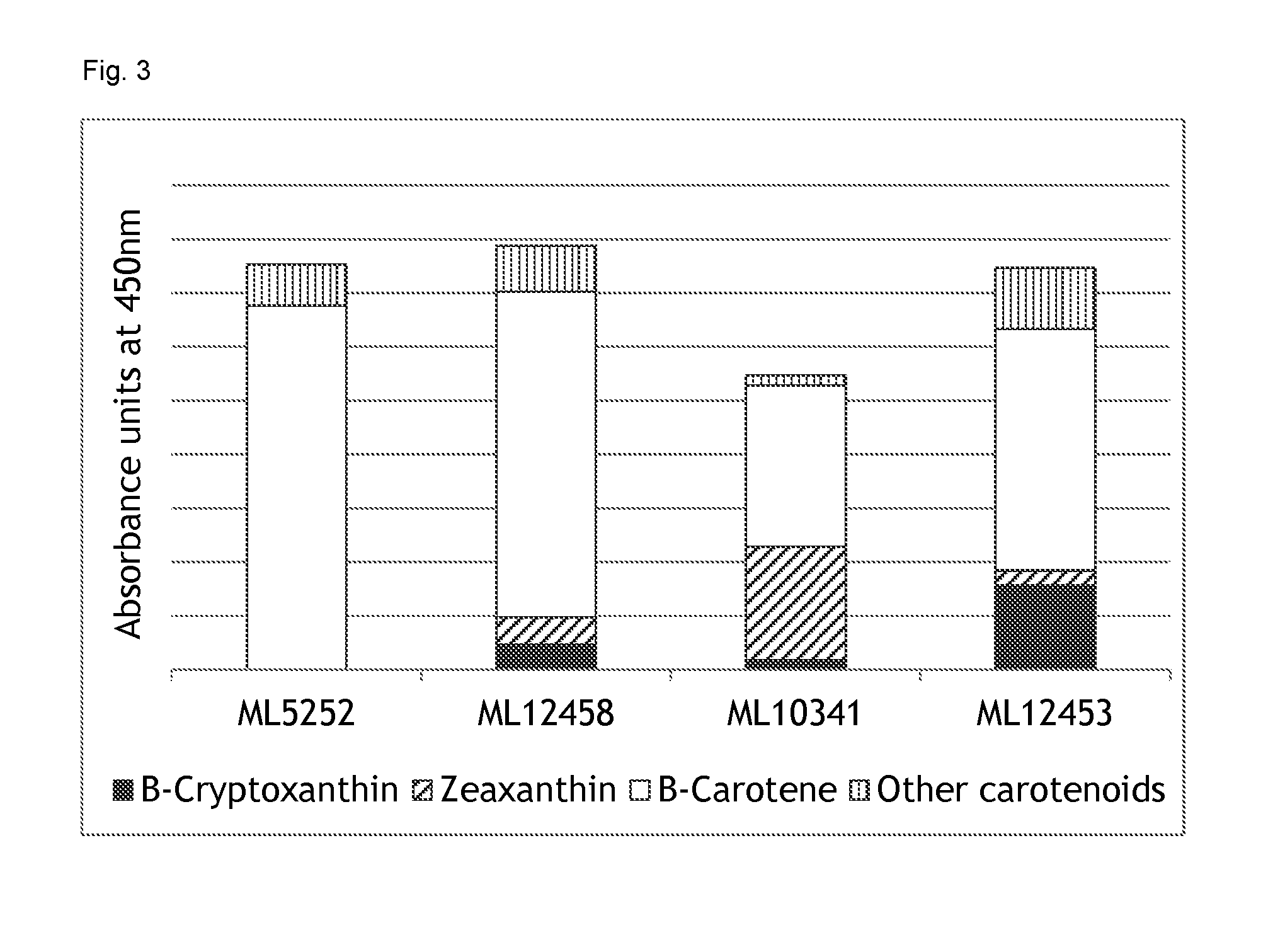
The invention provides a strawberry carotenoid component content determination method. According to the invention, with an ultra performance liquid chromatography method, content determinations upon 4 carotenoid components such as lutein, beta-carotene, beta-cryptoxanthin and lycopene in strawberry fruits are simultaneously carried out under a same chromatographic condition. According to the invention, acetone and petroleum ether with a ratio of 1:2 is adopted as an extraction agent, and carotenoids in strawberry fruits are subjected to saponification extraction; acetonitrile and methanol with a ratio of 1:9 are adopted as a mobile phase; a detection absorption wavelength is 450nm, and detection can be finished in 5min. The method provided by the invention has the advantages of high precision, high sensitivity, good repeatability, and high recovery rate. With the invention, separation and content determinations of the 4 carotenoid components in strawberry are simultaneously carried out. The method is simple and fast, the result is accurate and reliable, and reagent consumption is low. The method is an optimal method of qualitative and quantitative analysis of carotenoids of strawberry fruits.
Owner:CROP RES INST OF FUJIAN ACAD OF AGRI SCI
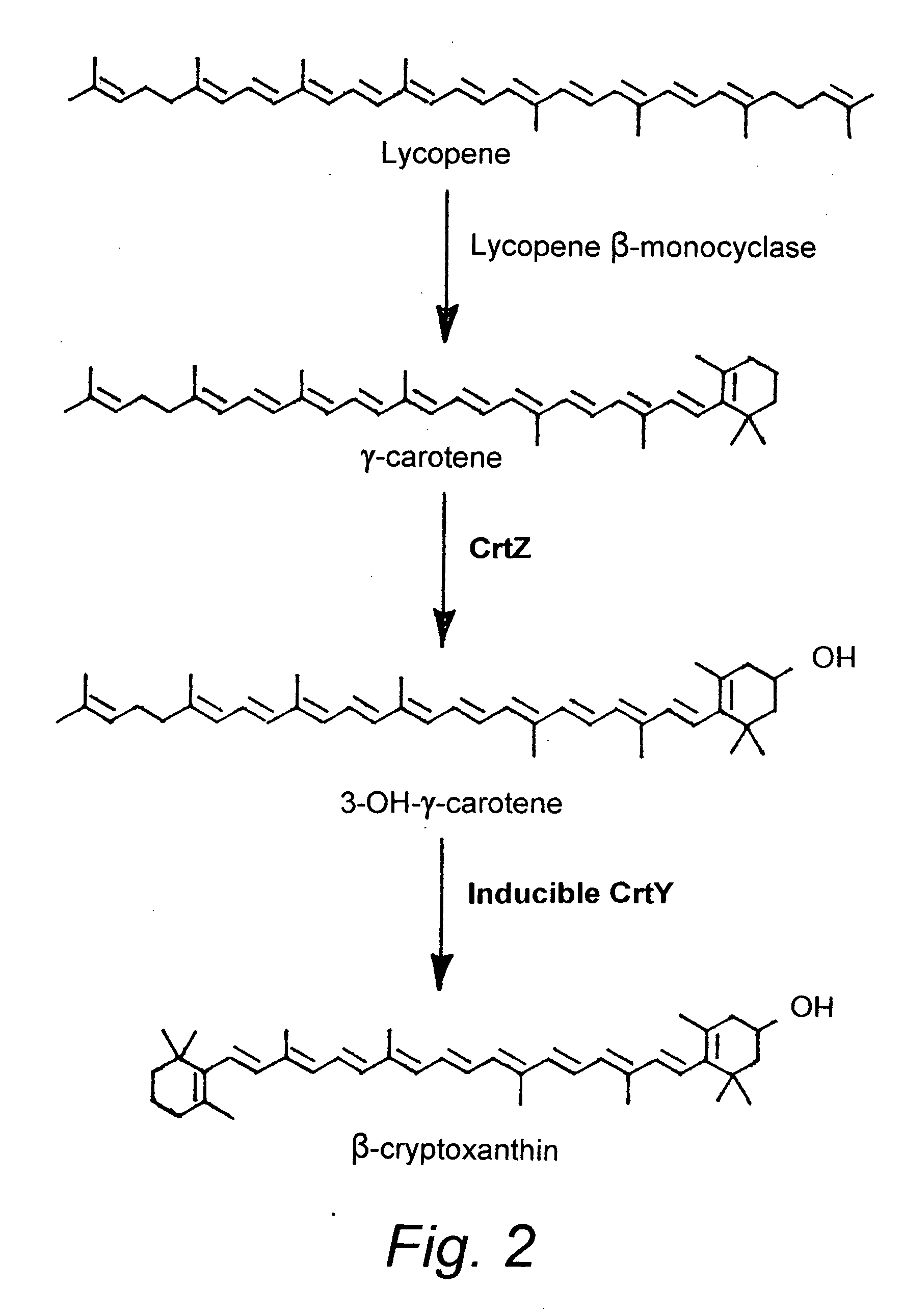
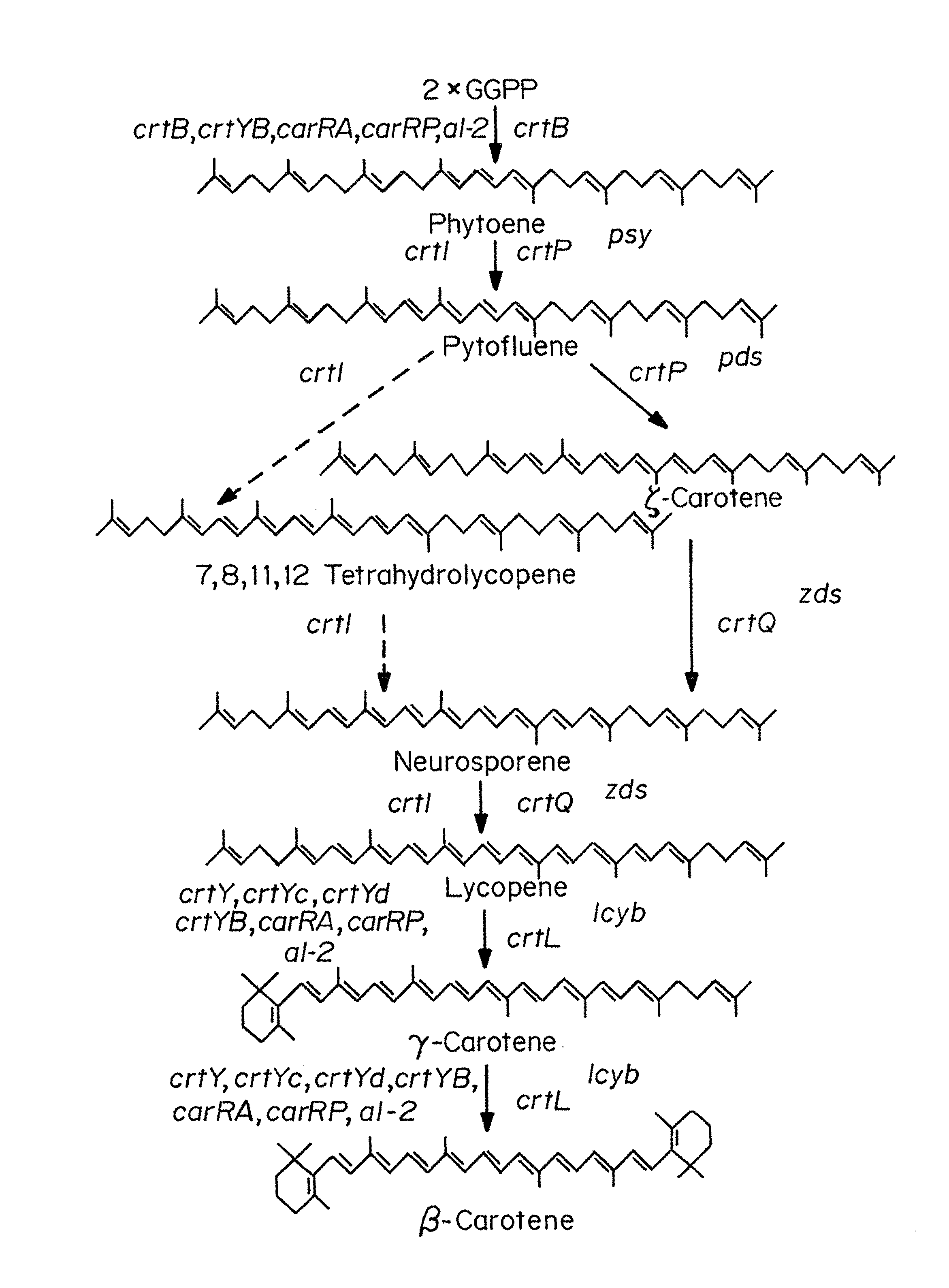
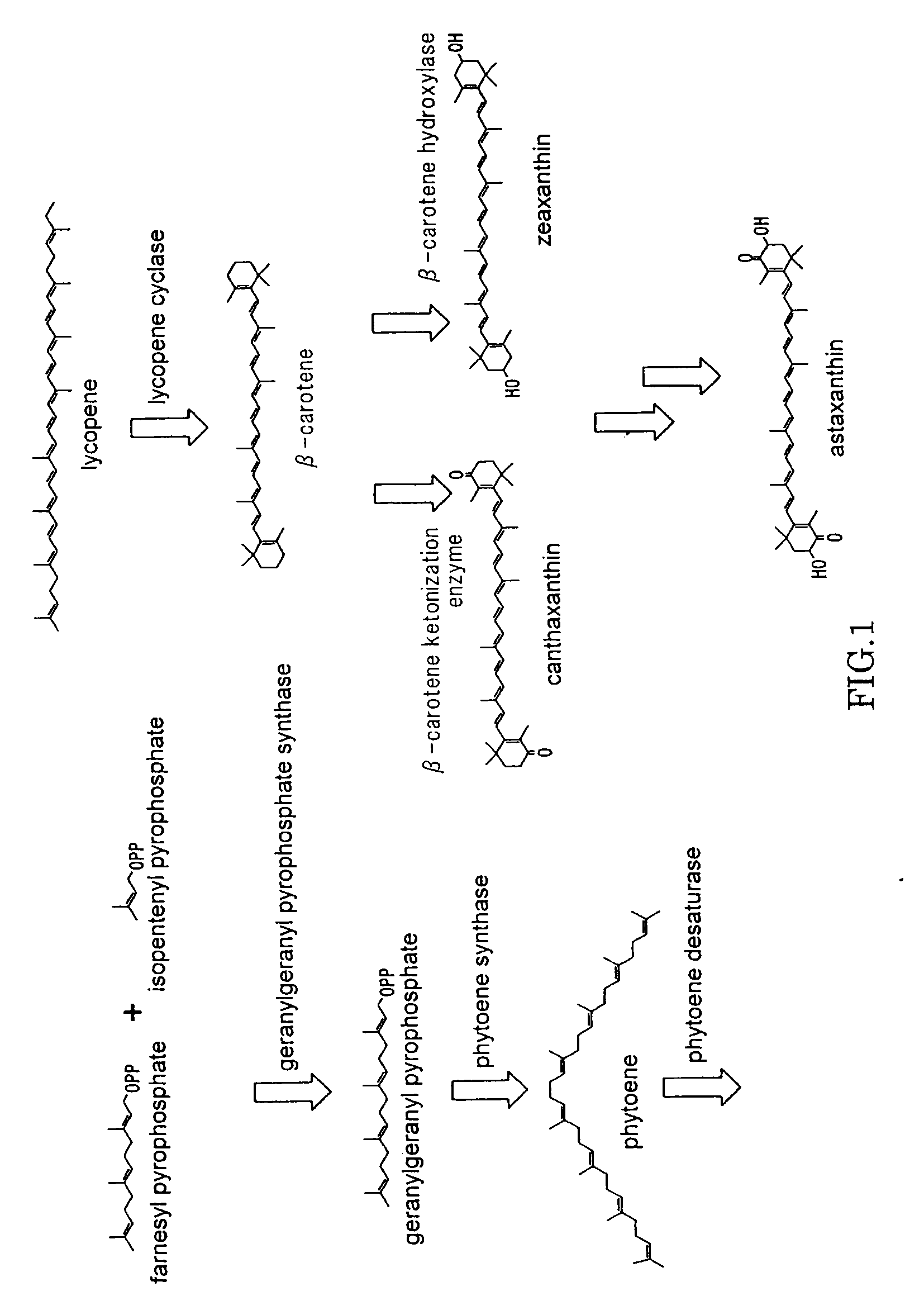
Beta-cryptoxanthin production using a novel lycopene beta-monocyclase gene
Novel lycopene beta-monocyclase genes were identified and used to transform a host cell to produce β-cryptoxanthin. The host cell produces lycopene and is transformed to express the novel lycopene β-monocyclase that converts lycopene into γ-carotene. The host cell is further transformed to express a lycopene hydroxylase that hydroxylates γ-carotene to 3-hydroxy-γ-carotene and a lycopene β-bicyclase that converts 3-hydroxy-γ-carotene to β-cryptoxanthin. The host cell is grown under conditions whereby γ-carotene is produced which is hydroxylated to 3-hydroxy-γ-carotene and which is converted into β-cryptoxanthin.
Owner:KEMIN FOODS L C
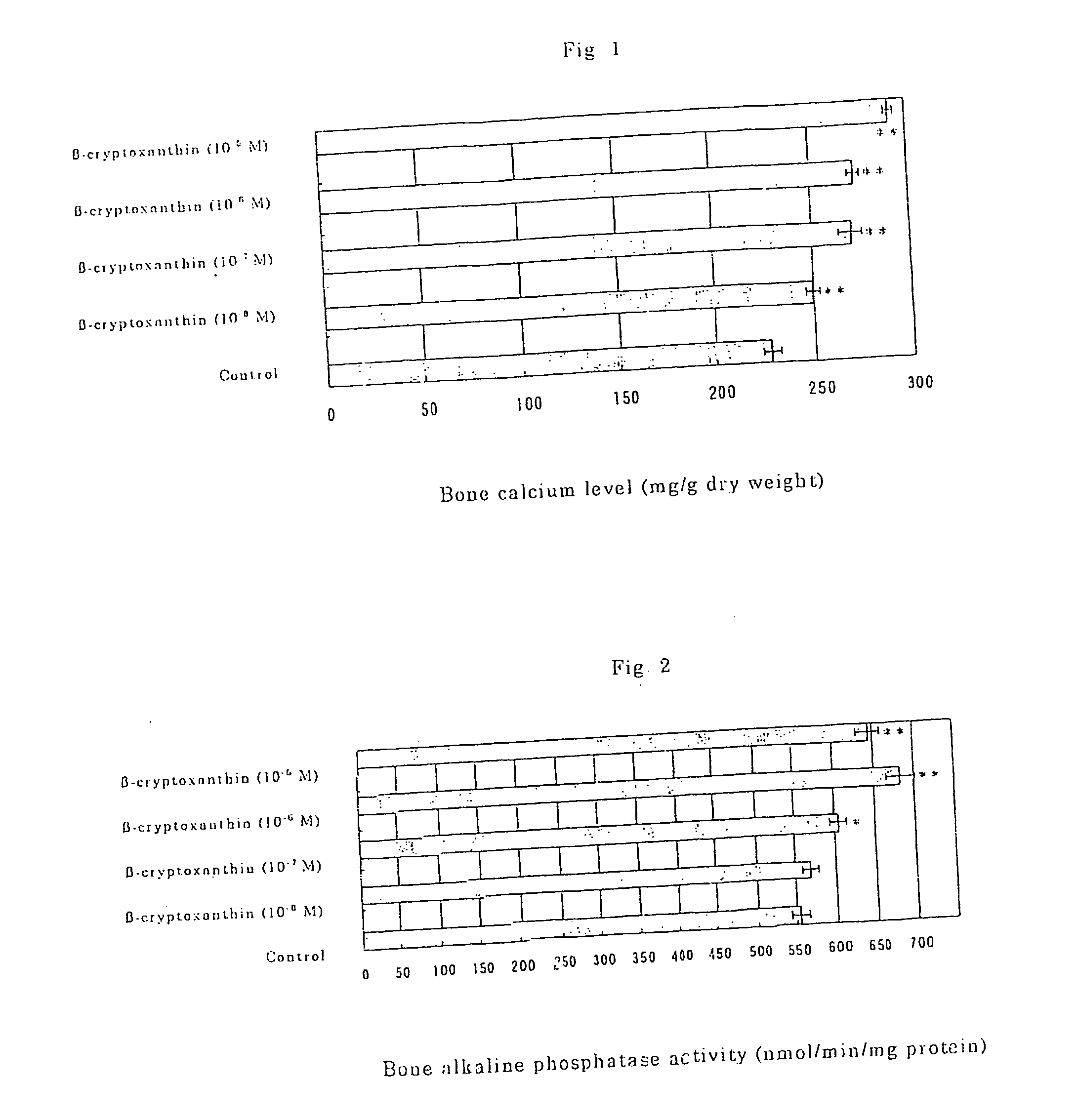
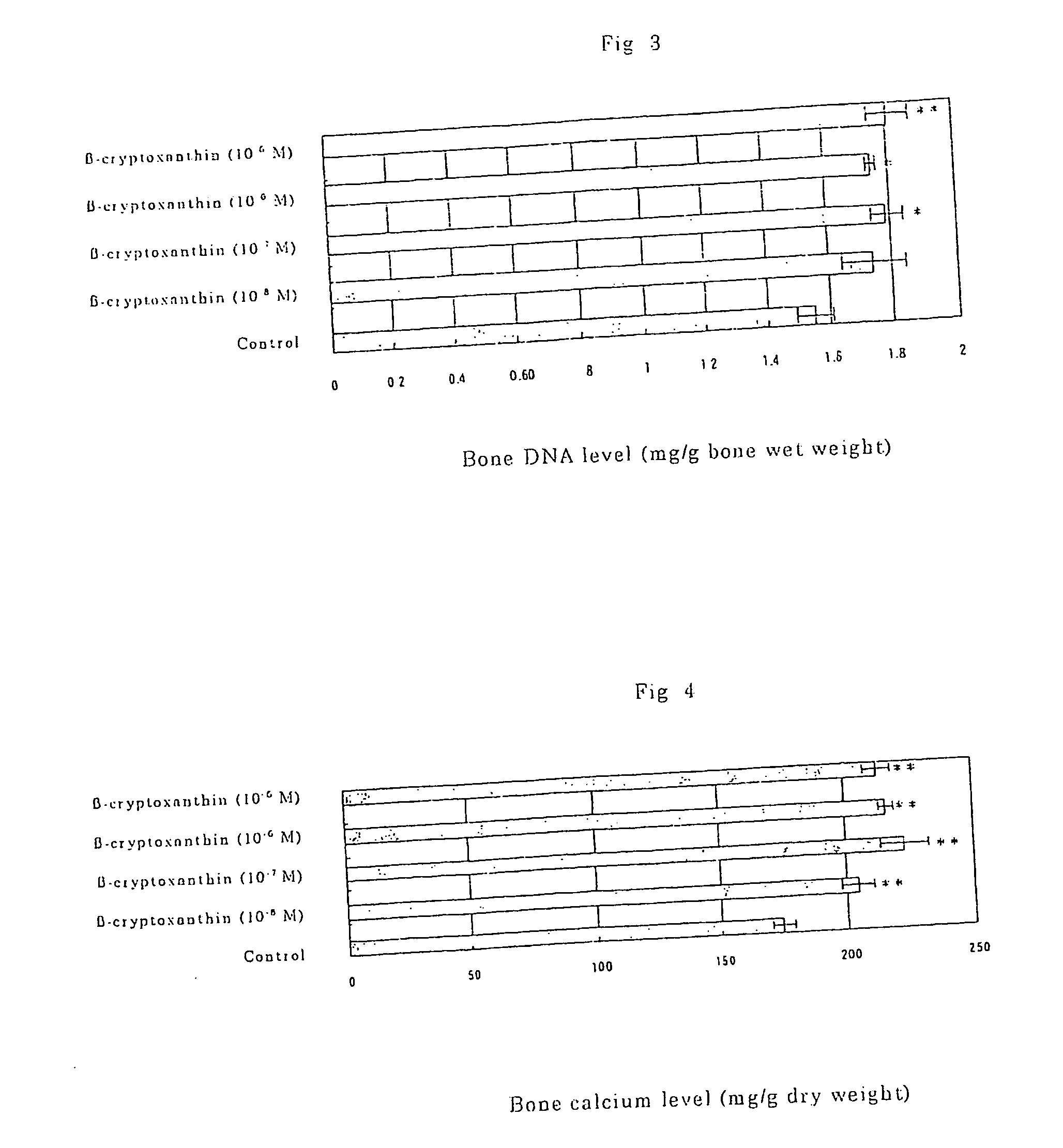
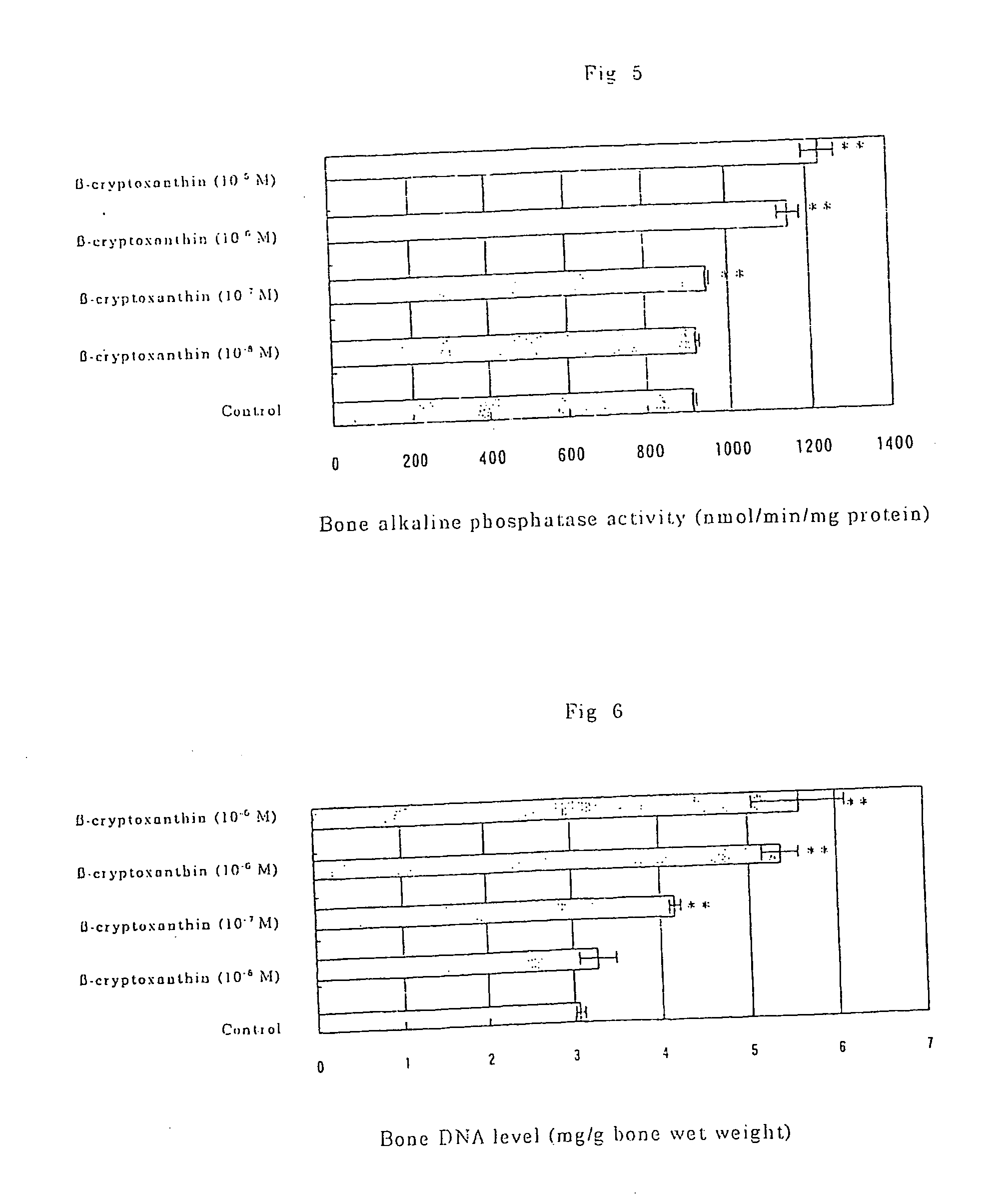
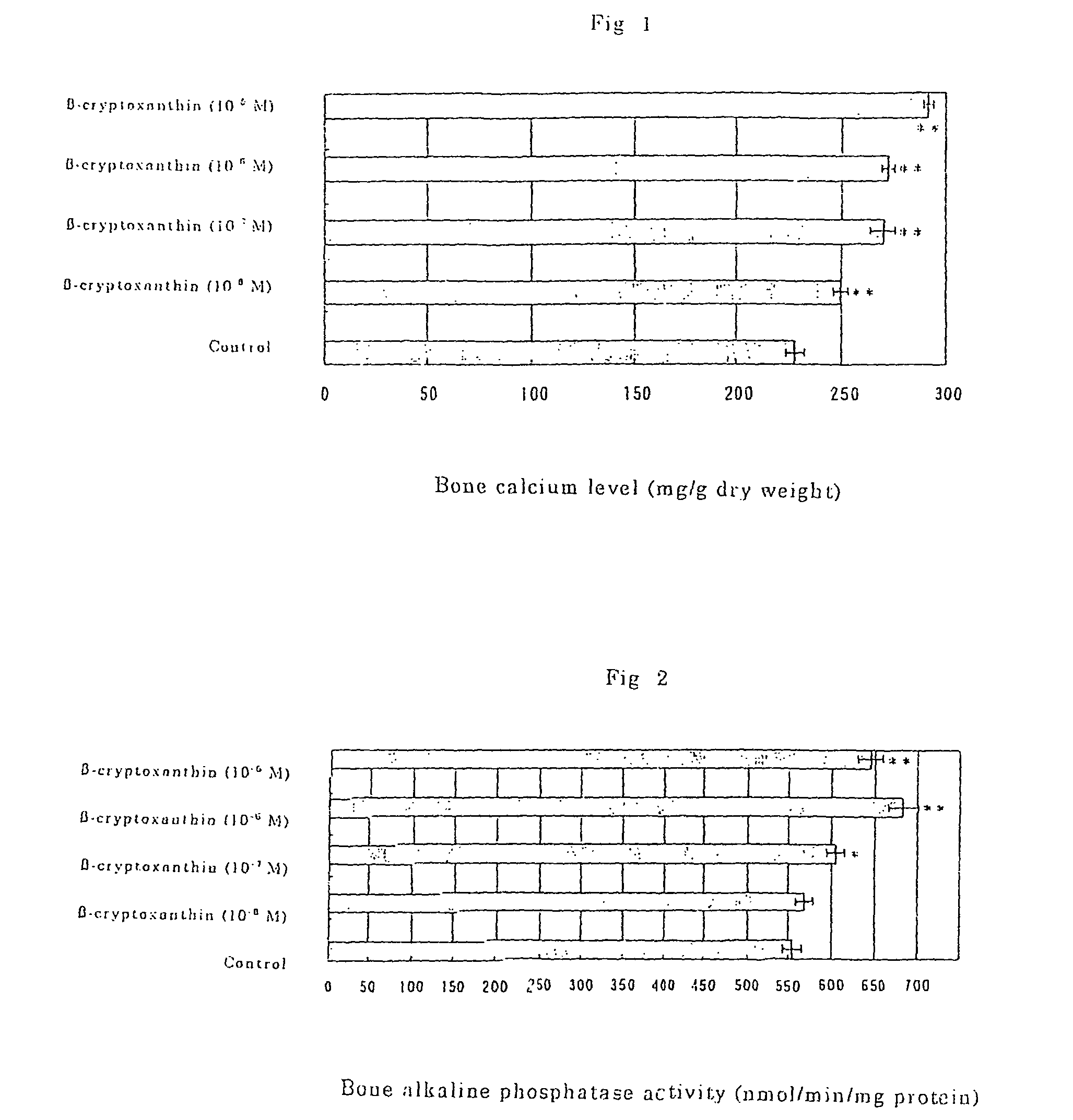
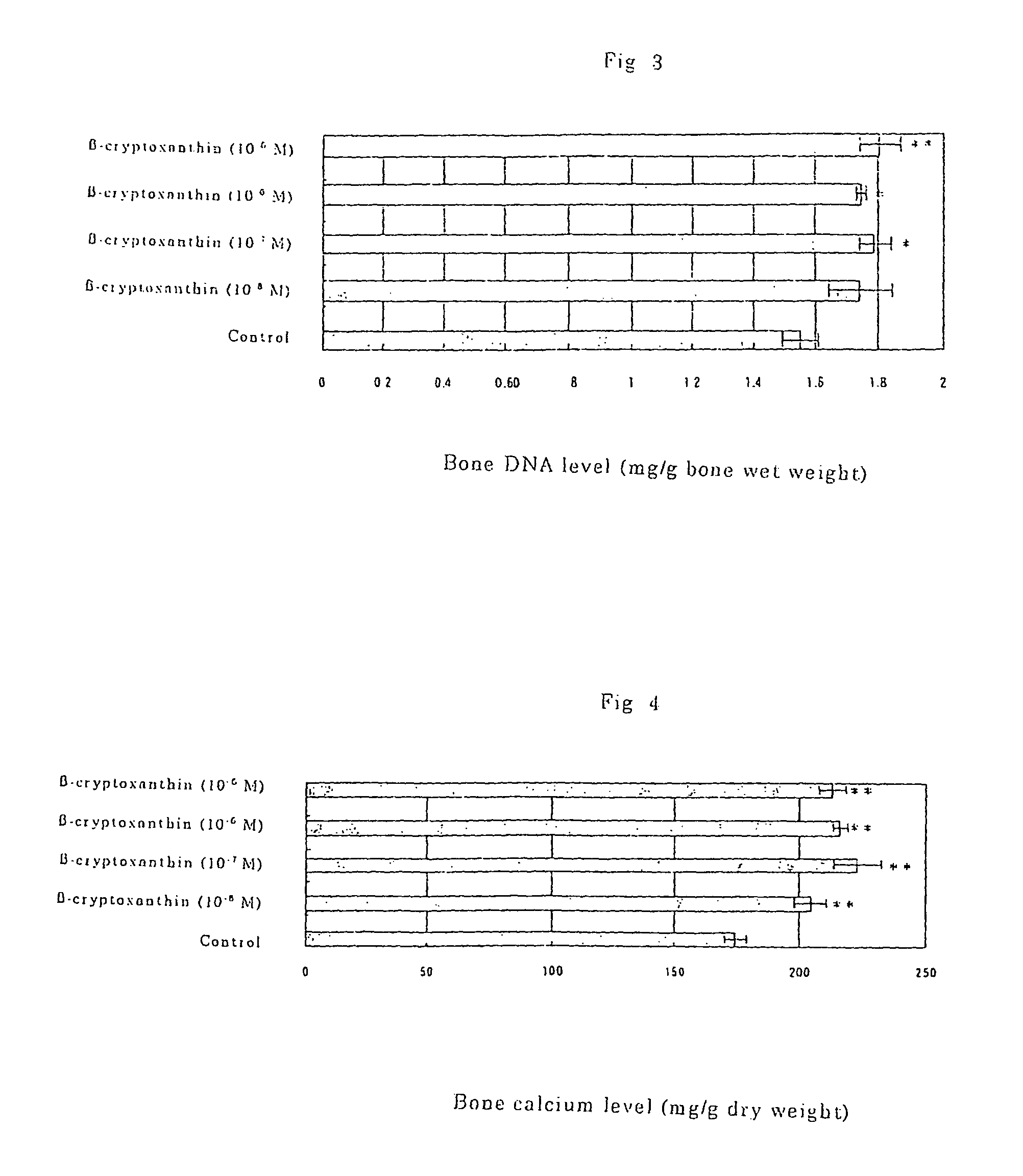
Osteogenesis promoter containing beta-cryptoxanthin as the active ingredient
ActiveUS20060106115A1Preventing/treating bone diseaseIncreasing protein synthesisBiocideHydroxy compound active ingredientsTreatment effectBeta-cryptoxanthin
Owner:BANK OF AMERICA N A
Extract containing beta-cryptoxanthin component from persimmon fruit
The present invention provides an extract containing a β-cryptoxanthin component from a persimmon fruit, a functional food and / or drink supplemented with the extract, and a process for producing the extract. The present invention produces the extract containing a β-cryptoxanthin component from a persimmon fruit by cutting an inexpensive raw material persimmon fruit, especially a persimmon skin, into fine pieces and extracting a β-cryptoxanthin component with an organic solvent such as ethanol. After the β-cryptoxanthin component is extracted with the organic solvent, it is preferred to eliminate the smell of dried persimmon by removing the organic solvent under reduced pressure. According to the present invention, an extract containing a β-cryptoxanthin component that does not raise consumers' anxiety even when added to foods, drinks, and the like can be produced at low cost.
Owner:TOYO SEIKAN KAISHA LTD
Extraction method for concentrate containing beta-cryptoxanthin, concentrate obtained by the method, and usage of the obtained concentrate
ActiveCN102219721AAbundant resourcesSolve the technical defect of low extraction efficiency of β-cryptoxanthinOrganic chemistryAnimal feeding stuffBeta-cryptoxanthinPre treatment
The invention relates to a method for extracting concentrate containing beta-cryptoxanthin from persistent calyx of Physalis alkekengi L. var. franchetii (Masters)Makino, the concentrate containing beta-cryptoxanthin obtained by the method, and the usage of the concentrate. The method comprises the steps of pretreatment, enzyme treatment, extraction, saponification, condensation and purification of raw materials. According to the method provided in the invention, the technical defect in the prior art that extraction efficiency of beta-cryptoxanthin is low is overcome; a beta-cryptoxanthin product with great value is obtained; and the content of beta-cryptoxanthin in the product provided in the invention is far more than the content of beta-cryptoxanthin in the product obtained in the prior art, thereby enabling a good prospect for the application of beta-cryptoxanthin products. The raw materials used in the method are easily available, the extraction process is simple, the content of beta-cryptoxanthin in the obtained product is high, and comprehensive cost for the extraction is controllable; therefore, the method is suitable for industrial production and can be used for lengthening manufacturing chain.
Owner:QINHUANGDAO DAHUI BIOLOGICAL TECH
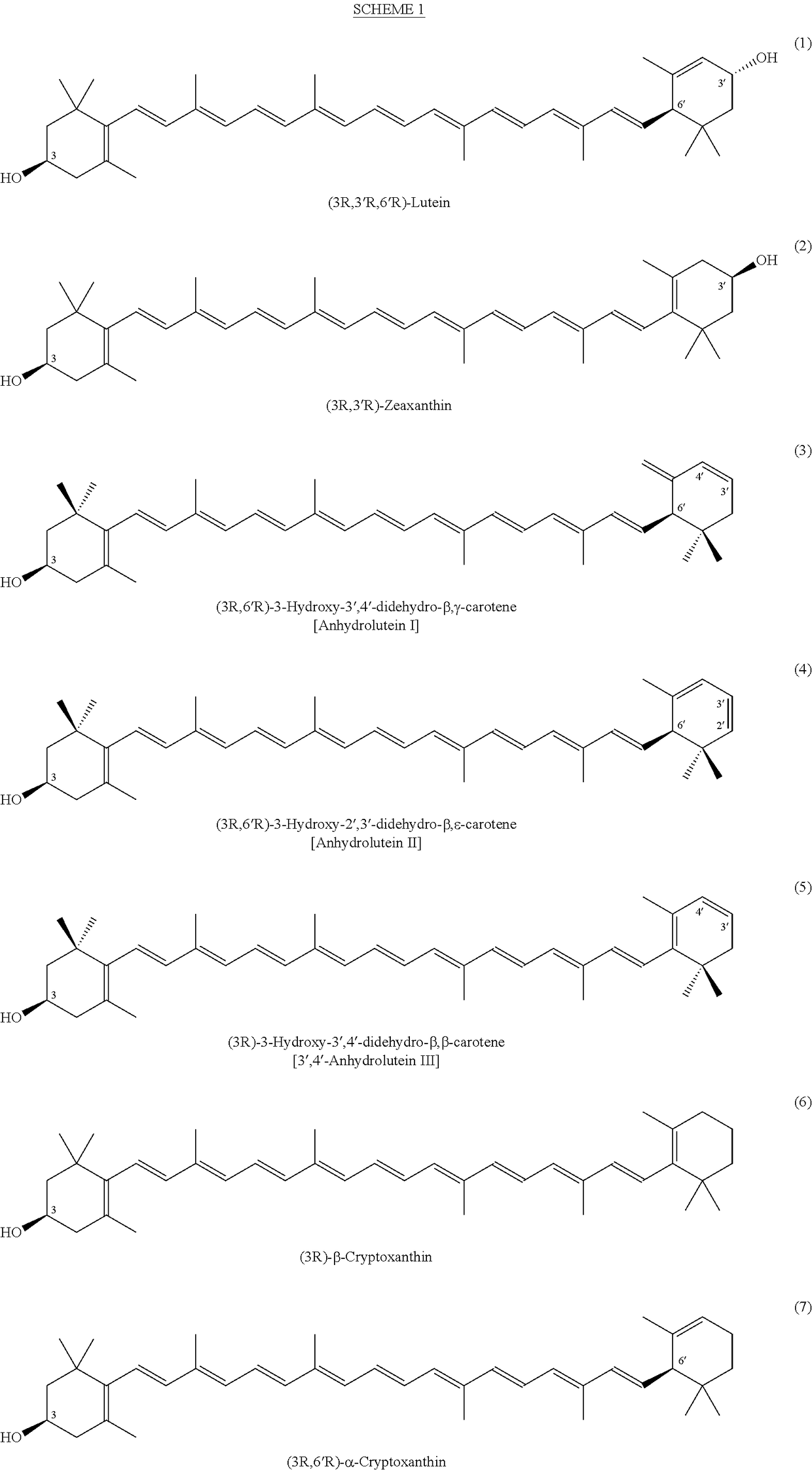
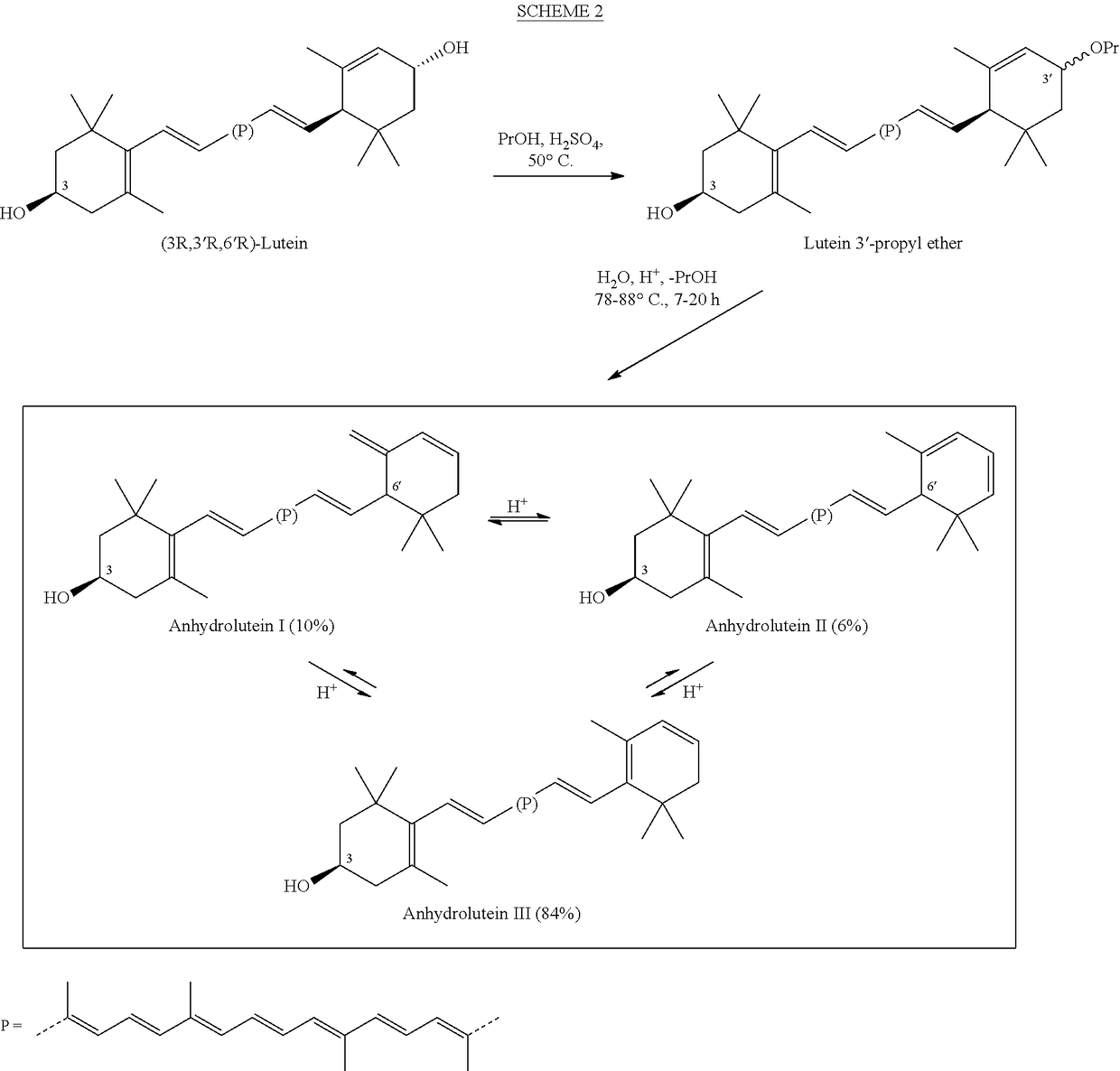
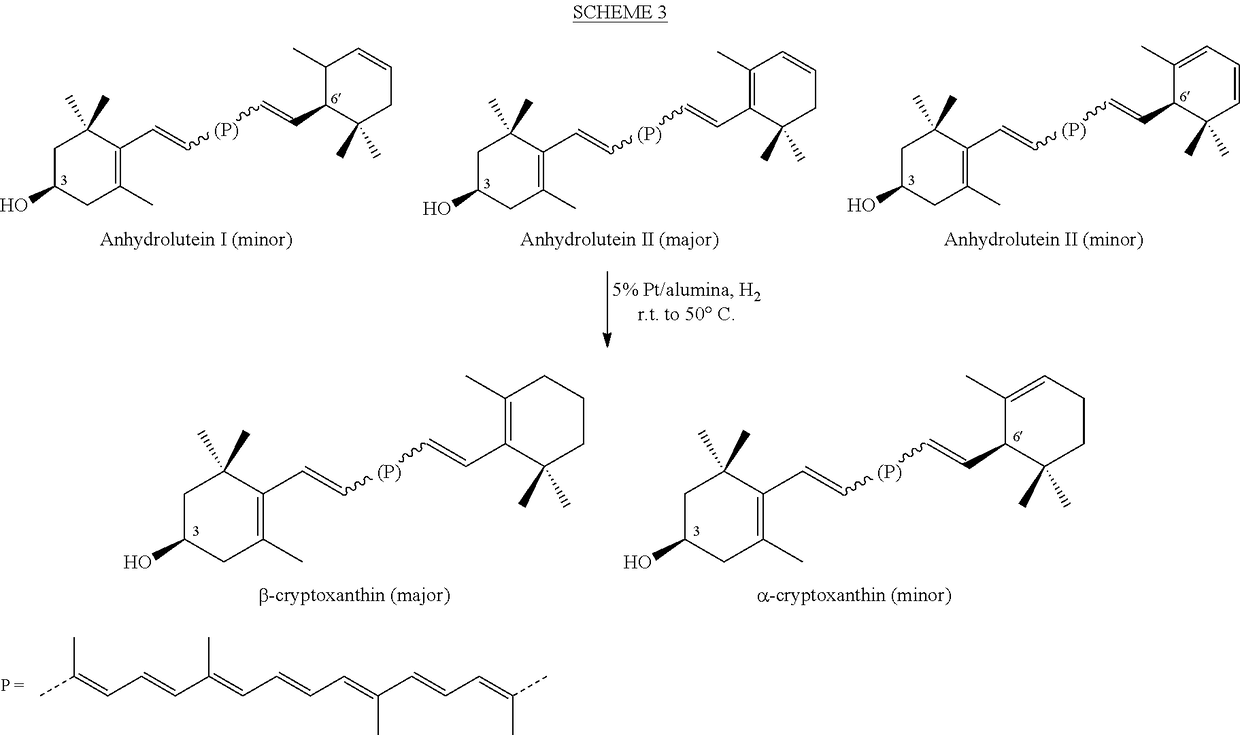
Process for the preparation of alpha- and beta-cryptoxanthin
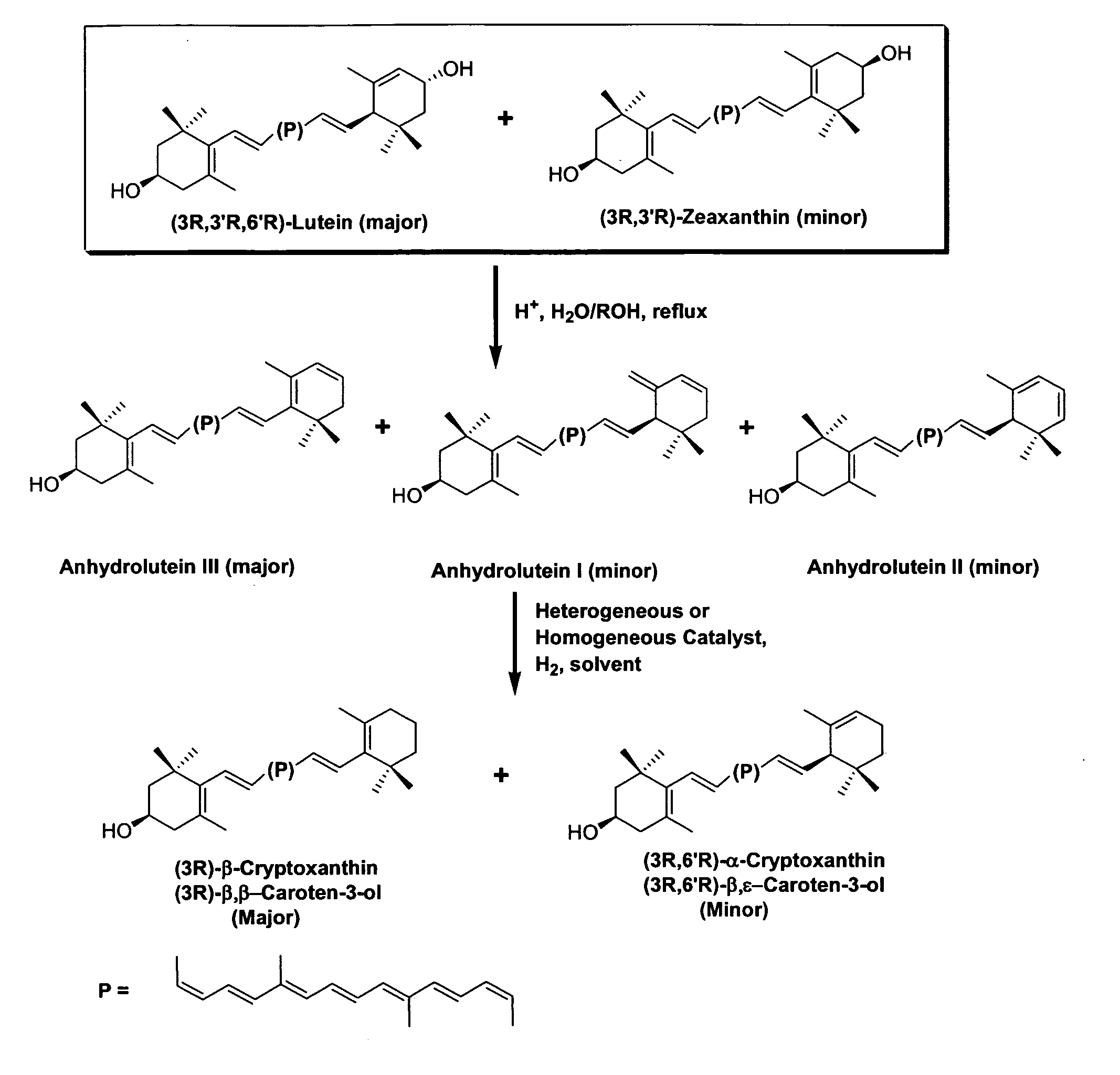
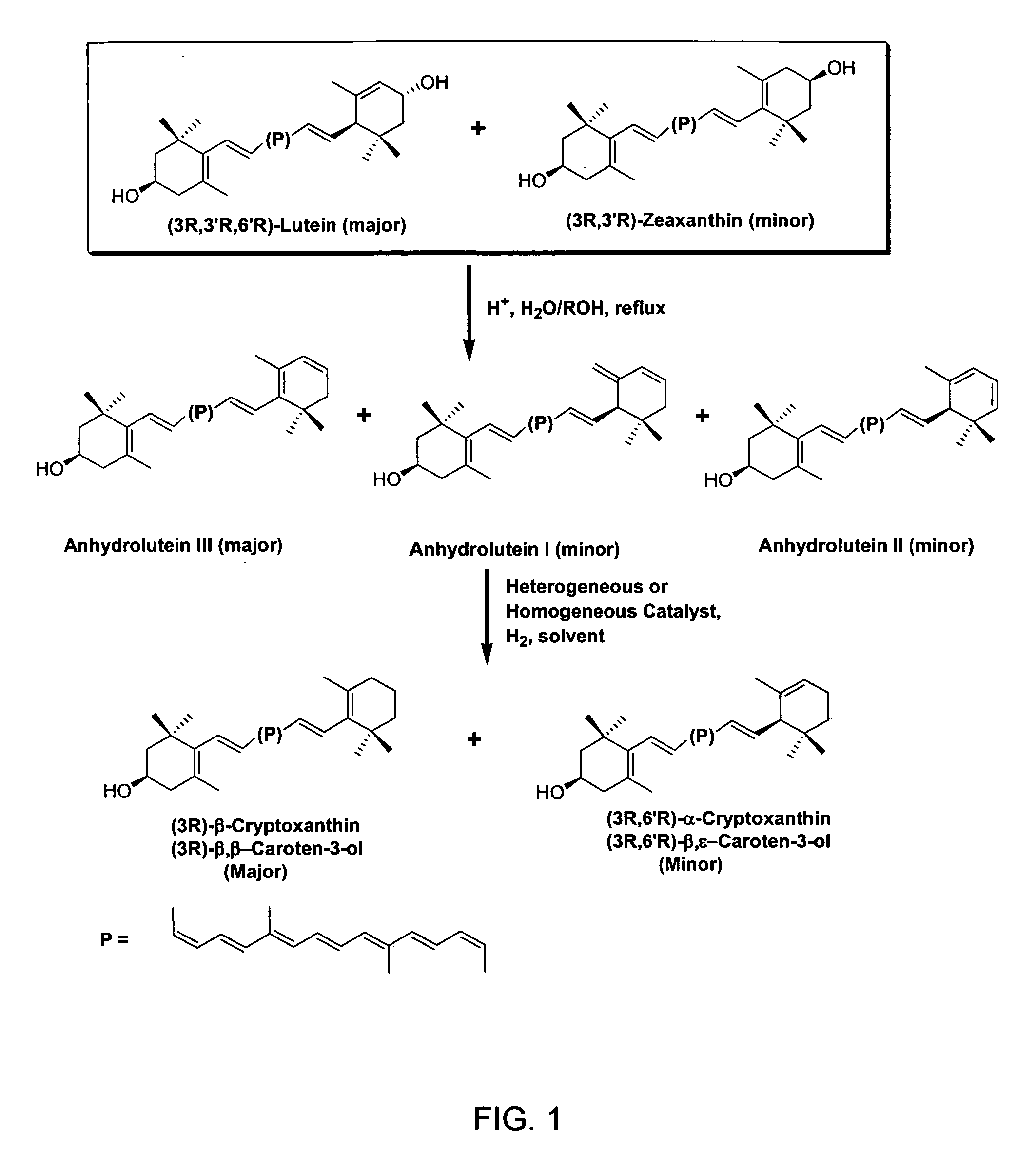
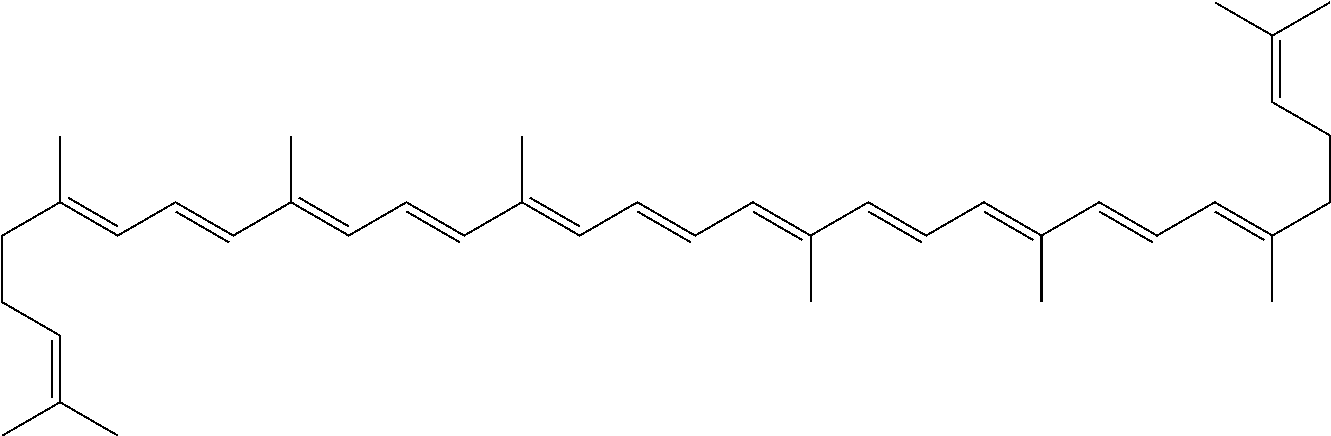
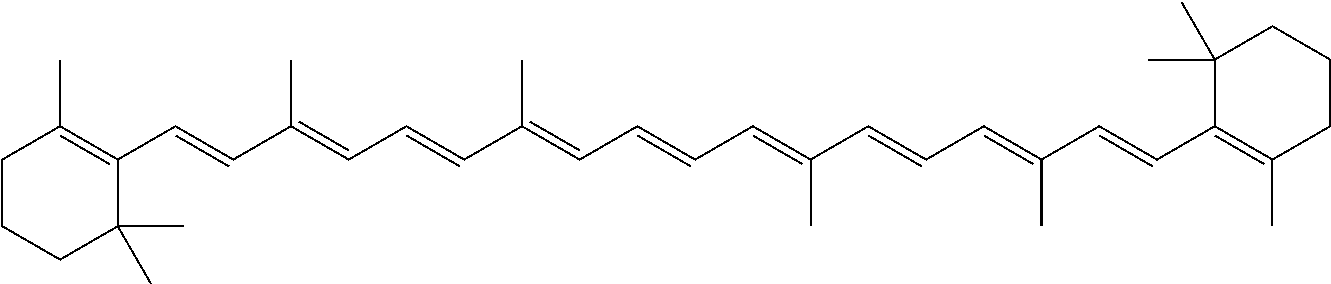
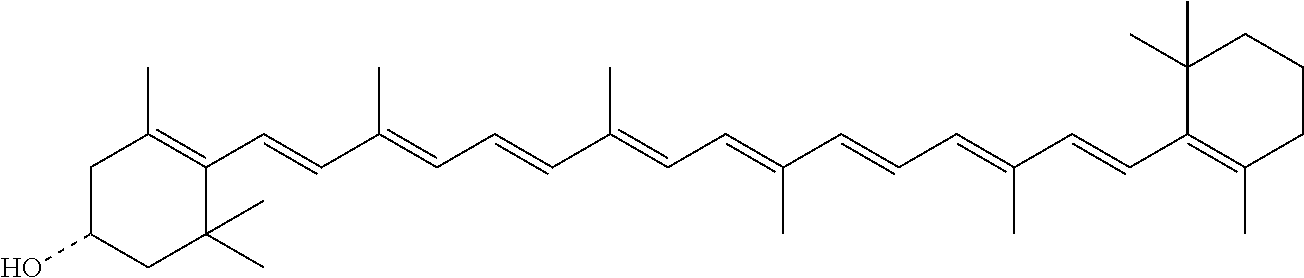
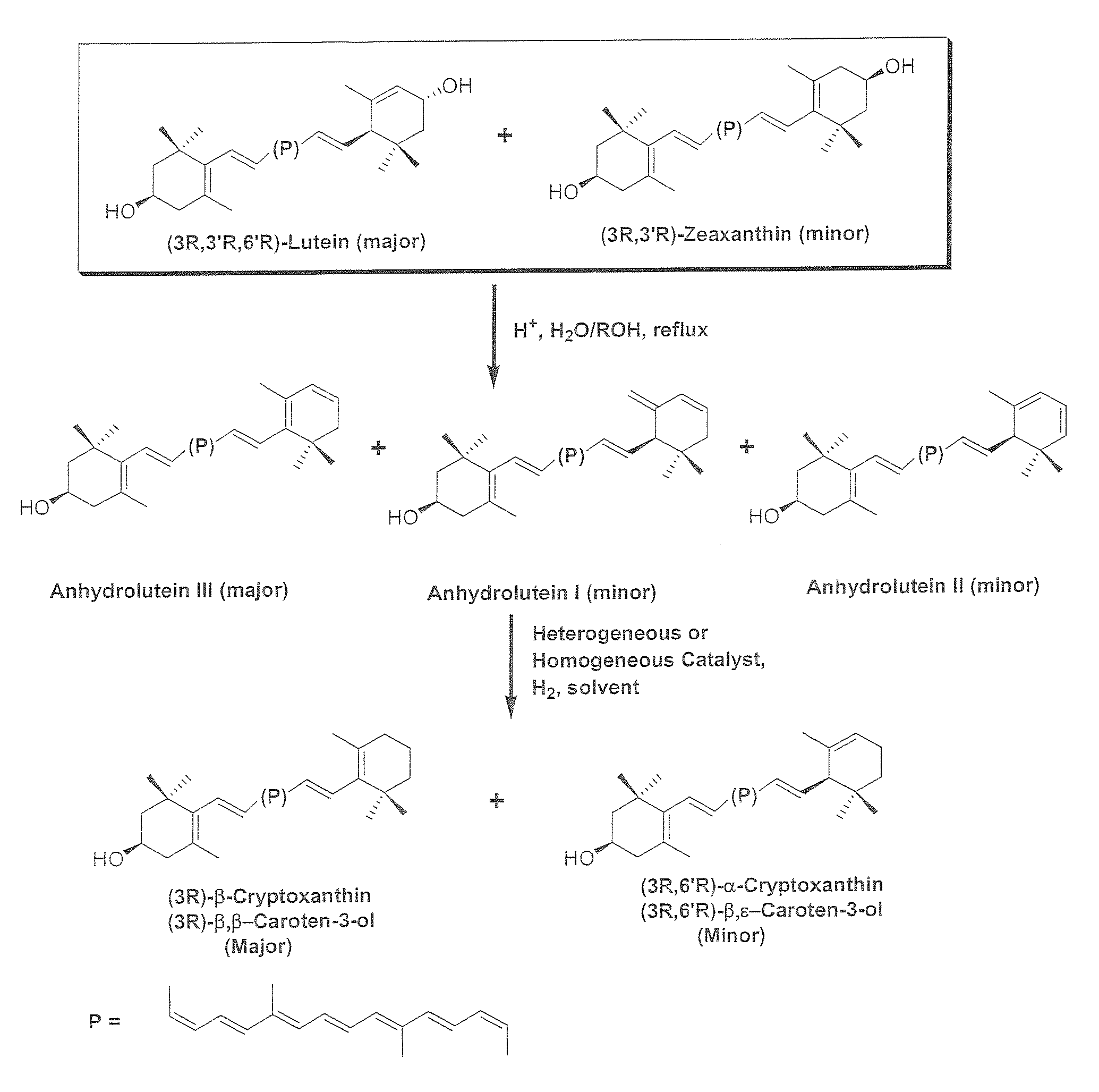
InactiveUS20060088631A1Easy to useHigh selectivityBeer brewingFood preparationDietary supplementCryptoxanthin
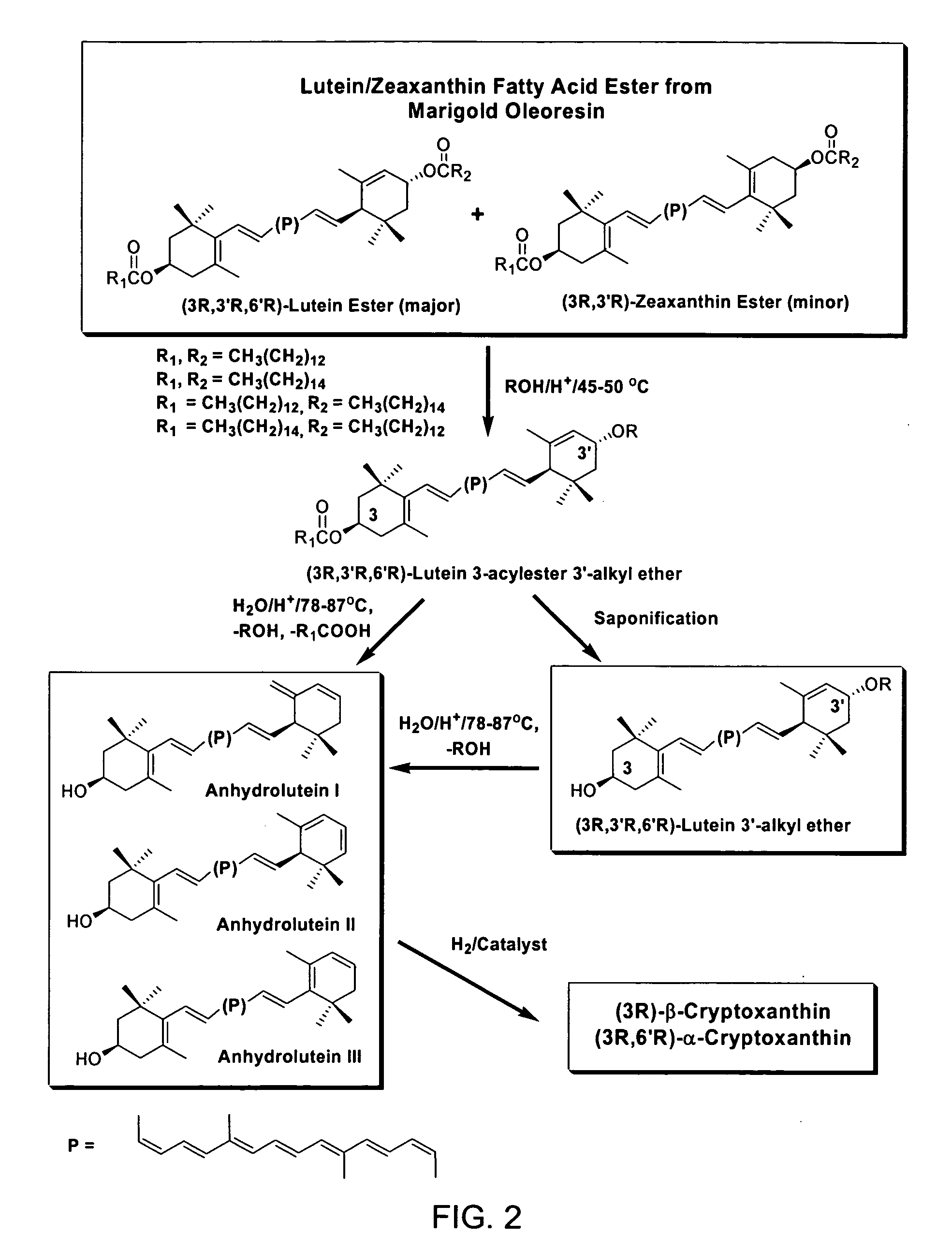
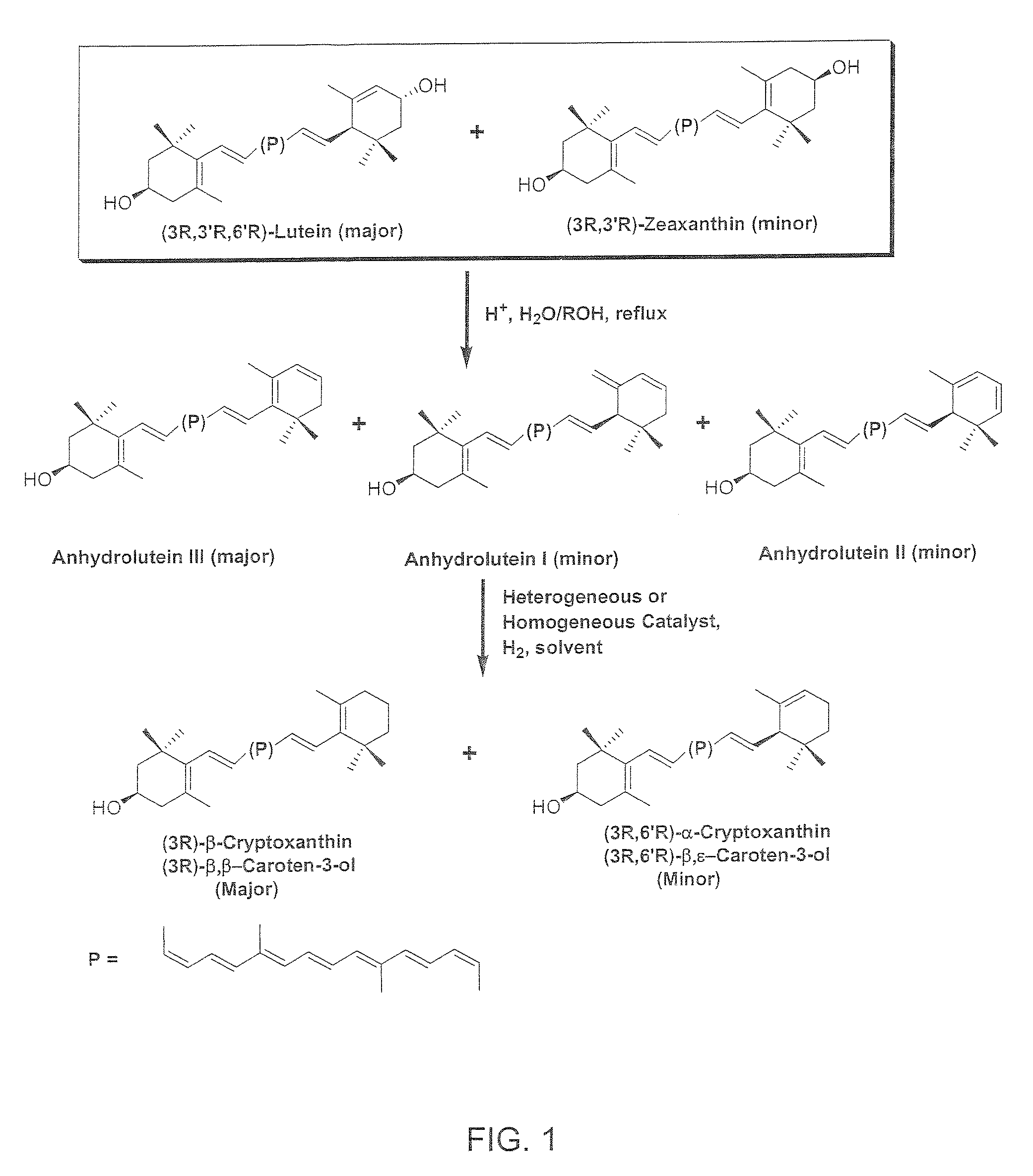
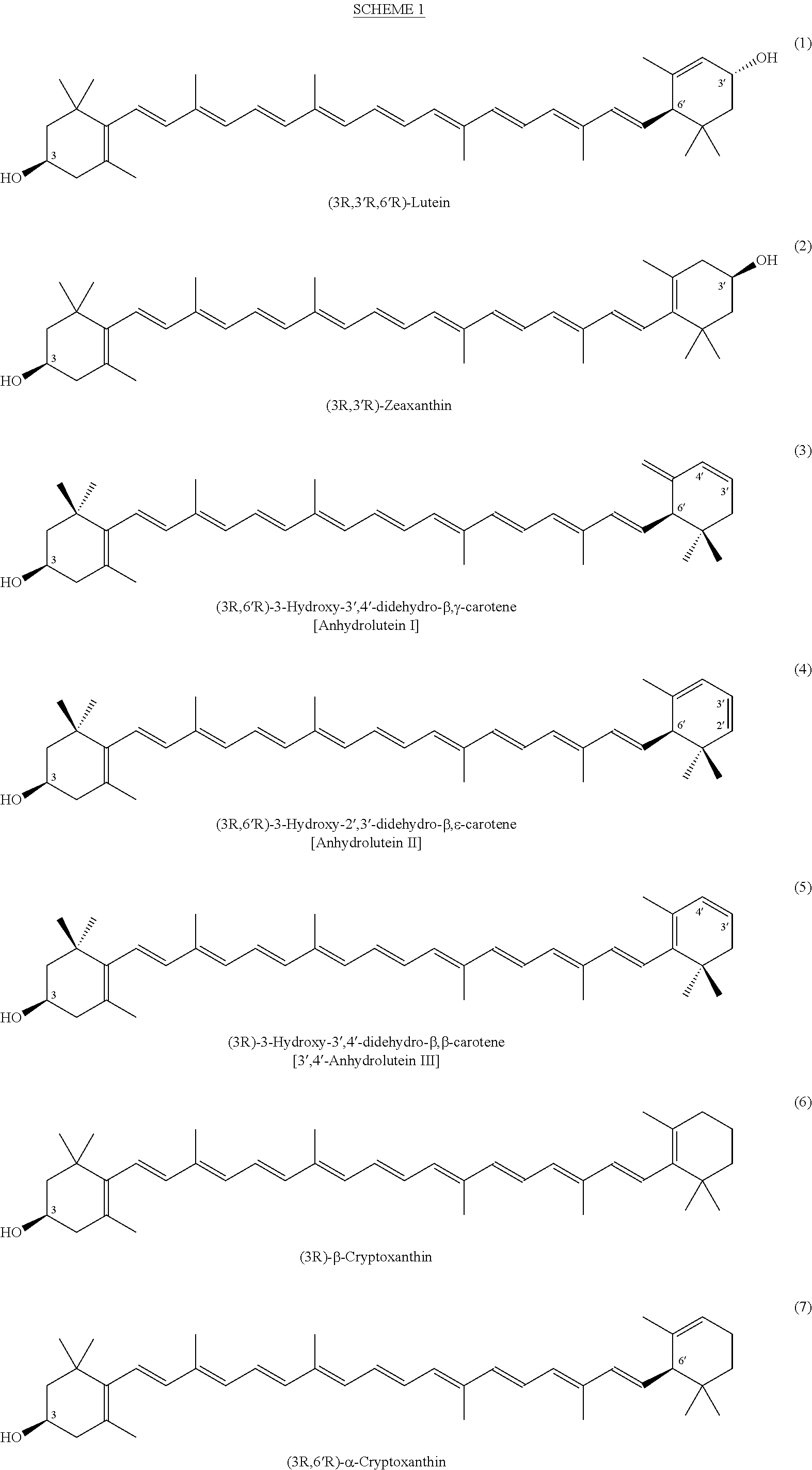
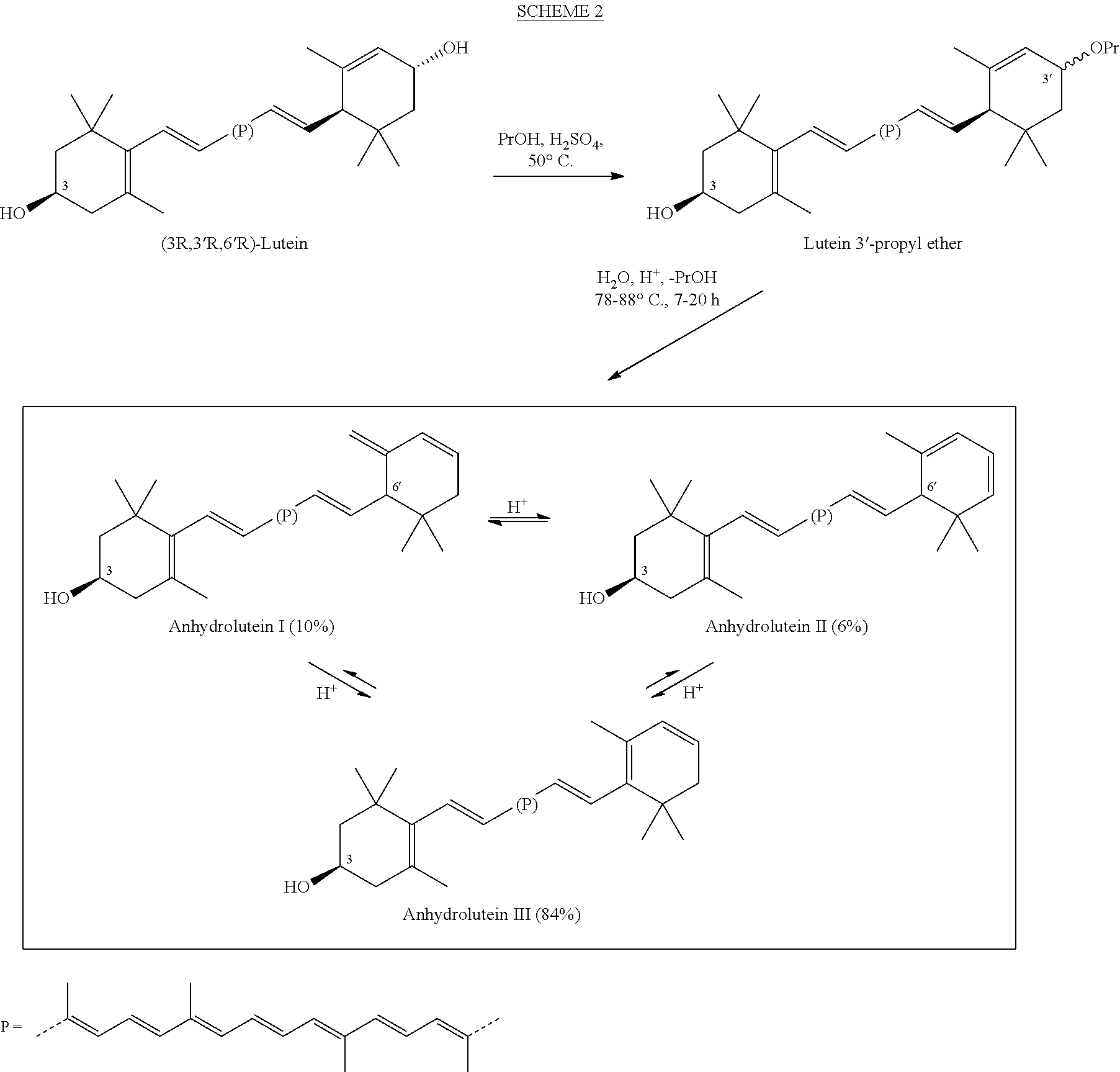
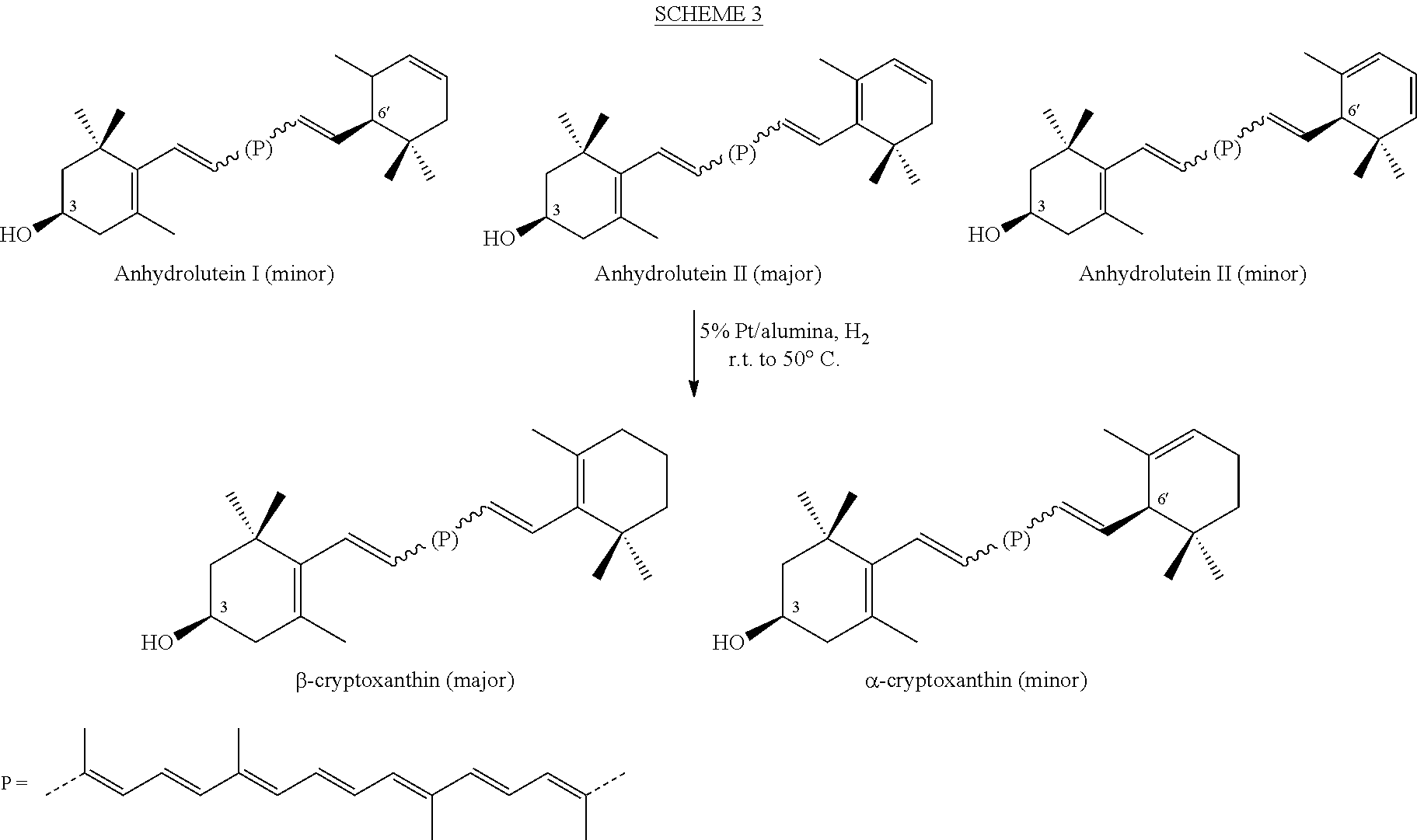
The present invention relates to a process for converting lutein and / or lutein esters to (3R)-β-cryptoxanthin and (3R,6′R)-α-cryptoxanthin, suitable for human consumption as dietary supplements, by employing safe and environmentally friendly reagents. (3R)-β-Cryptoxanthin and (3R,6′R)-α-cryptoxanthin are two rare food carotenoids that are not commercially available and the former exhibits vitamin A activity. In the first synthetic step, commercially available lutein and / or lutein esters are transformed into a mixture of dehydration products of lutein (anhydroluteins) in the presence of a catalytic amount of an acid. The resulting anhydroluteins are then converted to (3R)-β-cryptoxanthin (major product) and (3R,6′R)-α-cryptoxanthin (minor product) by heterogeneous catalytic hydrogenation employing transition elements of group VIII (Pt, Pd, Rh supported on alumina or carbon) in a variety of organic solvents under atmospheric pressure of hydrogen and at temperatures ranging from −15° C. to 40° C. Among these catalysts, Pt supported on alumina at 40° C. in ethyl acetate provides the best yield of (3R)-β-cryptoxanthin and (3R,6′R)-α-cryptoxanthin. Several homogeneous catalysts can also promote the regioselective hydrogenation of anhydroluteins to a mixture of (3R)-β-cryptoxanthin and (3R,6′R)-α-cryptoxanthin in low to moderate yields. The catalysts may be transition metal complexes such as palladium acetylacetonate, Rh(Ph3P)3Cl (Wilkinson's catalyst), [(C6H11)3P[C8H12][C5H5N]Ir+PF6− (Crabtree catalyst), or [C8H12][(MePh2P)2]Ir+PF6−. Among these, Wilkinson catalyst converts anhydroluteins to (3R)-β-cryptoxanthin and (3R,6′R)-α-cryptoxanthin in nearly quantitative yield. A novel feature of this invention is the regioselective hydrogenation of anhydroluteins while the highly conjugated polyene chain of these carotenoids remains intact.
Owner:UNIV OF MARYLAND
Process for Synthesis of (3S)- and (3R)-3-Hydroxy-Beta-Ionone, and Their Transformation to Zeaxanthin and Beta-Cryptoxanthin
InactiveUS20090311761A1High yieldOrganic compound preparationCarbonyl compound preparationMetaboliteIsomerization
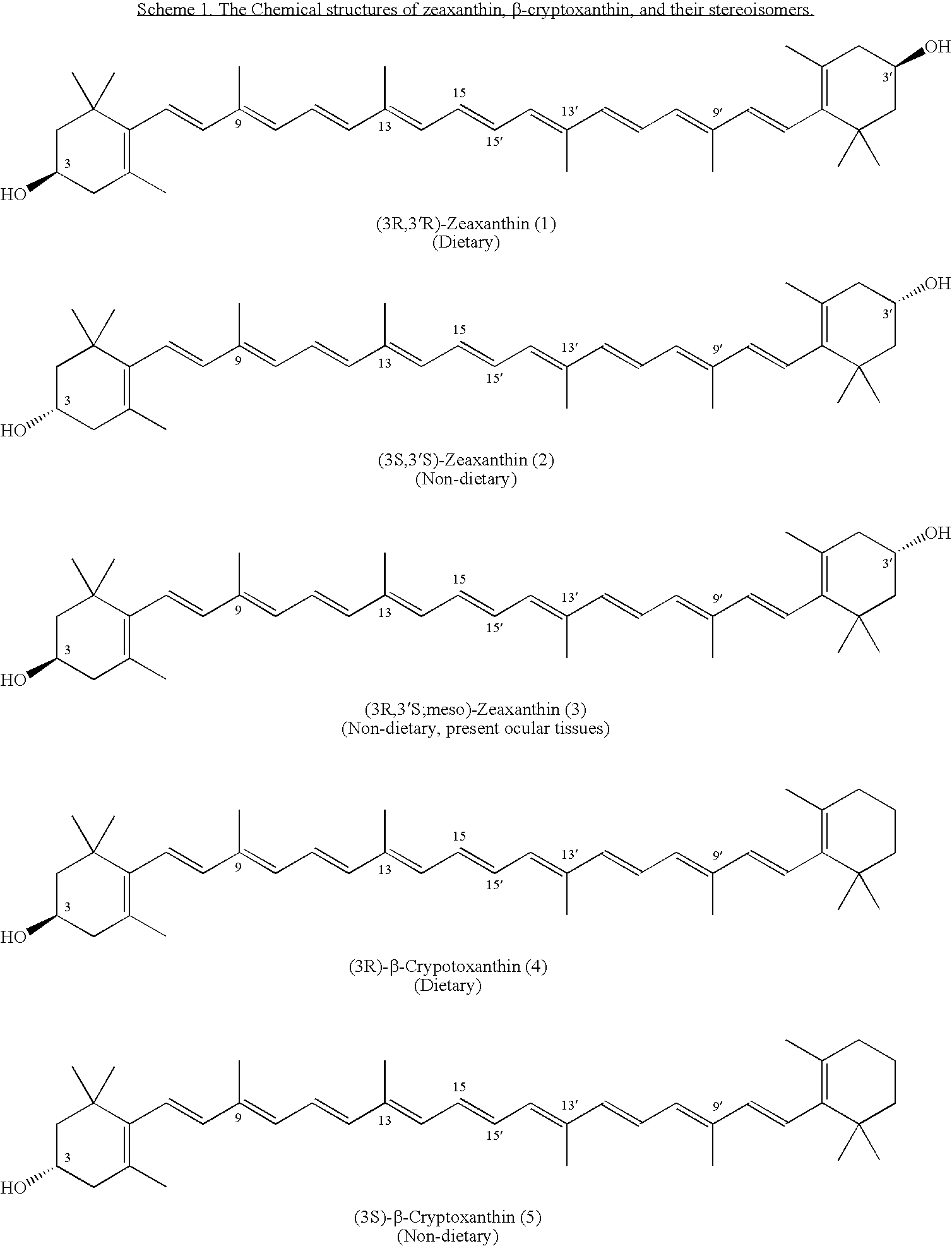
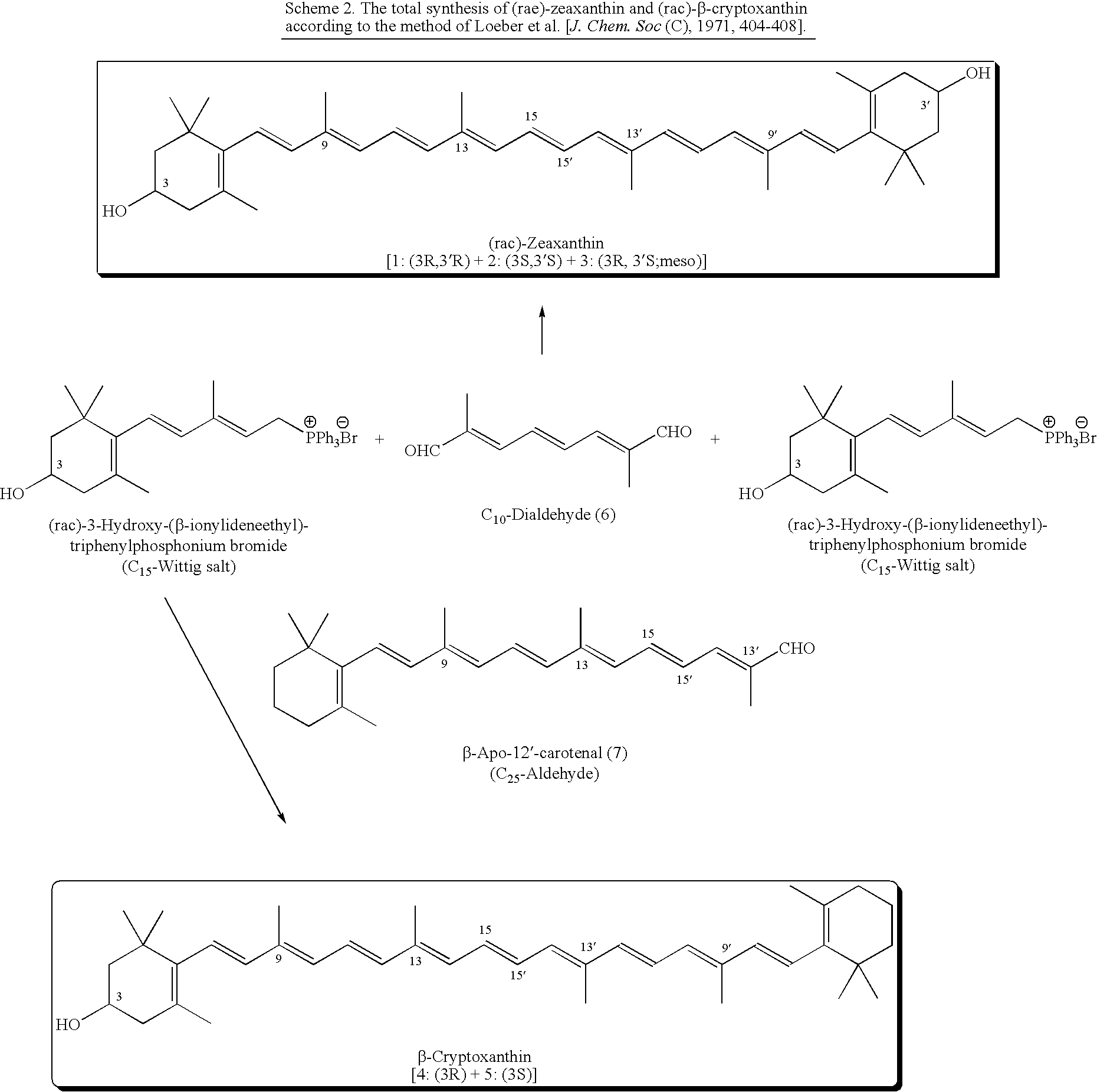
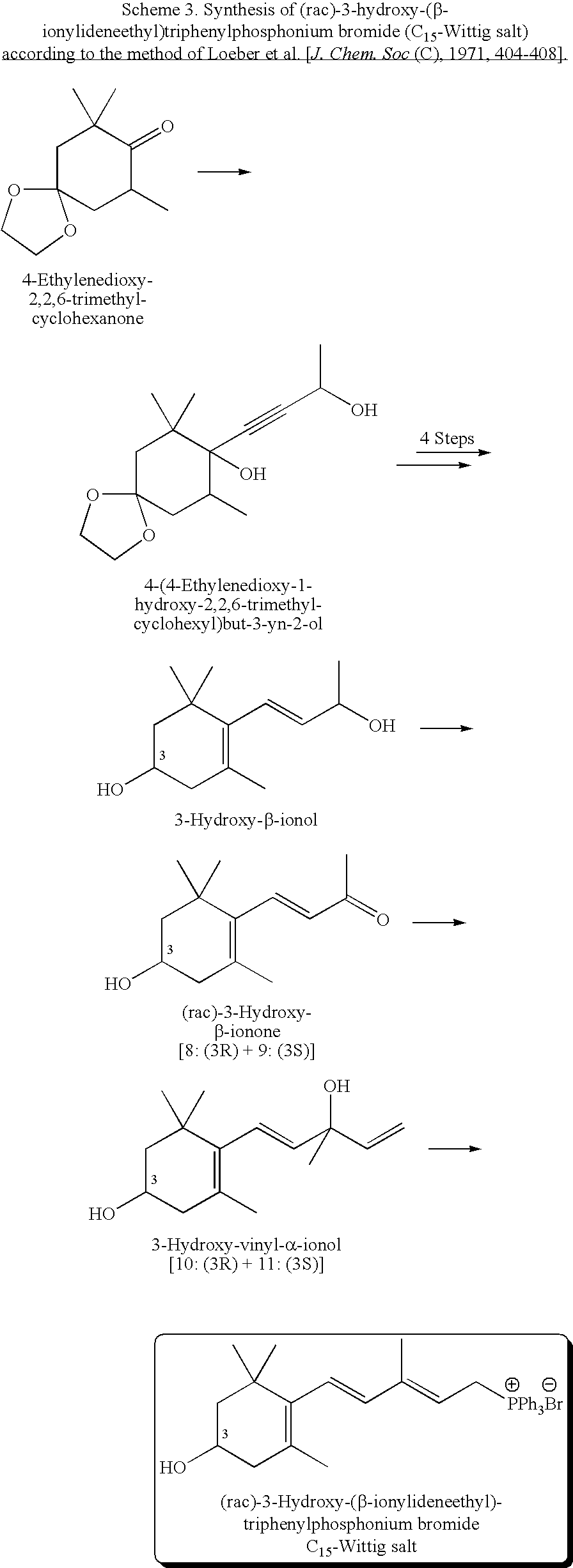
(3R)-3-Hydroxy-β-ionone and (3S)-3-hydroxy-β-ionone are two important intermediates in the synthesis of carotenoids with β-end group such as lutein, zeaxanthin, β-cryptoxanthin, and their stereoisomers. Among the various stereoisomers of these carotenoids, only (3R,3′R,6′R)-lutein, (3R,3′R)-zeaxanthin, and (3R)-β-cryptoxanthin are present in commonly consumed fruits and vegetables. There are 3 possible stereoisomers for zeaxanthin, these are: dietary (3R,3′R)-zeaxanthin (1), non-dietary (3S,3′S)-zeaxanthin (2), and non-dietary (3R,3′S;meso)-zeaxanthin (3) which is a presumed metabolite of dietary lutein. Dietary lutein as well as 1 and 3 are accumulated in the human macula and have been implicated in the prevention of age-related macular degeneration. (3R)-β-Cryptoxanthin (4) is also present in selected ocular tissues at a very low concentration whereas its enantiomer (3S)-β-cryptoxanthin (5) is absent in foods and human plasma.The present invention relates to a process for the synthesis of (3R)-3-hydroxy-β-ionone and its (3S)-enantiomer in high optical purity from commercially available (rac)-α-ionone. The key intermediate for the synthesis of these hydroxyionones is 3-keto-α-ionone ketal that was prepared from (rac)-α-ionone after protection of this ketone as a 1,3-dioxolane. Reduction of 3-keto-α-ionone ketal followed by deprotection, lead to 3-hydroxy-α-ionone that was transformed into (rac)-3-hydroxy-β-ionone by base-catalyzed double bond isomerization in 46% overall yield from (rac)-α-ionone. The racemic mixture of these hydroxyionones was then resolved by enzyme-mediated acylation in 96% ee. (3R)-3-Hydroxy-β-ionone and its (3S)-enantiomer were respectively transformed to (3R)-3-hydroxy-(β-ionylideneethyl)triphenylphosphonium chloride [(3R)—C15-Wittig salt] and its (3S)-enantiomer [(3S)—C15-Wittig salt] according to known procedures. Double Wittig condensation of these Wittig salts with commercially available 2,5-dimethylocta-2,4,6-triene-1,8-dial provided all 3 stereoisomers of zeaxanthin (1-3). Similarly, (3R)—C15-Wittig and its (3S)-enantiomer were each coupled with β-apo-12′-carotenal to yield 4 and 5.
Owner:UNIV OF MARYLAND
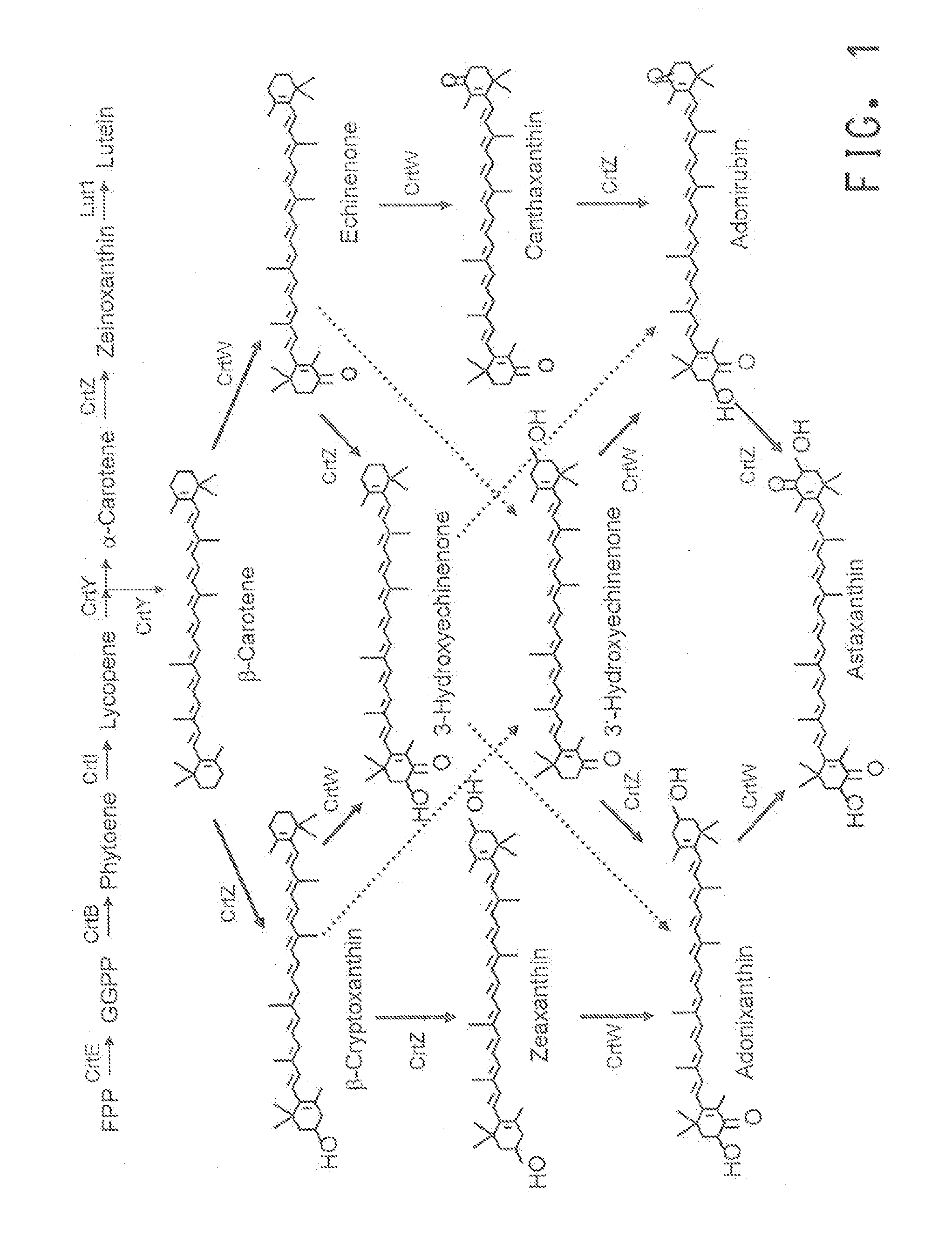
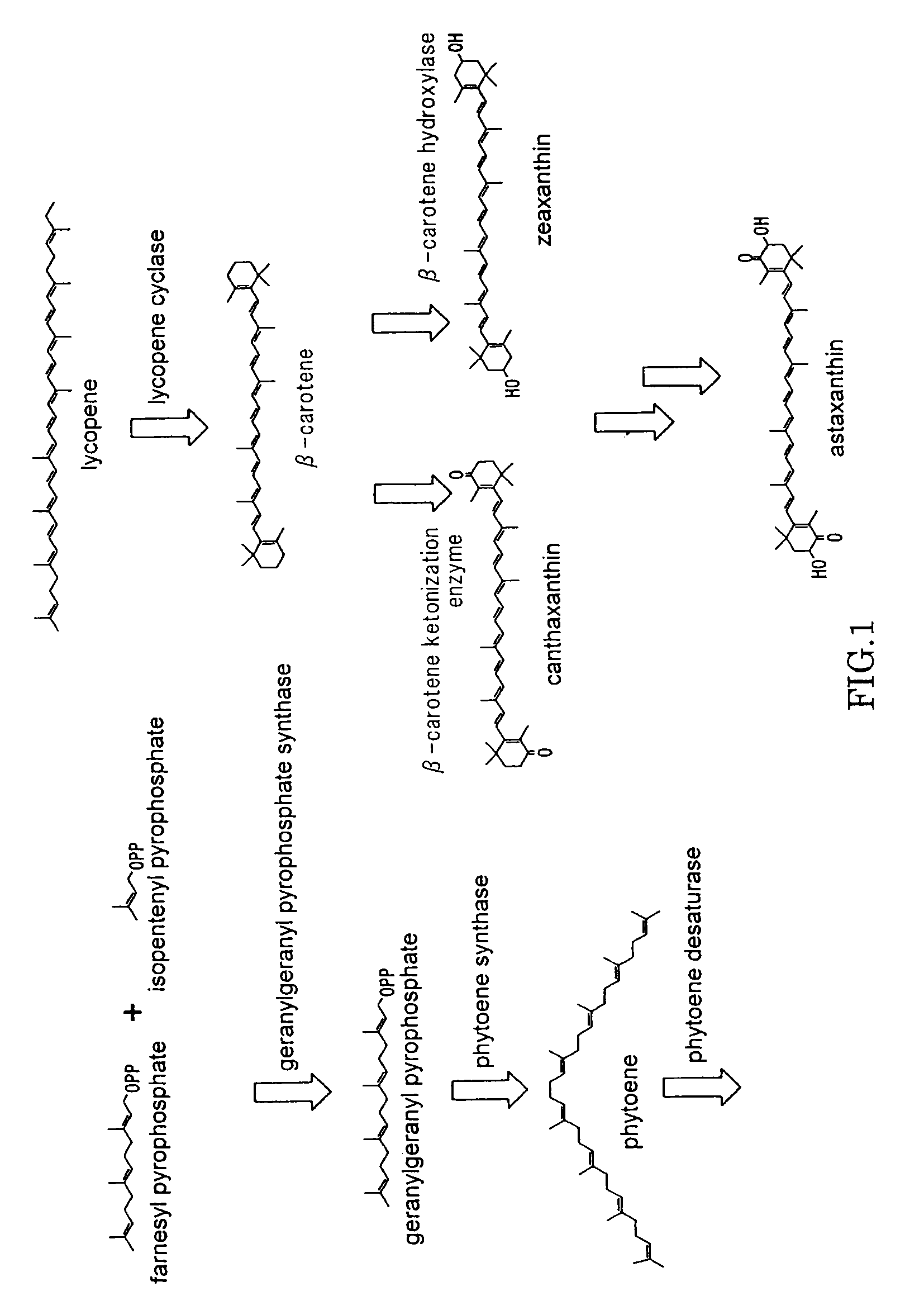
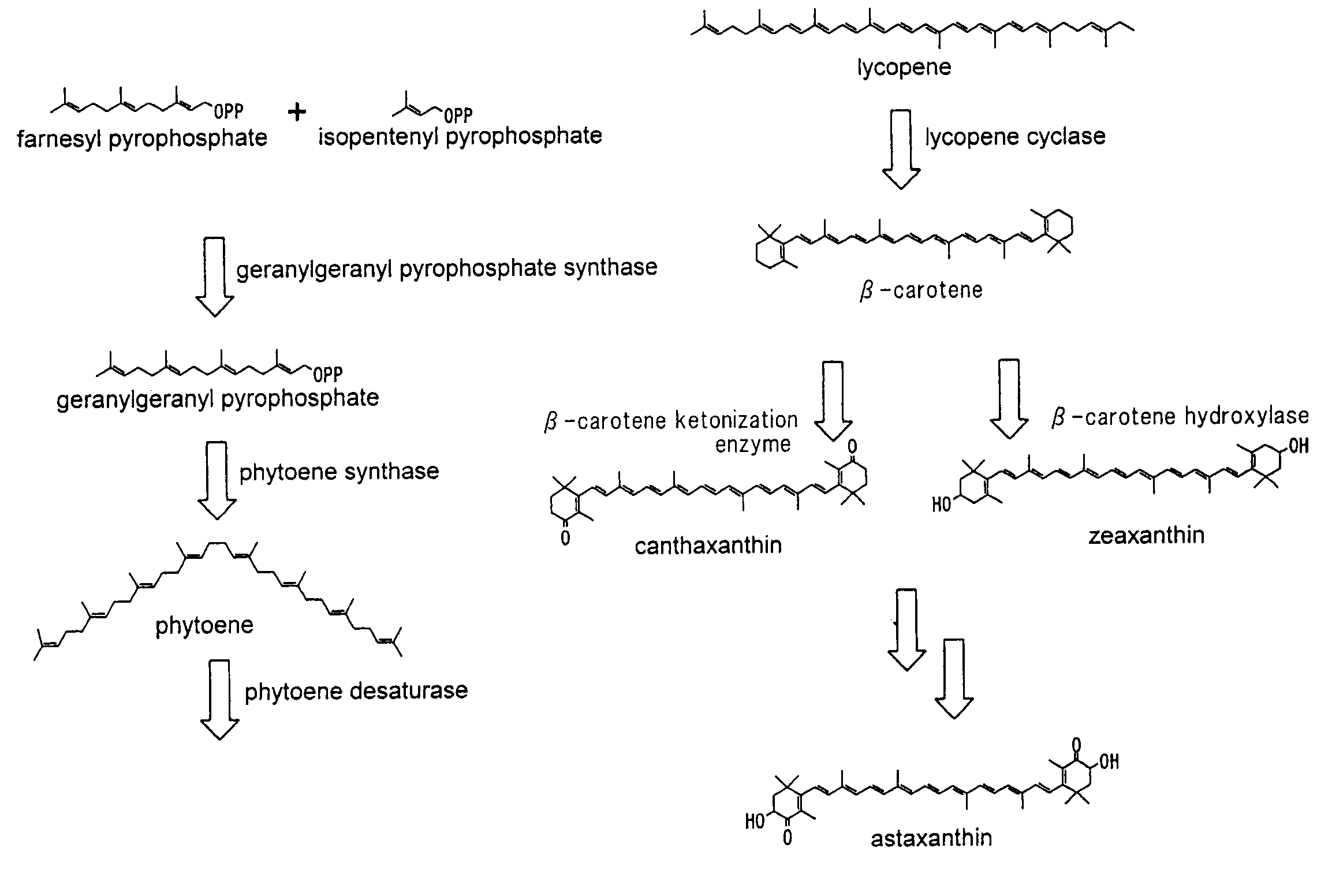
Astaxanthin production using a recombinant microbial host cell
A recombinant microbial host cell is provided capable of producing astaxanthin from β-carotene without a measurable concomitant accumulation of ketolated or hydroxylated intermediates such as adonixanthin, zeaxanthin, adonirubin, echinenone, 3-hydroxyechinenone, 3′-hydroxyechinenone, canthaxanthin, and β-cryptoxanthin. Specifically, a β-carotene producing microbial host cell was engineered to express two heterologous genes, a β-carotene ketolase from Chlamydomonas reinhardtii in combination with a carotenoid hydroxylase from Brevundimonas vesicularis or Arabidopsis thaliana.
Owner:EI DU PONT DE NEMOURS & CO
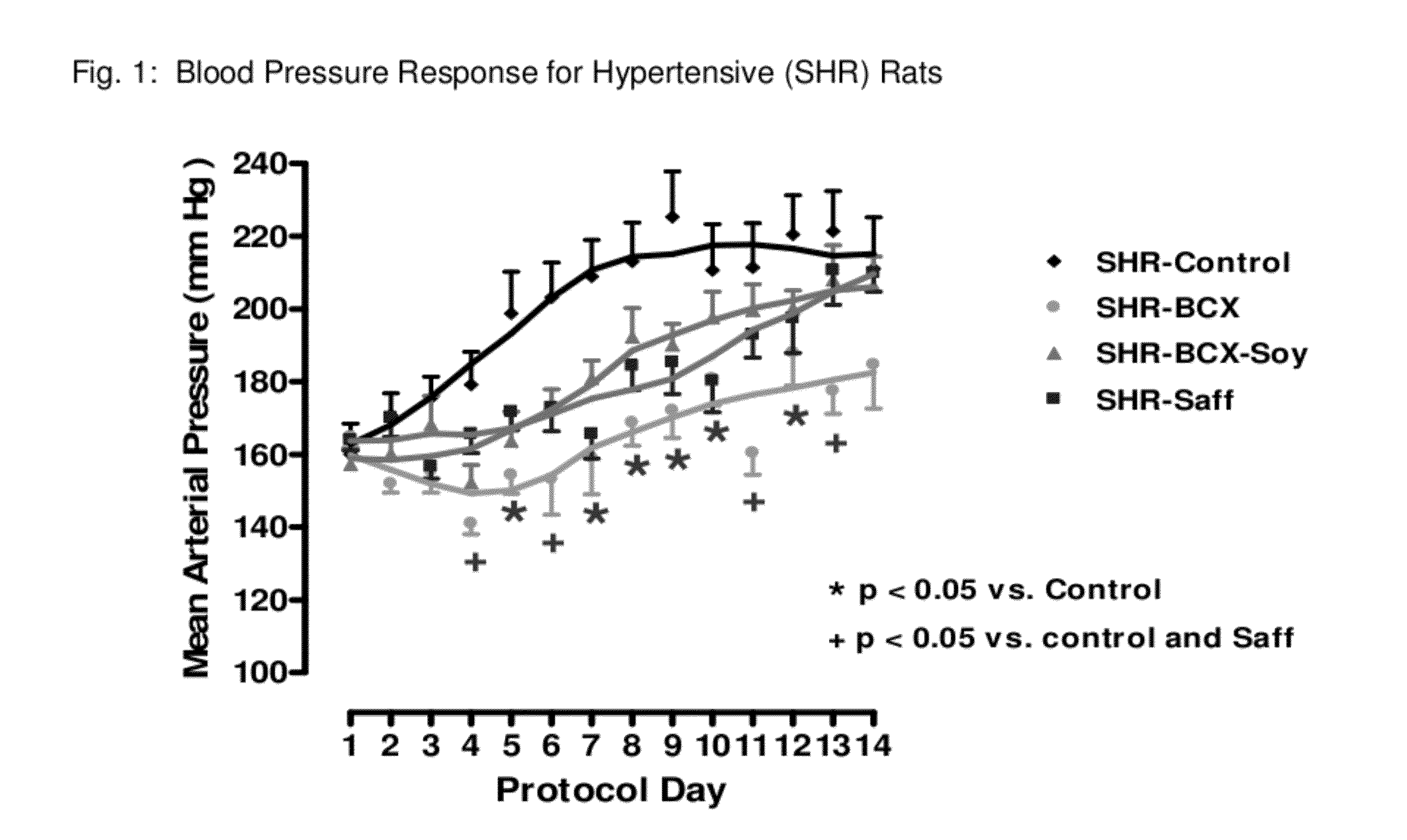
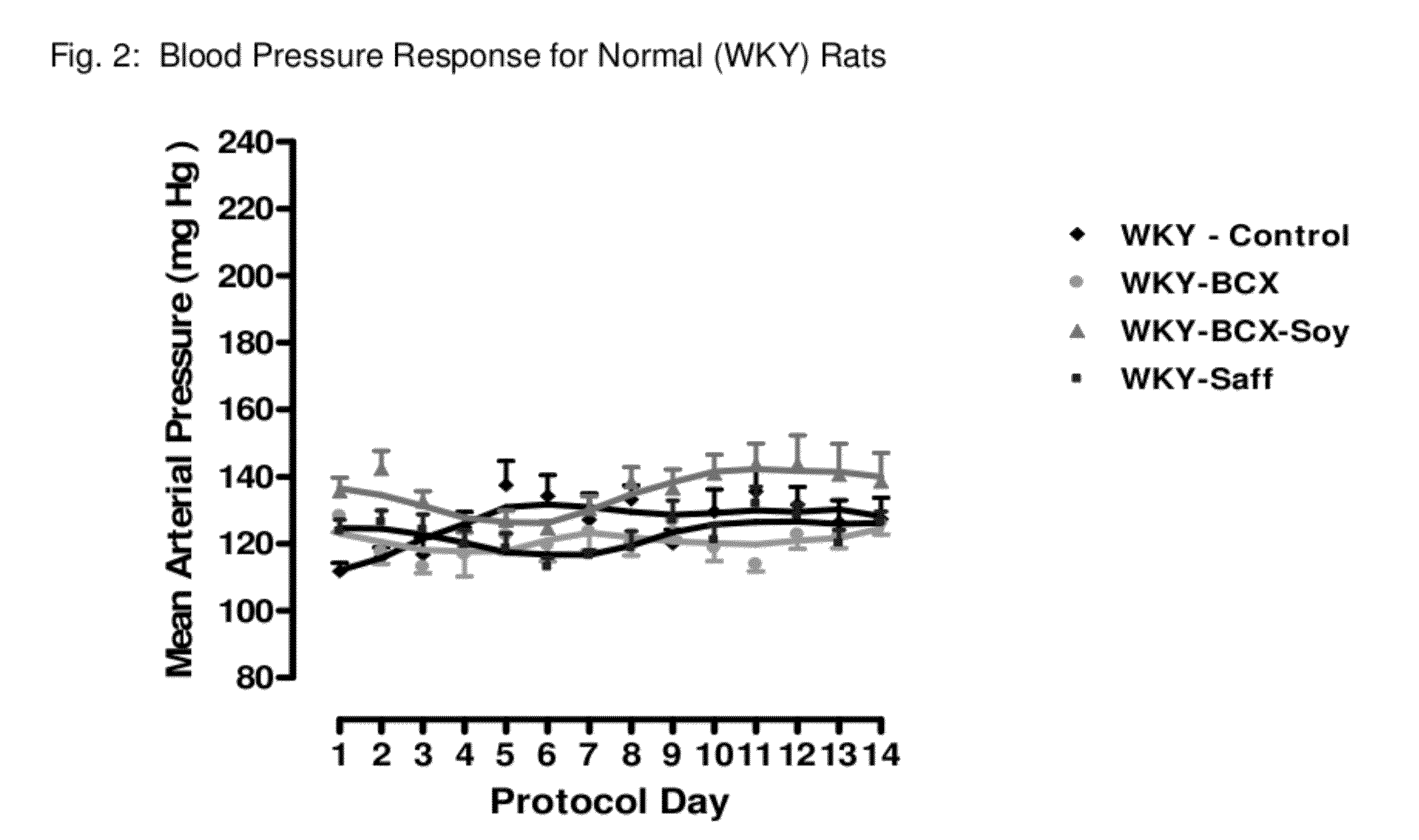
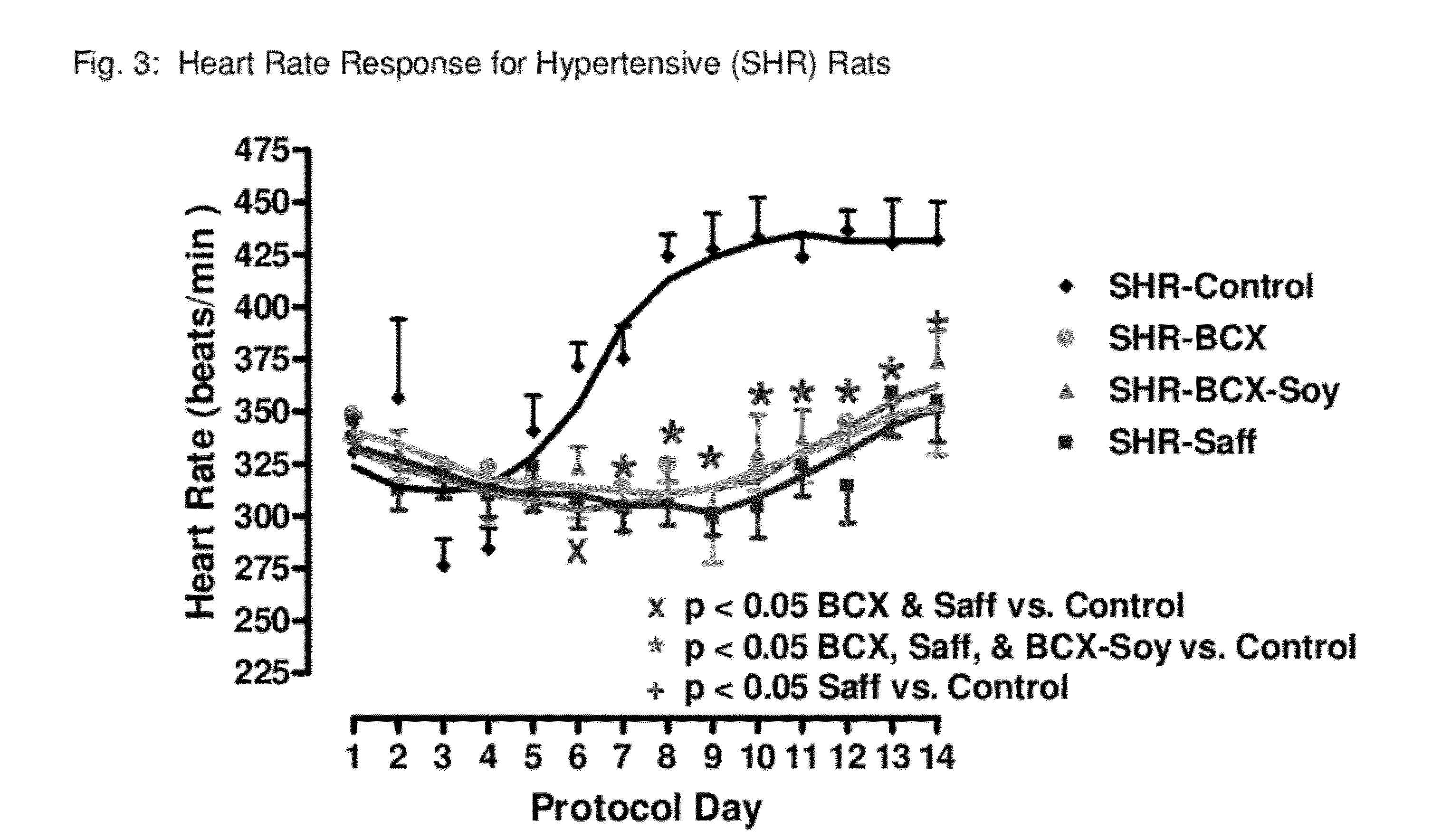
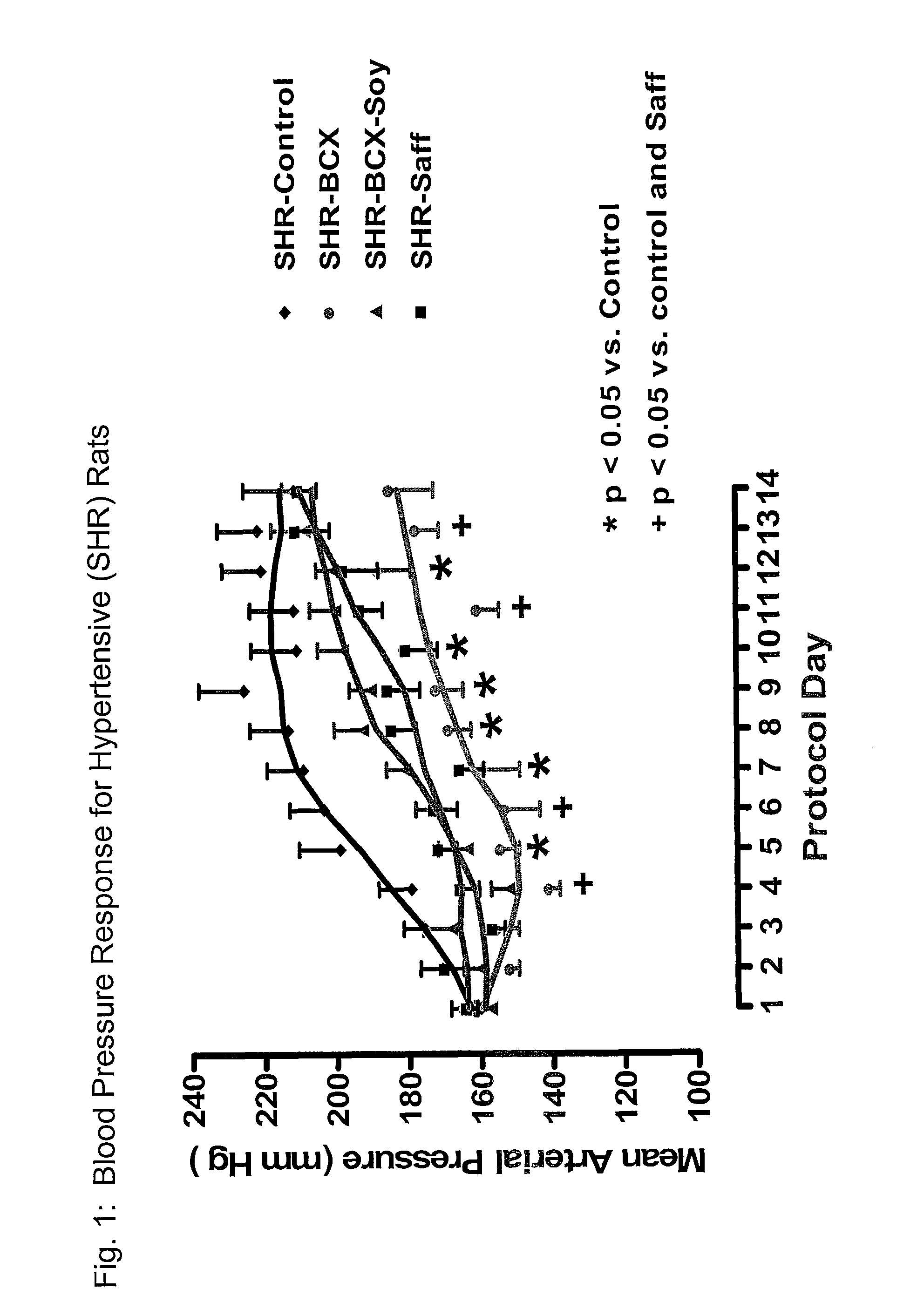
Method of improving cardiovascular health
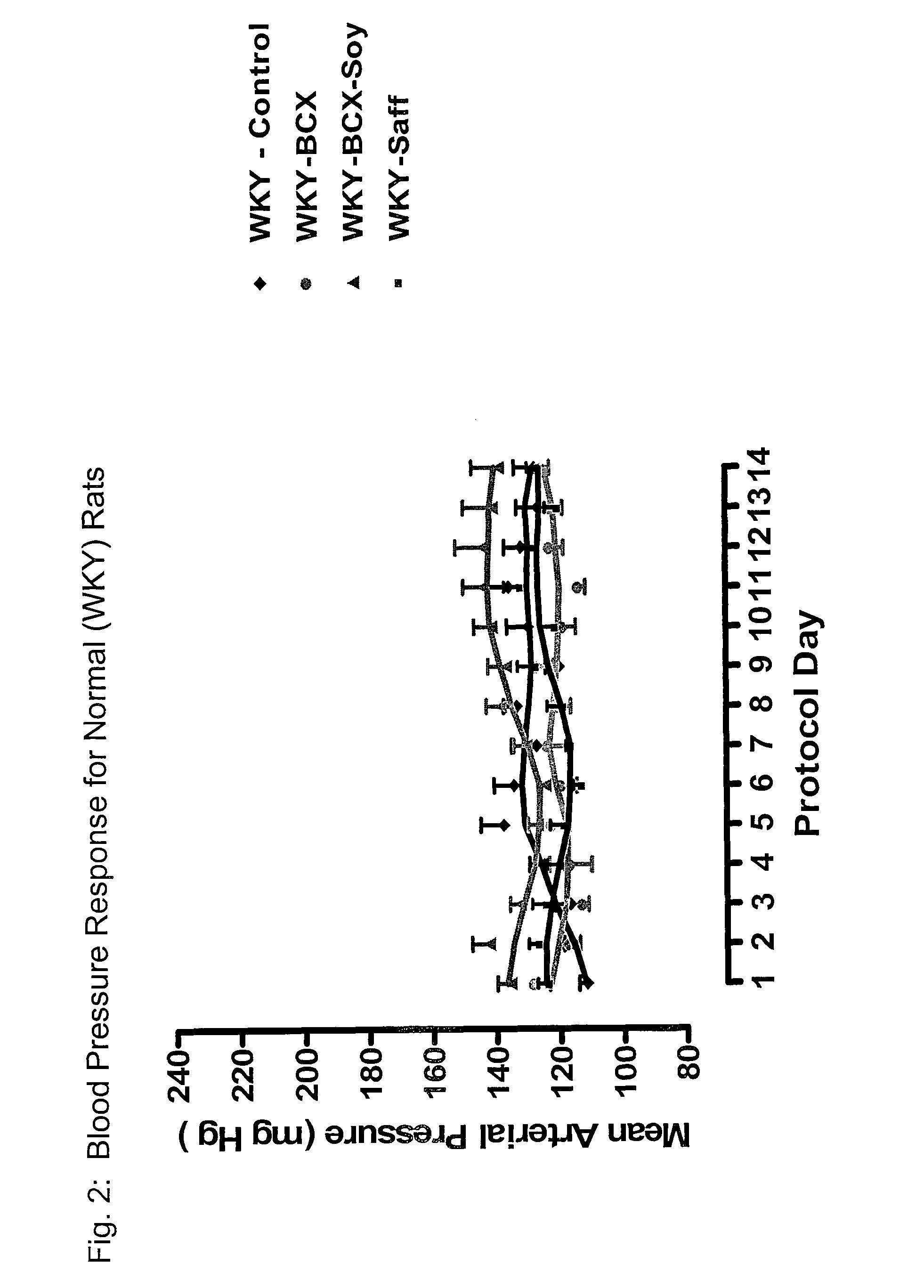
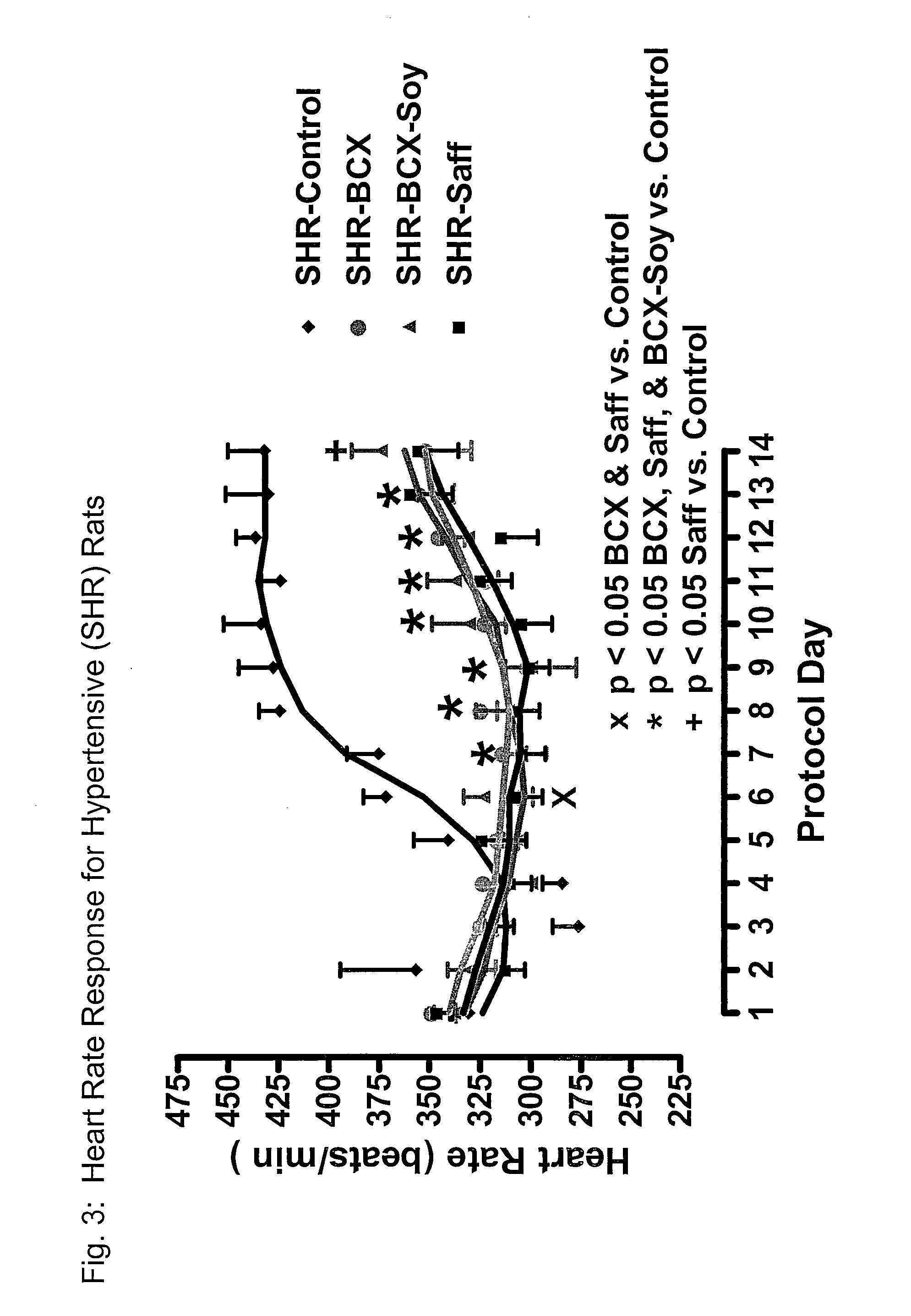
ActiveUS20120053247A1Promotes cardiovascular healthLower blood pressureBiocideHydroxy compound active ingredientsΒ cryptoxanthinBeta-cryptoxanthin
A nutritional supplement including β-cryptoxanthin is found to be effective at lowering blood pressure in mammals. β-cryptoxanthin therefore may be used to maintain cardiovascular health by lowering blood pressure, preventing high, elevated blood pressure and / or maintaining healthy blood pressure. Administration of β-cryptoxanthin in combination with safflower oil is particularly effective.
Owner:BANK OF AMERICA NAT TRUST & SAVINGS ASSOC
Extract liquid containing -cryptoxanthin ingredient, and food or beverage and soap or cosmetic each containing the extract liquid
There are provided an extract solution to be added to food and drink containing a Beta-cryptoxanthin component consisting of an extract containing a Beta-cryptoxanthin component dissolved or dispersed in fat or oil or in a solution comprising an emulsifier and an extract solution to be added to soaps or cosmetics containing a Beta-cryptoxanthin component consisting of an extract containing a Beta-cryptoxanthin component dissolved or dispersed in fat or oil or in a solution comprising an emulsifier. There are also provided food and drink or soaps or cosmetics added with such extract solution containing a Beta-cryptoxanthin component. An extract containing a Beta-cryptoxanthin component derived from persimmon peel is preferable.
Owner:TOYO SEIKAN KAISHA LTD
Method and device for preparing highly-pure capsicum pigment capsaicin through supercritical fluid column chromatography
InactiveCN107163618ANo pollution in the processSafe and efficient processSolid sorbent liquid separationCarboxylic acid amide separation/purificationChromatographic separationBeta-cryptoxanthin
The invention relates to a method and a device for preparing a highly-pure capsicum pigment capsaicin through supercritical fluid column chromatography. The method comprises the following steps: mixing and dissolving supercritical carbon dioxide or modified supercritical carbon dioxide and capsicum oleoresin or saponification products thereof in a feeding tank, filtering the obtained solution, carrying out adsorption and desorption separation through a preparative chromatography column filled with solid adsorbent particles, collecting an eluate containing different solutes to corresponding collectors according to the signal of a detector, separating the solute components and carbon dioxide in a separator, carrying out filtering dedusting, condensation liquid removal, adsorption column purification and compression on tail gas to form a liquid, and returning the liquid to a storage tank in order to carry out cycle use. Extremely low solvent residual and even no solvent residual products with a high purity, such as capsanthin, capsorubin, beta-carotene, beta-cryptoxanthin, zeaxanthine, capsanthin ester, capsorubin ester and cryptoxanthin ester, and highly pure products, such as nordihydrocapsaicin, capsaicin and dihydrocapsaicin can be prepared through the method.
Owner:GUANGZHOU LEADER BIO TECH
Method for separating and purifying main carotenoids in orange peel
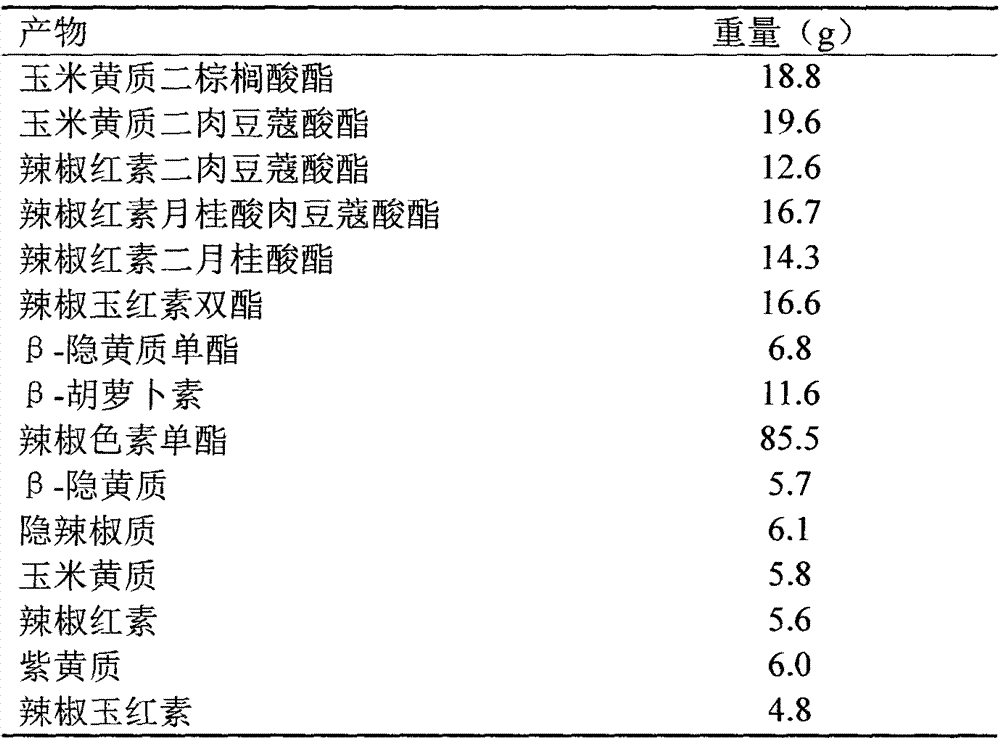
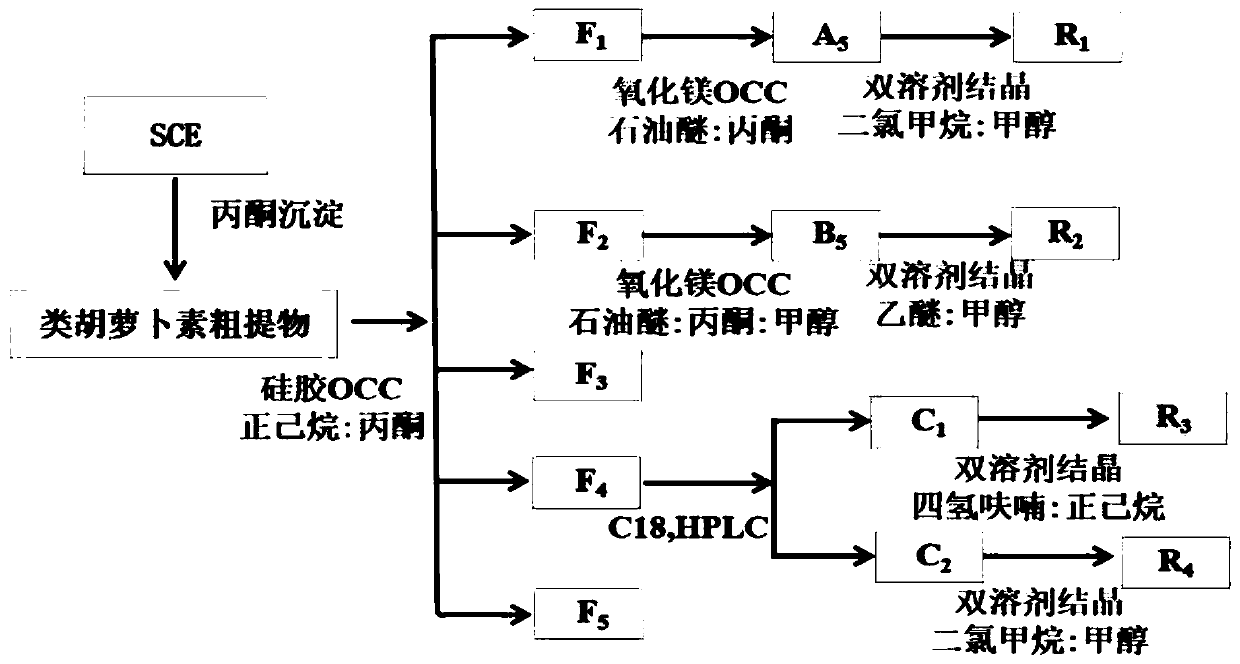
The invention belongs to the technical field of separation and purification. The invention discloses a separation and purification method of main carotenoids in orange peel. An orange peel carotenoidsaponification extract liquid is used as a raw material; acetone precipitation-silica gel column chromatography-magnesium oxide column chromatography / semi-preparative liquid phase-crystallization andother means are combined to purify the orange peel carotenoids to obtain crystals of four high-purity carotenoids which are R1, R2, R3 and R4; and the purified carotenoids obtained are subjected to structure identification through thin-layer chromatography, a C30 column, high performance liquid chromatography-diode array detection-atmospheric pressure chemical ionization tandem mass spectrometry and nuclear magnetic resonance spectrometry. The purities of beta-carotene, beta-cryptoxanthin, xanthophyll and zeaxanthine obtained by the method reach 98.79%, 99.11%, 96.59% and 96.77% respectively,and the yields are 0.0025 per mill, 0.0275 per mill, 0.0066 per mill and 0.0092 per mill respectively.
Owner:SOUTHWEST UNIVERSITY
Carotenoid-containing compositions and methods
Owner:MEAD JOHNSON NUTRITION
Natural food preservative
InactiveCN106616206AImprove securityNo side effectsFood ingredient as antioxidantFood preservationSide effectBeta-cryptoxanthin
The invention discloses a natural food preservative, which consists of the following raw materials in parts by weight: 65 to 75 parts of antioxidant and 25 to 35 parts of bacteriostatic agent; and the antioxidant is one or a mixture of multiple of Alpha-carotene, Beta-cryptoxanthin and capsanthin. The natural food preservative disclosed by the invention, which is added with the natural and safe antioxidant and bacteriostatic agent, is synergistic, does not have toxicity or side effects, has high stability, and can be stored in the dark for a long term. The natural food preservative cannot be affected by temperature and pH, can remarkably prolong the shelf lives of products, and increases food safety.
Owner:李光
Process For The Preparation of Beta and Alpha Cryptoxanthin
ActiveUS20100280286A1Easy to useGood choiceOrganic compound preparationCarbonyl compound preparationDietary supplementCryptoxanthin
The present invention relates to a process for converting lutein and / or lutein esters to (3R)-β-cryptoxanthin and (3R,6′R)-α-cryptoxanthin, suitable for human consumption as dietary supplements, by employing safe and environmentally friendly reagents. (3R)-β-Cryptoxanthin and (3R,6′R)-α-cryptoxanthin are two rare food carotenoids that are not commercially available and the former exhibits vitamin A activity. In the first synthetic step, commercially available lutein and / or lutein esters are transformed into a mixture of dehydration products of lutein (anhydroluteins) in the presence of a catalytic amount of an acid. The resulting anhydroluteins are then converted to (3R)-β-cryptoxanthin (major product) and (3R,6′R)-α-cryptoxanthin (minor product) by heterogeneous catalytic hydrogenation employing transition elements of group VIII (Pt, Pd, Rh supported on alumina or carbon) in a variety of organic solvents under atmospheric pressure of hydrogen and at temperatures ranging from −15° C. to 40° C. Among these catalysts, Pt supported on alumina at 40° C. in ethyl acetate provides the best yield of (3R)-β-cryptoxanthin and (3R,6′R)-α-cryptoxanthin. Several homogeneous catalysts can also promote the regioselective hydrogenation of anhydroluteins to a mixture of (3R)-β-cryptoxanthin and (3R,6′R)-α-cryptoxanthin in low to moderate yields. The catalysts may be transition metal complexes such as palladium acetylacetonate, Rh(Ph3P)3Cl (Wilkinson's catalyst), [(C6H11)3P[C8H12][C5H5N] Ir+PF6− (Crabtree catalyst), or [C8H12][(MePh2P)2]Ir+PF6−. Among these, Wilkinson catalyst converts anhydroluteins to (3R)-β-cryptoxanthin and (3R,6′R)-α-cryptoxanthin in nearly quantitative yield. A novel feature of this invention is the regioselective hydrogenation of anhydroluteins while the highly conjugated polyene chain of these carotenoids remains intact.
Owner:UNIV OF MARYLAND
Peroxisome Proliferator-Activated Receptor (Ppar) Activator, and Drugs, Supplements, Functional Foods and Food Additives Using the Same
InactiveUS20070218147A1Reduce heatNot impair unique tasteBiocideHydroxy compound active ingredientsFood additiveSide effect
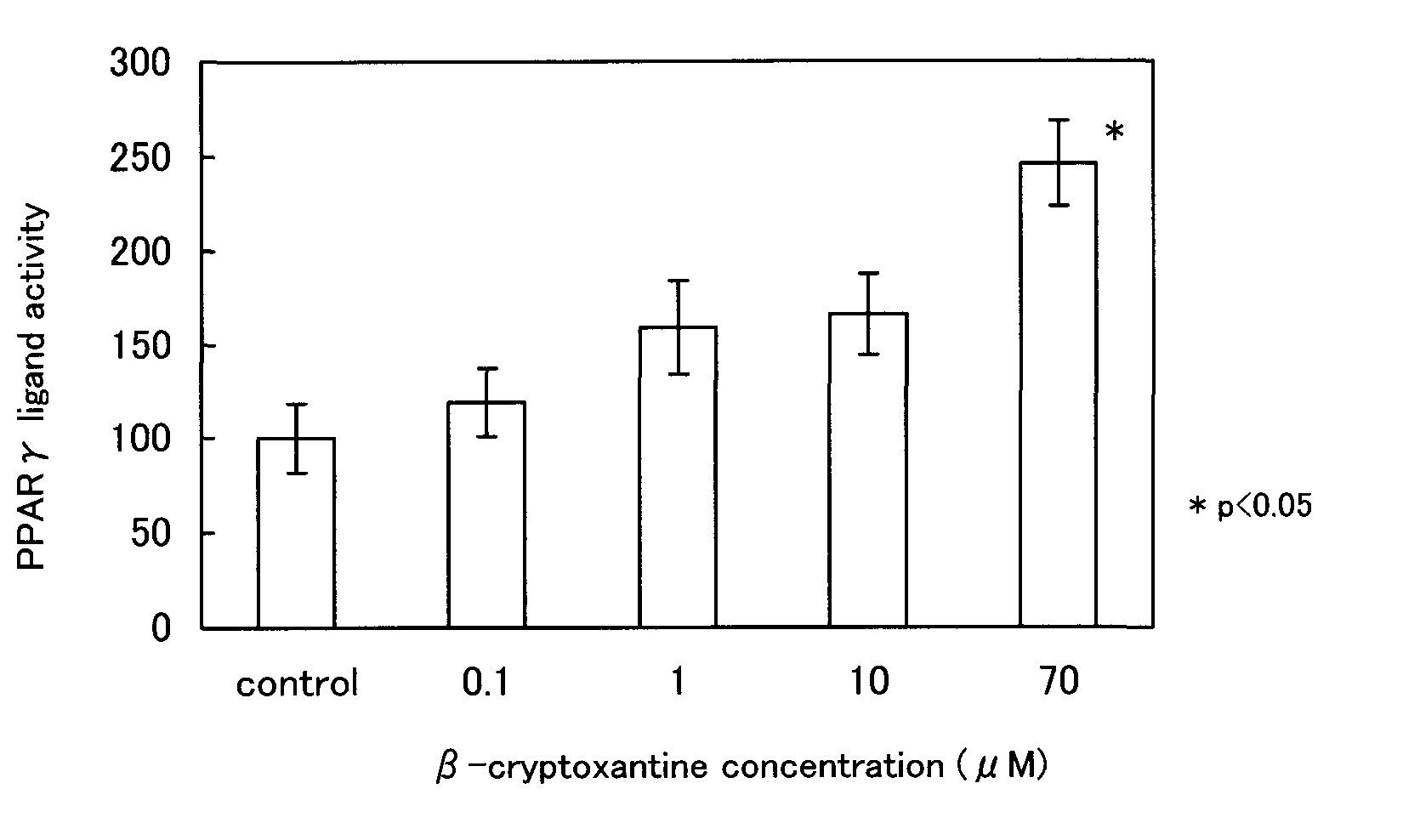
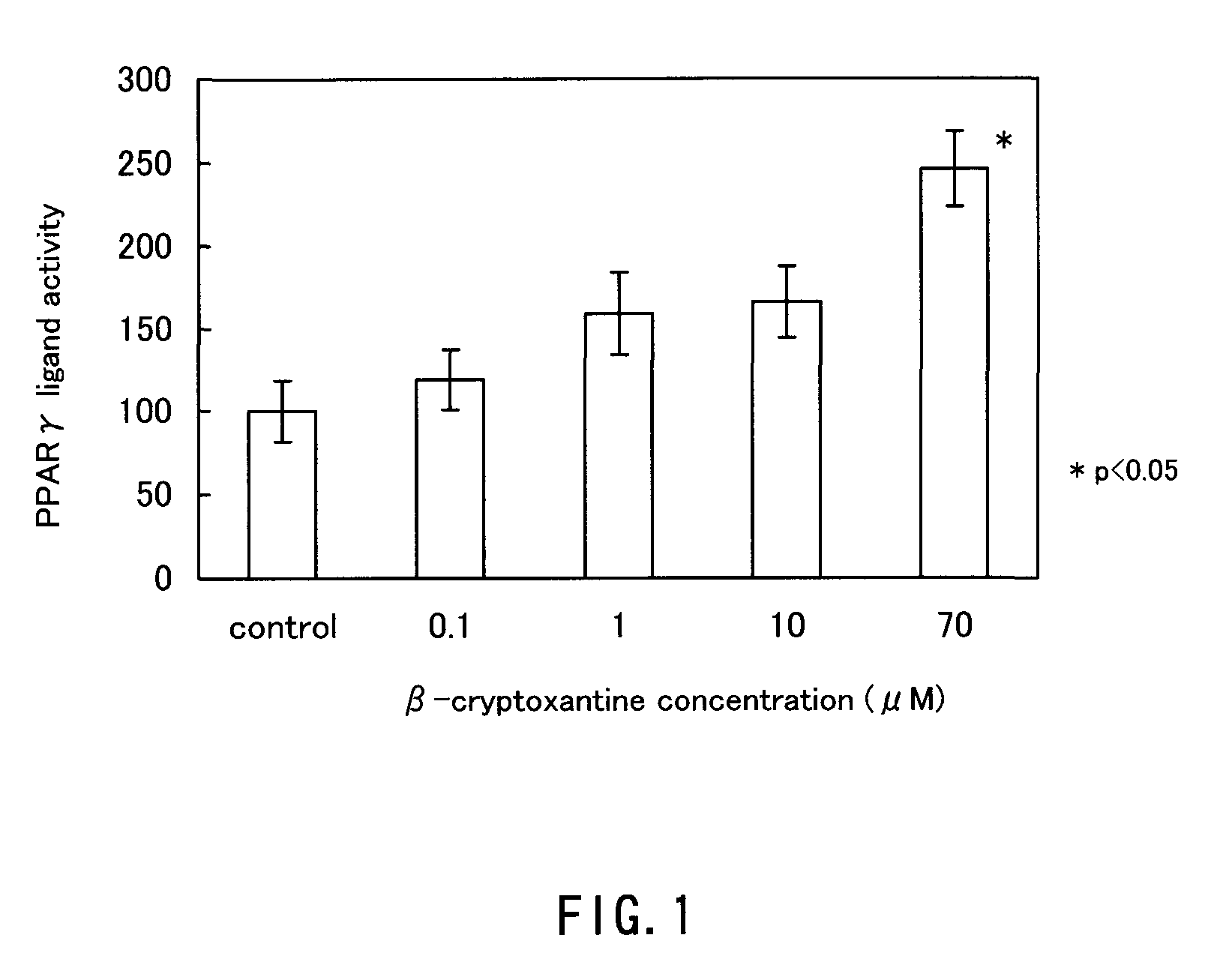
It is intended to provide a peroxisome proliferator-activated receptor (PPAR) activator, which is free from the problem of side effects, can be taken over a long term and has no characteristics taste. β-cryptoxantine is employed as a PPAR activator. β-cryptoxantine, which is contained in a large amount in the pulp of citrus fruits (in particular, mandarin-type citrus fruits) such as satsuma oranges, for example, has been consumed for many years. Thus, it is free from any problem in safety and has a low calorie content. Therefore, it can be taken over a long term. Because of being tasteless and odorless, moreover, β-cryptoxantine would not damage the unique taste when added to a food. Therefore, it can be added to foods and taken.
Owner:ARKRAY INC
Osteogenesis promoter containing β-cryptoxanthin as the active ingredient
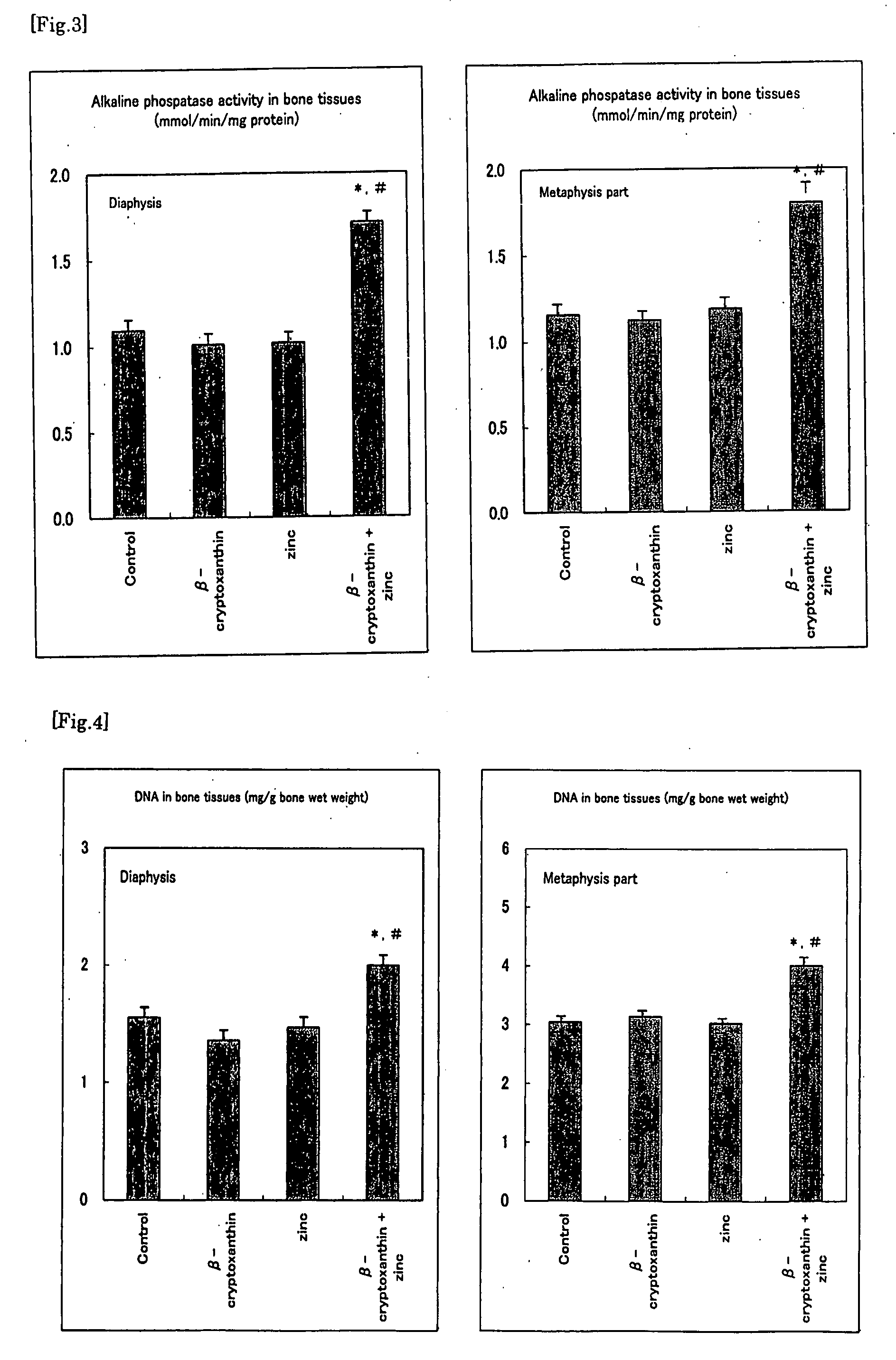
It is intended to provide an osteogenesis promoter having a remarkable effect of positively promoting osteogenesis and thus preventing / treating bone diseases; a preventive / a remedy for bone diseases such as osteoporosis having both of an osteogenesis-promoting effect and a bone resorption-inhibiting effect; and a method of screening an active ingredient for preventing / treating bone diseases by using a compound having both of an osteogenesis-promoting effect and a bone resorption-inhibiting effect as a lead compound. It is confirmed that beta-cryptoxanthin, which occurs in a large amount in peel and sarcocarp of satsuma orange, has an osteogenesis-promoting effect and a therapeutic effect on bone disease. Thus, beta-cryptoxanthin or its composition is used as an osteogenesis promoter, a preventive / a remedy for bone diseases, a functional food or a food material for preventing / treating bone diseases and a feed composition.
Owner:BANK OF AMERICA NAT TRUST & SAVINGS ASSOC
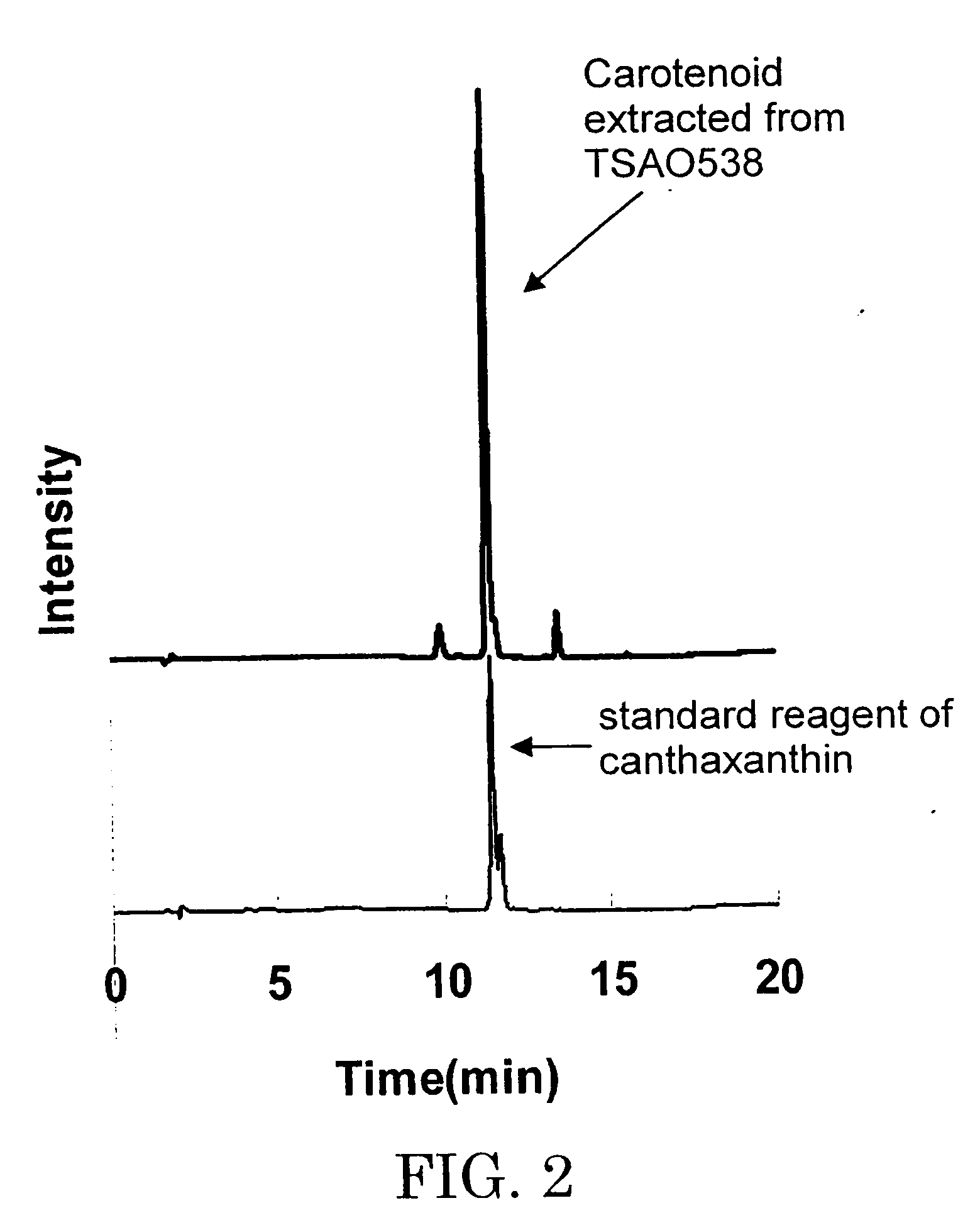
Microorganism and method for producing carotenoid using it
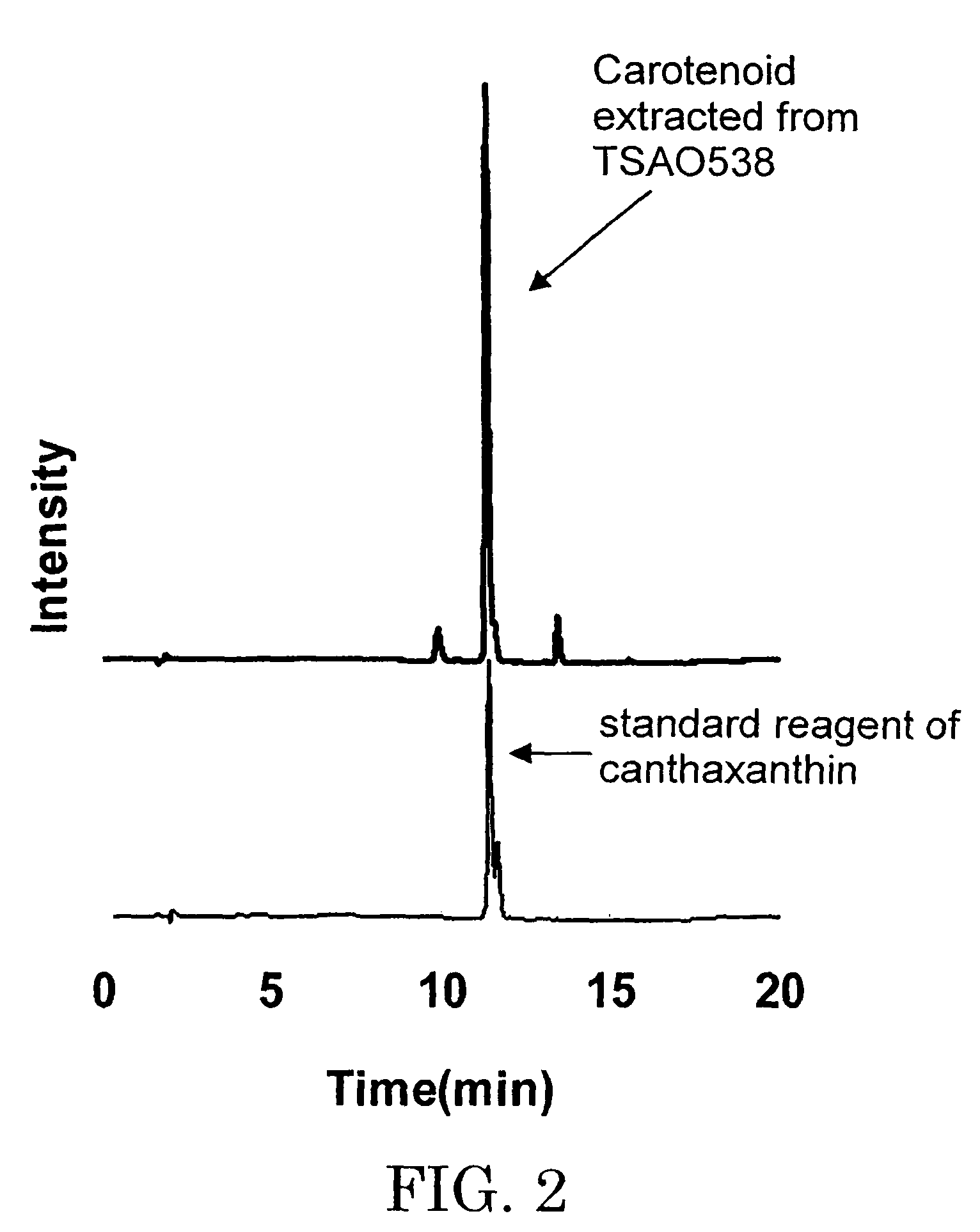
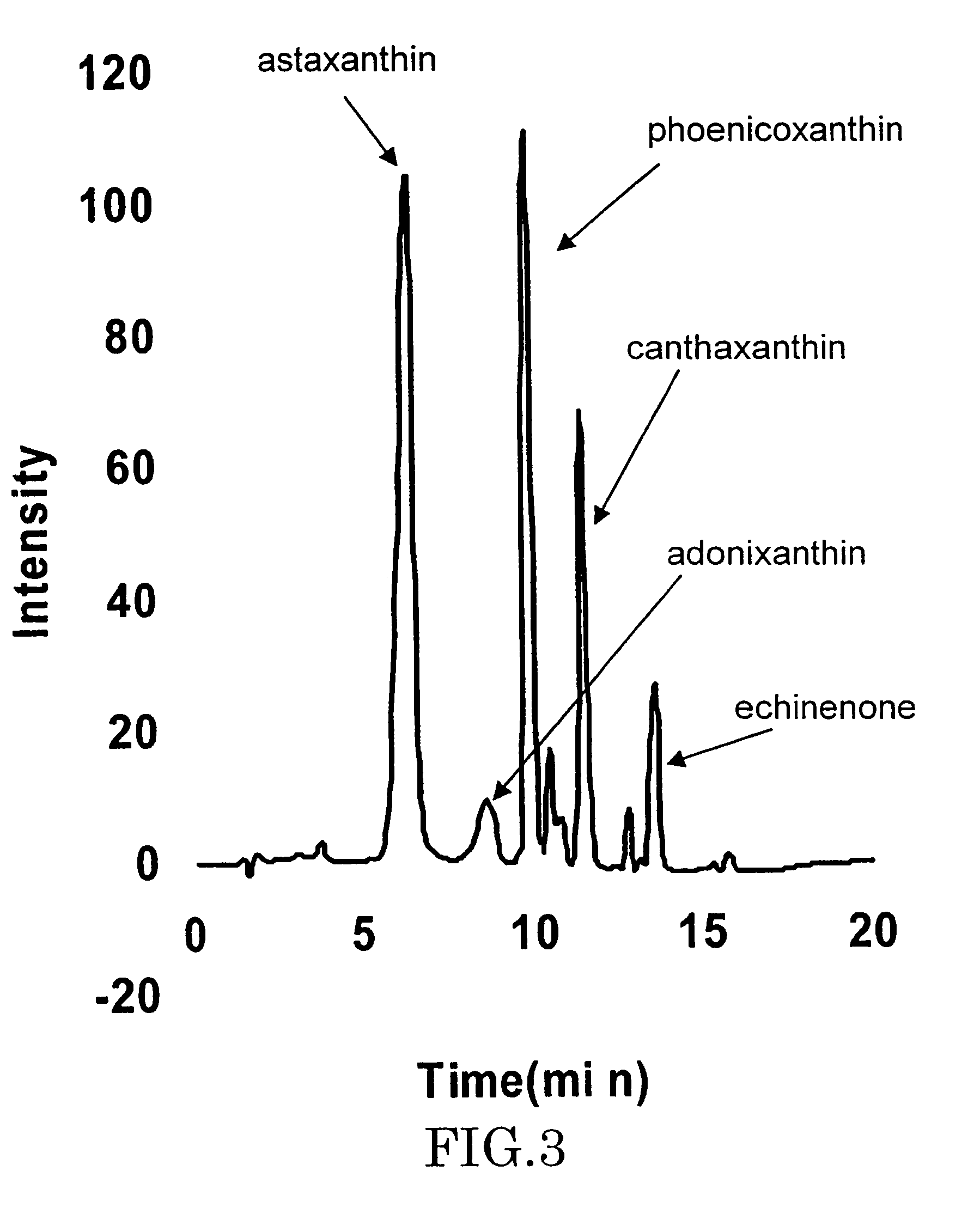
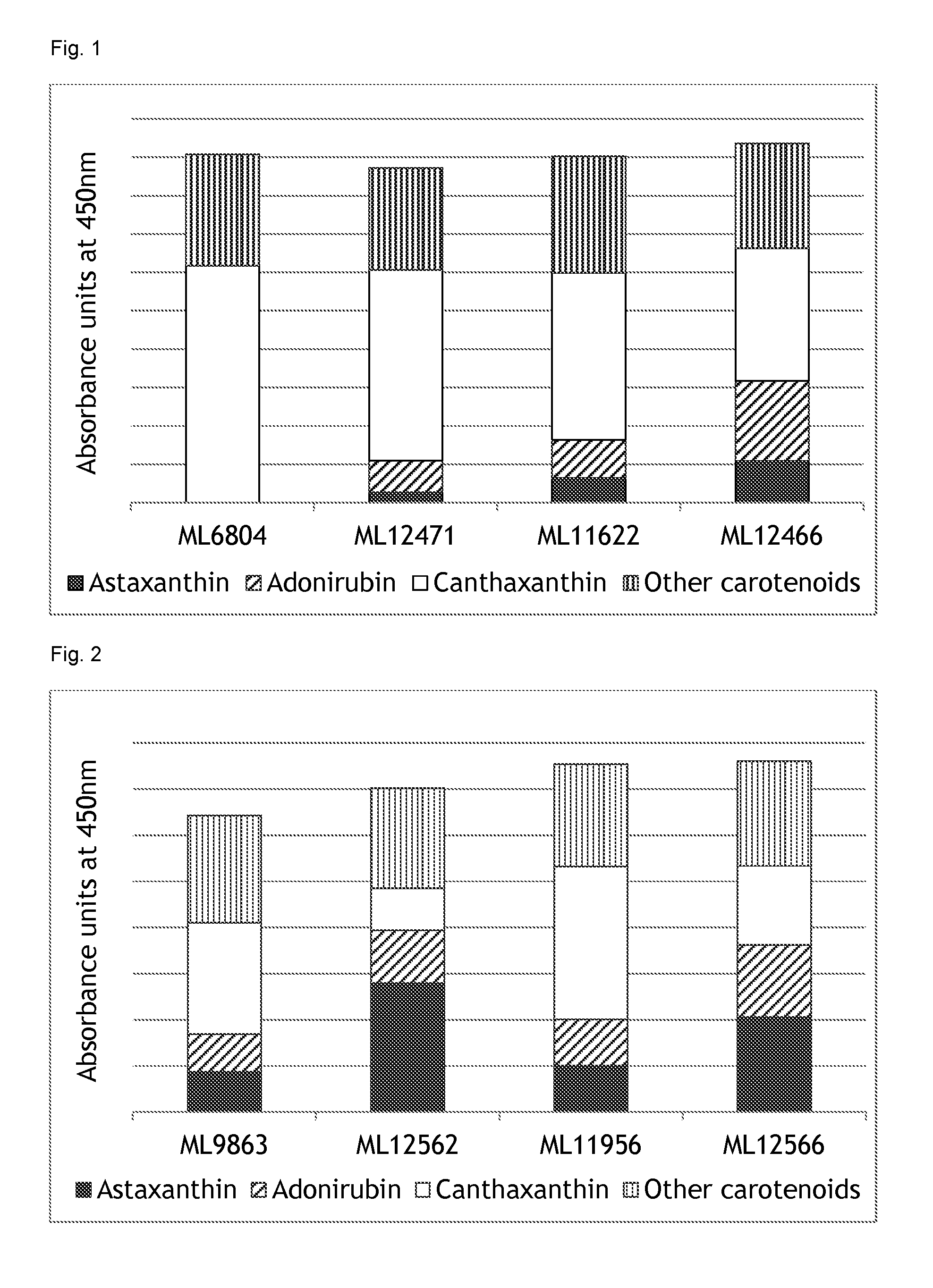
A carotenoid producing bacterium belonging to the genus Paracoccus that selectively produces canthaxanthin so that the amount thereof is not less than 90 percent by weight of the total amount of produced carotenoids including β-carotene, β-cryptoxanthin, echinenone, canthaxanthin, 3-hydroxyechinenone, 3′-hydroxyechinenone, zeaxanthin, phoenicoxanthin, adonixanthin, and astaxanthin. A method for producing canthaxanthin by culturing the above bacterium, and then collecting carotenoids from bacterial cells or a culture solution after the culturing.
Owner:TOSOH CORP
Carotene hydroxylases and their use for producing carotenoids
ActiveUS20150315550A1Improve propertiesImproved propertySugar derivativesMicroorganismsMicroorganismBeta-Carotene
The present invention relates to novel hydroxylases, nucleic acid sequences coding therefore, expression constructs and vectors comprising these sequences, microorganisms transformed therewith, processes for the microbiological hydroxylation of isoprenoids, for example processes for converting beta-carotene into beta-cryptoxanthin and zeaxanthin, or canthaxanthin into astaxanthin.
Owner:DSM IP ASSETS BV
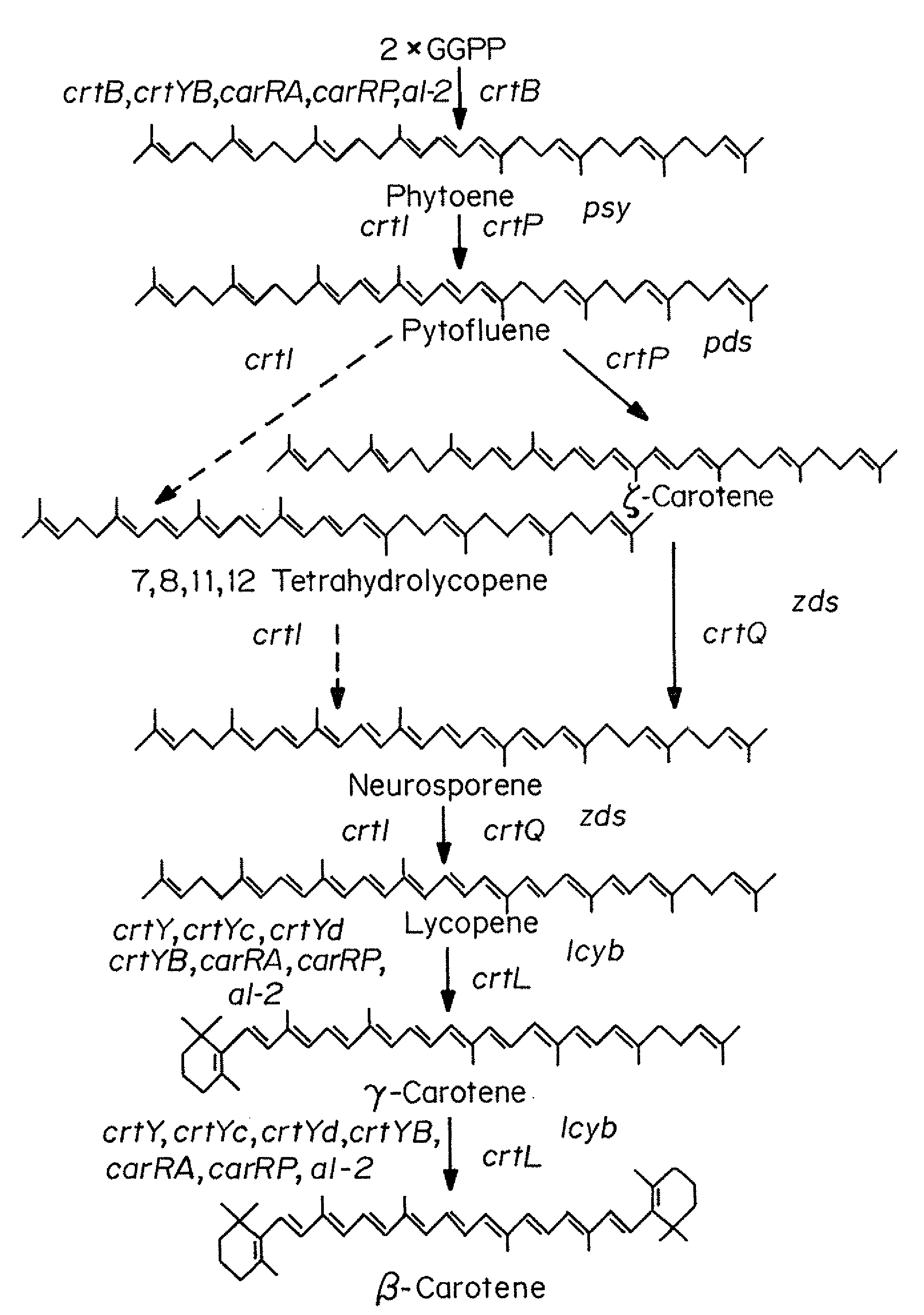
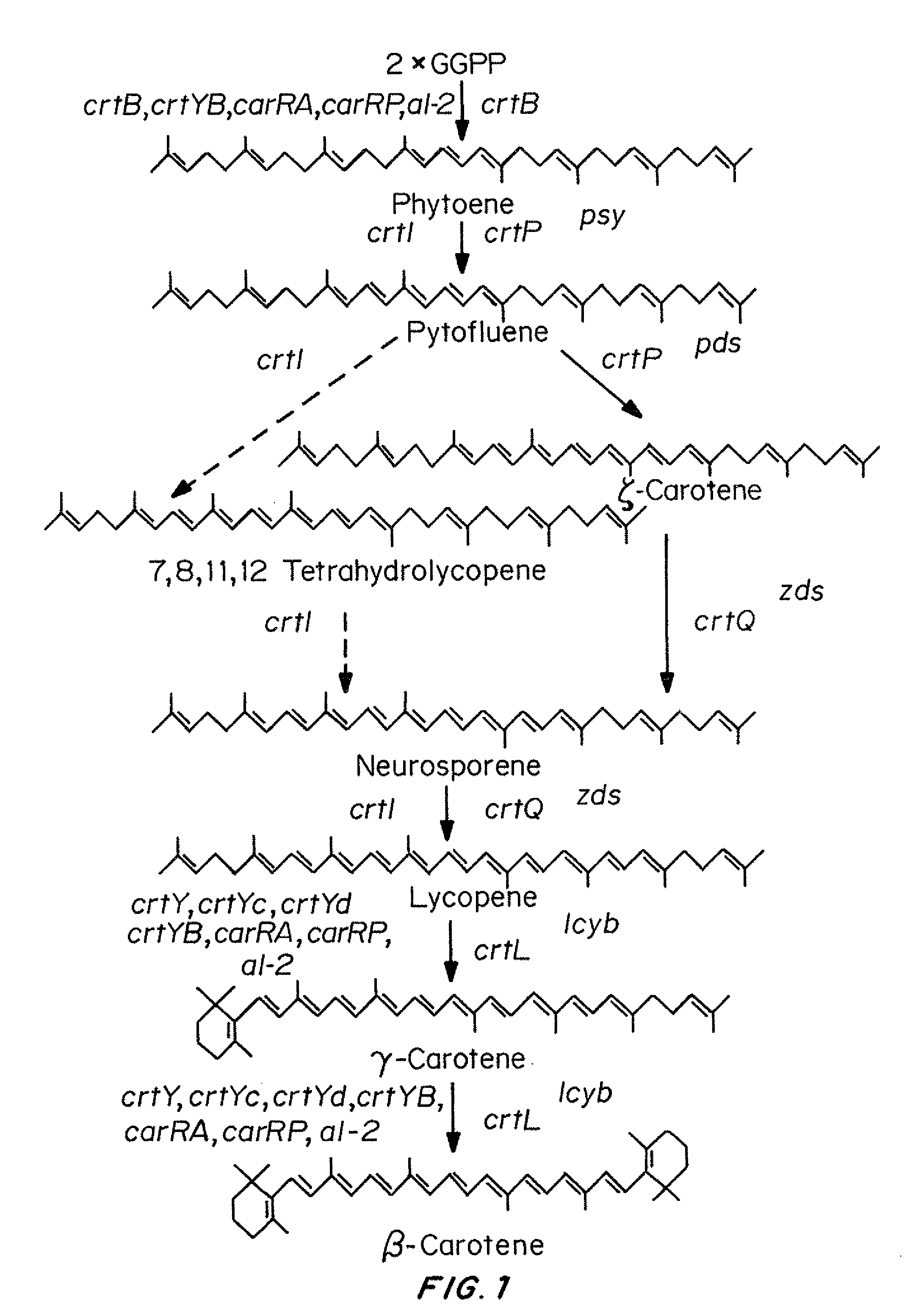
Production of provitamin A carotenoids in mushrooms and uses thereof
Owner:MEDICINE & NEED CORP
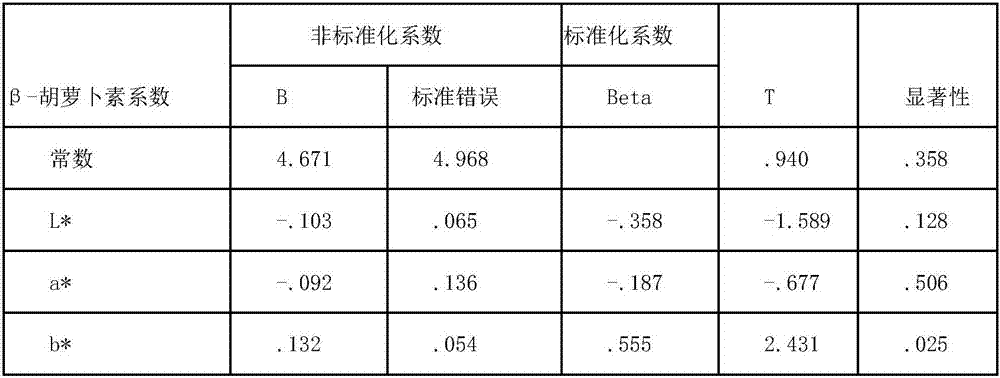
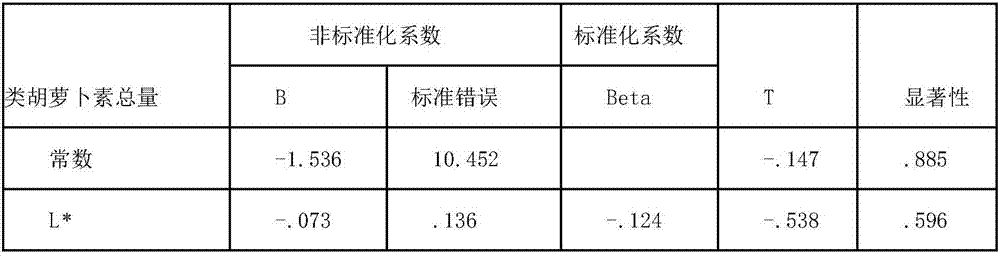
Method for predicting content of beta-carotene and total content of carotenoids in yellow peach pulp correlated with color difference
InactiveCN107024435AQuick forecastImprove accuracyComponent separationColor/spectral properties measurementsPattern recognitionBeta-cryptoxanthin
The invention discloses a method for predicting content of beta-carotene and total content of carotenoids in yellow peach pulp correlated with color difference. The method comprises the following steps of measuring the color difference value of the yellow peach pulp, including brightness value L*, red and green color difference value a* and yellow and blue color difference value b*; using HPLC (high performance liquid chromatography) to measure the content of carotenoid in the pulp, including the contents of xanthophyll, zeaxanthine, beta-cryptoxanthin, alpha-carotene and beta-carotene, wherein the sum of contents is the total content of the carotenoids; analyzing and fitting the content of beta-carotene, the total content of the carotenoids and the color difference value, and establishing a predicting equation of the content of the beta-carotene and the total content of the carotenoids. After repeated testing, the method has the advantages that the difference between the predicting value and the actual measurd value through the equation is not obvious; the accuracy is higher, the measuring and calculation method is simple, and the content of the beta-carotene and the total content of the carotenoids in the yellow peach pulp can be quickly predicted.
Owner:JIANGSU ACAD OF AGRI SCI
Novel microorganism and method for producing carotenoid using the same
[Object] To provide a canthaxanthin selective producing bacterium being able to easily extract and purify canthaxanthin, which is one of carotenoids, and a method for producing canthaxanthin by a culture method.[Solving Means] A carotenoid producing bacterium belonging to the genus Paracoccus is provided which selectively produces canthaxanthin so that the amount thereof is not less than 90 percent by weight of the total amount of produced carotenoids including β-carotene, β-cryptoxanthin, echinenone, canthaxanthin, 3-hydroxyechinenone, 3′-hydroxyechinenone, zeaxanthin, phoenicoxanthin, adonixanthin, and astaxanthin. In addition, a method for producing canthaxanthin is provided which has the steps of culturing the above bacterium, and then collecting carotenoids from bacterial cells or a culture solution after the culturing.
Owner:TOSOH CORP
Production of provitamin a carotenoids in mushrooms and uses thereof
Mushrooms genetically engineered to produce provitamin A carotenoids including α-carotene, β-carotene, γ-carotene, and β-cryptoxanthin are provided. In some embodiments, mushrooms are transformed with genes that encode enzymes that have phytoene synthase, pyhtoene dehydrogenase and lycopene cyclase activities and function to convert GGPP to one or more provitamin A carotenoids. Mushrooms are transformed using known methods, including Agrobacterium-mediated transformation. Transgenic mushrooms producing provitamin A carotenoids are useful to treat, alleviate, reduce, and / or inhibit one or more symptoms of a disease or disorder associated with vitamin A deficiency (VAD).
Owner:MEDICINE & NEED CORP
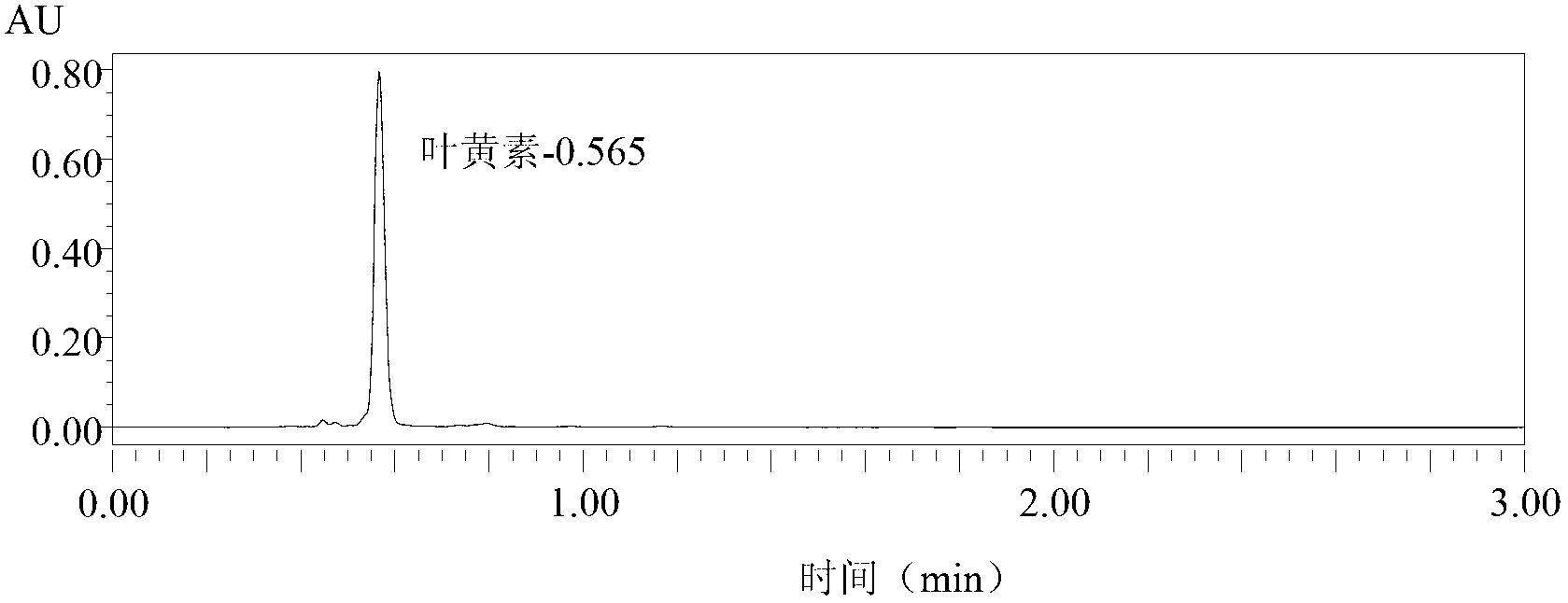
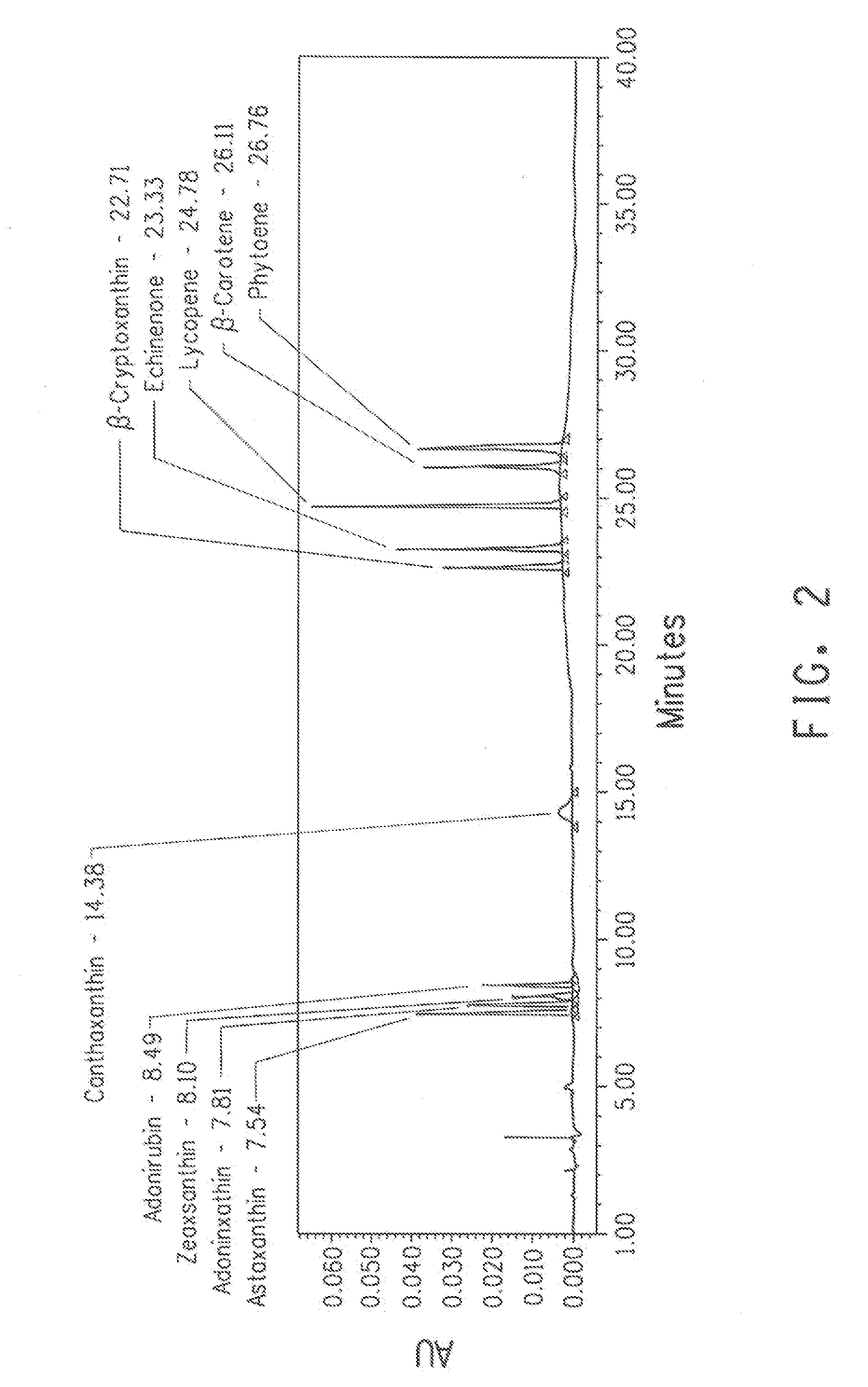
Detection method of beta-cryptoxanthin single-cis-form and two-cis-form isomer and ketone oxidation product
ActiveCN104181267AGuaranteed Response SignalEasy to separateComponent separationBeta-cryptoxanthinAmpere
The invention relates to a detection method of beta-cryptoxanthin single-cis-form and two-cis-form isomer and ketone oxidation product, belonging to the technical field of the analysis. The detection method is characterized in that a YMC Carotenoid C30 chromatographic column is adopted, the flowing phase is 1.5% ammonium acetate water, methyl tert-butyl ether and methanol in a ratio of 5 / 25 / 70 and 1.5% ammonium acetate water, methyl tert-butyl ether and methanol in a ratio of 5 / 85 / 10, the flow rate is 0.6mL / min, the feeding amount is 20 micro liters, the column temperature is 25 DEG C, the detection wavelength is 200nm to 550nm, the beta-cryptoxanthin isomer and oxidation product can be remarkably separated, an APCI<+> positive ion mass spectrum is adopted, the flow rate of outflow components, entering a mass spectrometer, of the chromatographic column is 10 micro liters per minute, the scanning range is 80m / z to 1000m / z, the capillary voltage is 2500V, the drying gas is 5L, the amount of atomized gas is 20psi, the vaporization temperature is 300DEG C, the steam temperature is 400DEG C, and corona current is 4 micro amperes. The qualitative analysis of the beta-cryptoxanthin single-cis-form and two-cis-form isomer and ketone oxidation product is conducted according to an elution sequence, mass spectral information and spectrum information of a sample, and then the quantitative analysis can be conducted according to a peak area. The method has the advantages of rapidness, efficiency, reproducibility, high yield and the like.
Owner:JIANGSU ACADEMY OF AGRICULTURAL SCIENCES
Method of improving cardiovascular health
InactiveUS8021698B2Promotes cardiovascular healthLower blood pressureBiocideHydroxy compound active ingredientsΒ cryptoxanthinBeta-cryptoxanthin
A nutritional supplement including β-cryptoxanthin is found to be effective at lowering blood pressure in mammals. β-cryptoxanthin therefore may be used to maintain cardiovascular health by lowering blood pressure, preventing high, elevated blood pressure and / or maintaining healthy blood pressure. Administration of β-cryptoxanthin in combination with safflower oil is particularly effective.
Owner:KEMIN FOODS L C
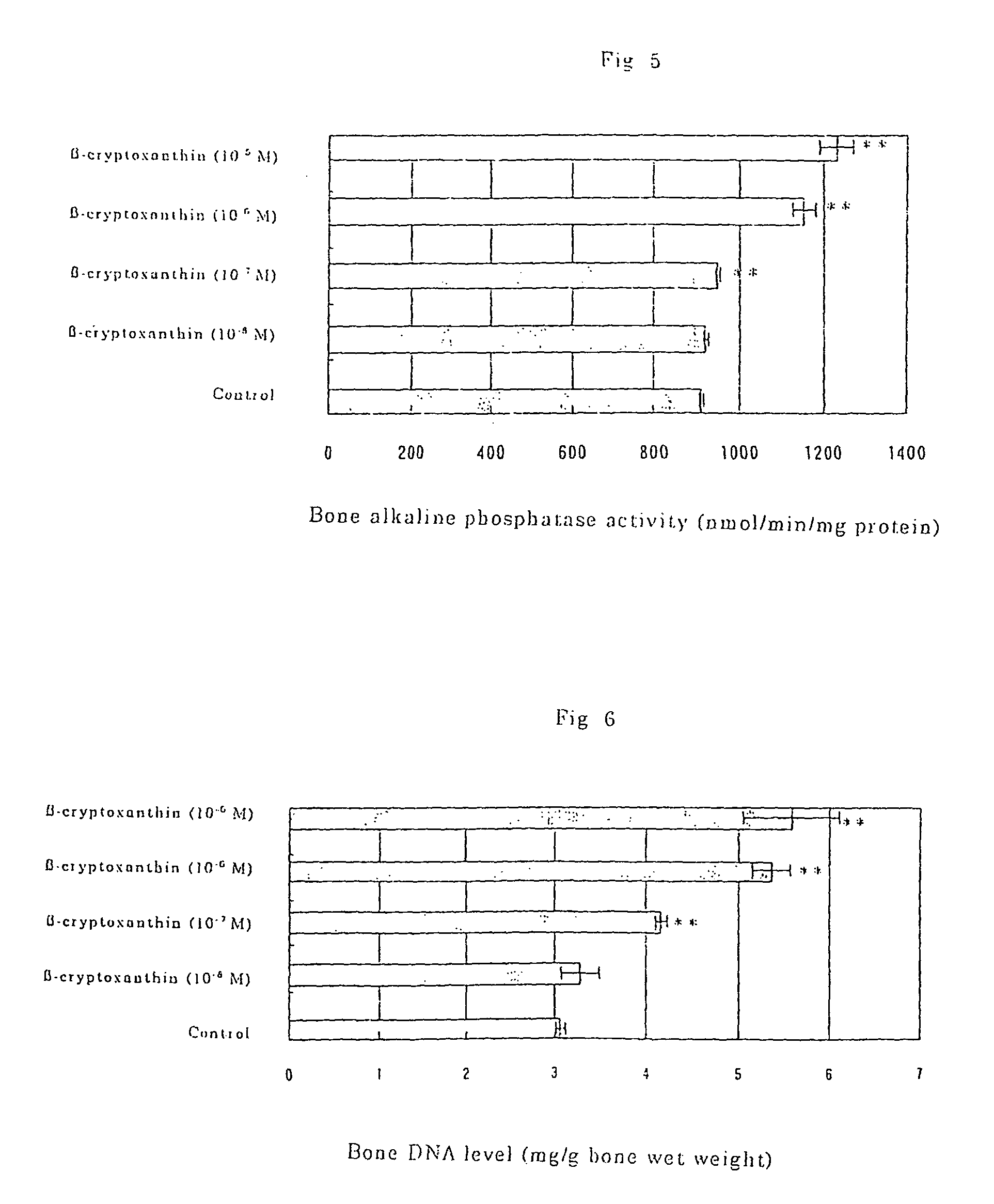
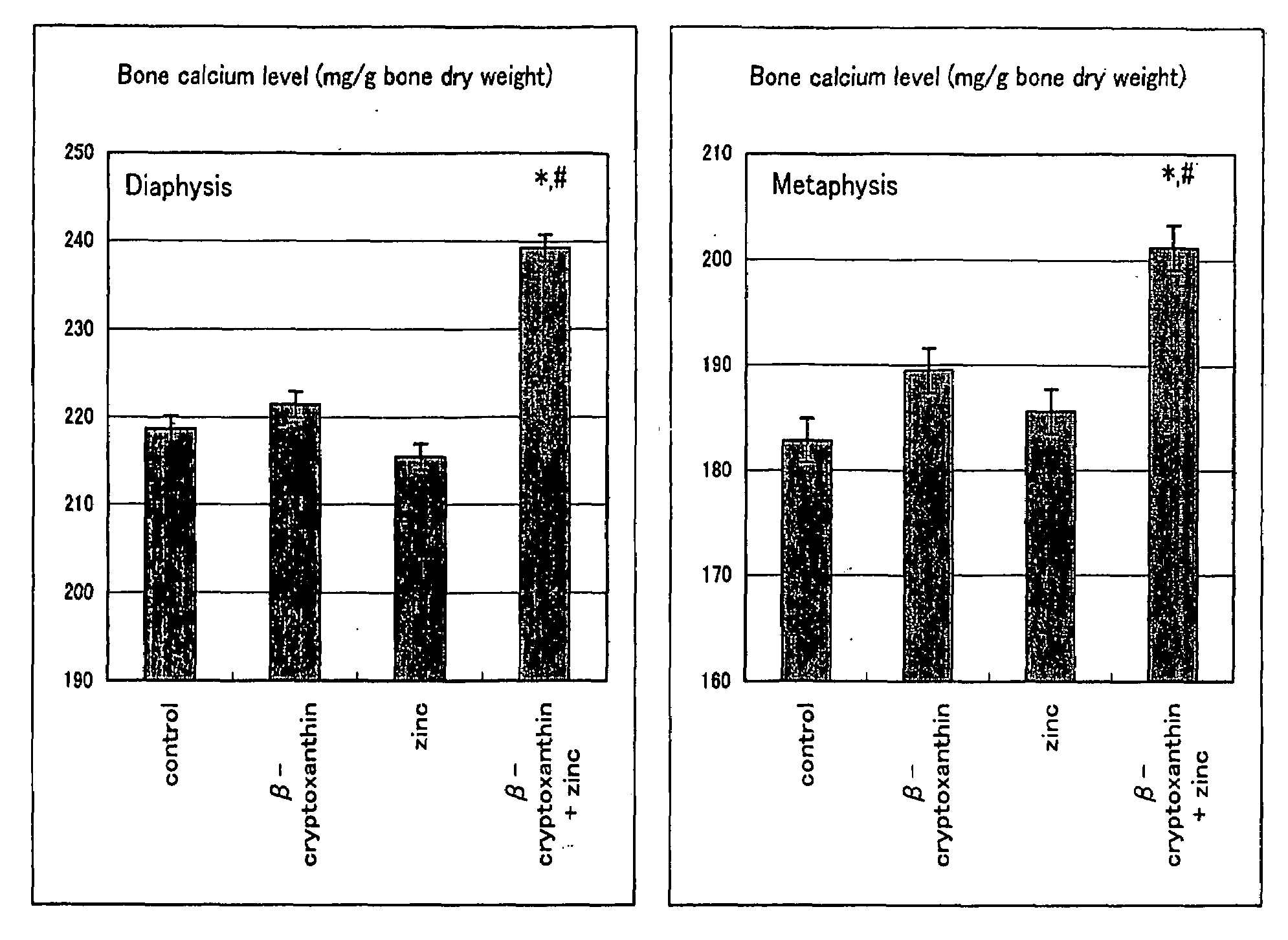
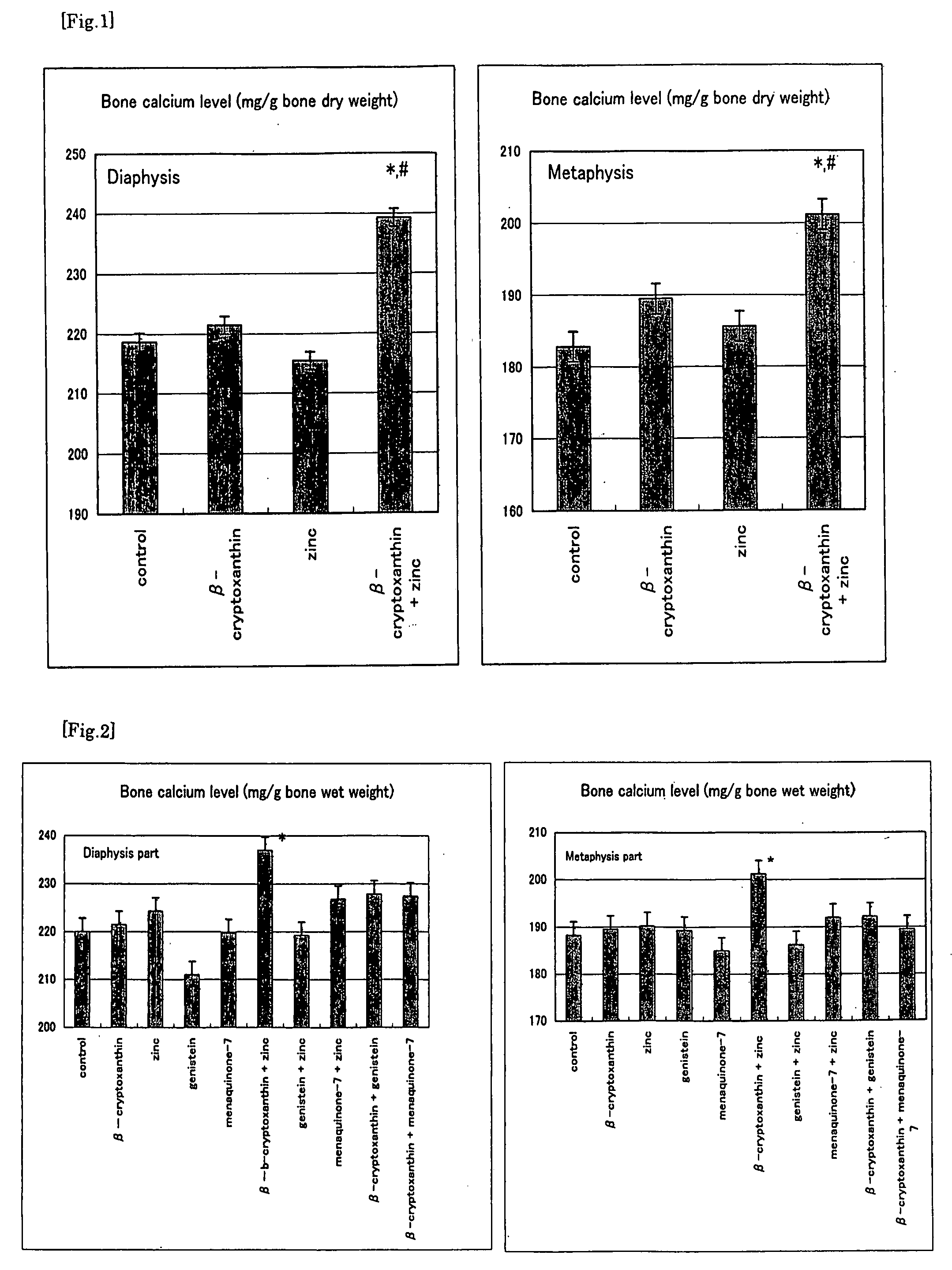
Composition For Promoting Osteogenesis And Increasing Bone Mineral Content
InactiveUS20080113038A1Increasing salt mineral contentPromote osteogenesisBiocideHydroxy compound active ingredientsZinc compoundsBeta-cryptoxanthin
It is to provide a composition for promoting osteogenesis and increasing bone mineral content with a function of (promoting) osteogenesis and increasing bone mineral content, having a significant effect, which promotes actively osteogenesis to prevent / treat bone diseases, a preventative / therapeutic agent for bone diseases, a functional food or food material for preventing / treating bone diseases, and feed composition. A composition for promoting osteogenesis and increasing bone mineral content and a preventative / therapeutic agent for bone diseases comprising β-cryptoxanthin and zinc compound at a concentration that does not exhibit a significant effect of increasing bone calcium level when used separately, as active ingredients are prepared. Further, β-cryptoxanthin and zinc compound are added / compounded to a food or food material, or feed, at a concentration that does not exhibit a significant increasing effect of bone calcium level, when used separately.
Owner:YAMAGUCHI MASAYOSHI
Process for a direct one-pot transformation of lutein to beta-cryptoxanthin via its acetate ester
InactiveUS20160115122A1Organic compound preparationPreparation by transesterificationBeta-cryptoxanthinDietary supplement
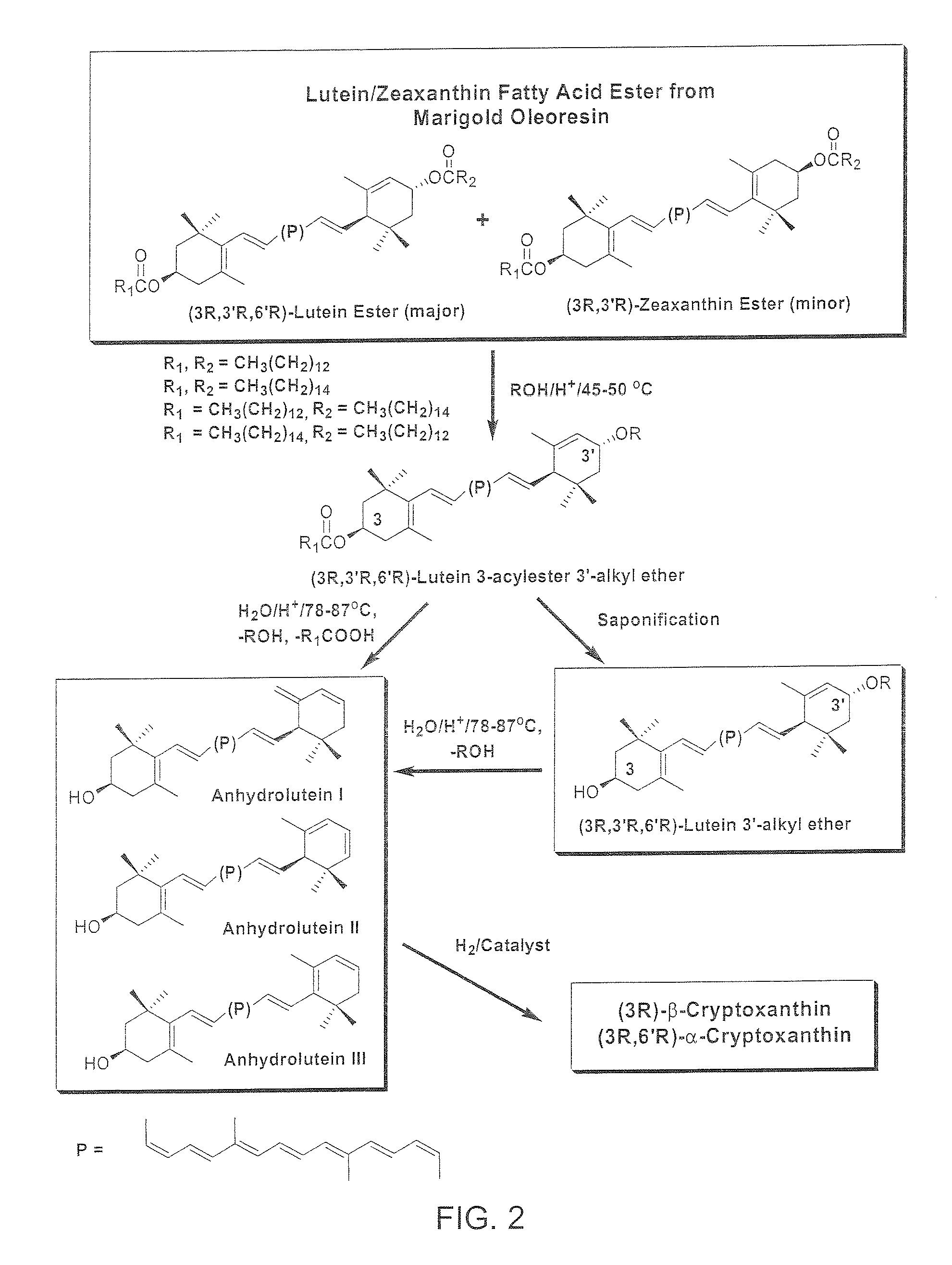
The present invention relates to a process for converting commercially available lutein and / or lutein esters from extracts of marigold flower petals to (3R)-β-cryptoxanthin (major) and (3R,6′R)-α-cryptoxanthin (minor) in ratios ranging from 95:5 to 98:2 in a one-pot reaction at room temperature. Because the entire process can be carried out by employing safe and environmentally friendly food-grade reagents, the resulting mixture of these carotenoids is suitable for human consumption as a dietary supplement.
Owner:UNIV OF MARYLAND
Process for a direct one-pot transformation of lutein to β-cryptoxanthin via its acetate ester
The present invention relates to a process for converting commercially available lutein and / or lutein esters from extracts of marigold flower petals to (3R)-β-cryptoxanthin (major) and (3R,6′R)-α-cryptoxanthin (minor) in ratios ranging from 95:5 to 98:2 in a one-pot reaction at room temperature. Because the entire process can be carried out by employing safe and environmentally friendly food-grade reagents, the resulting mixture of these carotenoids is suitable for human consumption as a dietary supplement.
Owner:UNIV OF MARYLAND
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com