Medicine ranitidine hydrochloride compound for treating stomach illness and preparation method of medicine ranitidine hydrochloride compound
A technology of ranitidine hydrochloride and compound, which is applied in the field of drug ranitidine hydrochloride for treating gastric diseases and its preparation field, can solve the problems of easy moisture absorption and discoloration, poor stability, difficult preparation of preparations, etc., and achieves low moisture absorption and stability. Good performance and easy preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
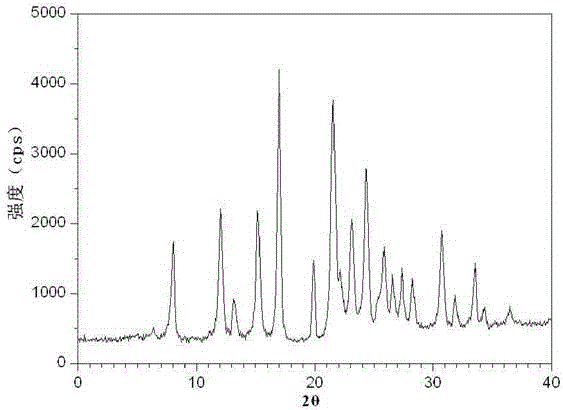
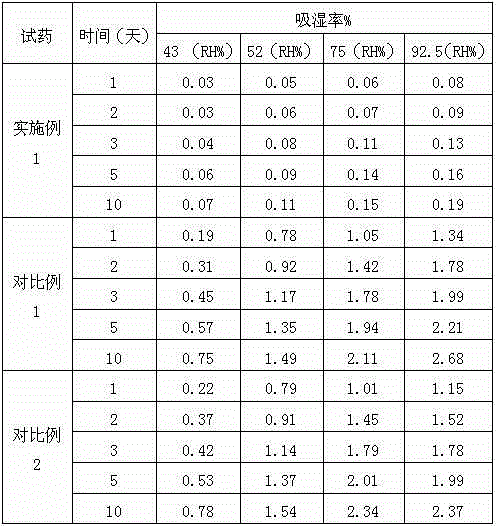
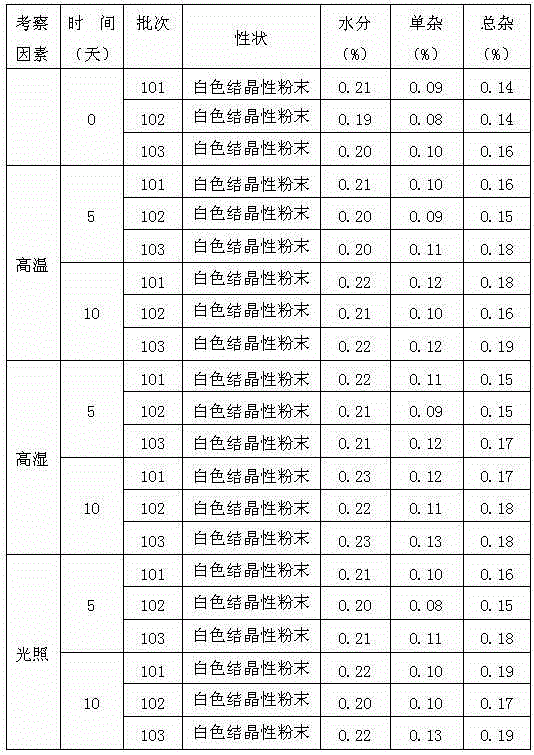
Image
Examples
Embodiment 1
[0024] Example 1: The preparation method of ranitidine hydrochloride compound, the steps are as follows:
[0025] Dissolve the solid ranitidine hydrochloride in a methanol solution with a volume of 8 times the weight of ranitidine hydrochloride at 20°C; first add a mixed solvent of ethanol and acetone with a total volume of 10 times the weight of ranitidine hydrochloride. The volume ratio to acetone is 1.5:1. Stir while adding, control the temperature at 20°C, and cultivate the crystal for 0.5 hours; then add acetone and 2,4-dimethyl in a total volume of 12 times the weight of ranitidine hydrochloride The mixed solvent of base pyridine, the volume ratio of acetone to 2,4-lutidine is 3:2. After the crystal is grown for 1 hour, the temperature is reduced to -5°C at a rate of 10°C / hour, and then the stirring speed is maintained at 120 rpm. Stirring for crystallization and crystallization for 2 hours; filtration, 40° C. and drying under reduced pressure to obtain ranitidine hydrochl...
Embodiment 2
[0027] Example 2: The preparation method of ranitidine hydrochloride compound, the steps are as follows:
[0028] Dissolve the solid ranitidine hydrochloride in a methanol solution with a volume of 9 times the weight of ranitidine hydrochloride at 22.5°C; first add a mixed solvent of ethanol and acetone with a total volume of 11 times the weight of ranitidine hydrochloride. The volume ratio to acetone is 1.5:1. Stir while adding, control the temperature at 22.5°C, and grow the crystal for 0.75 hours; then add acetone and 2,4-dimethyl in a total volume of 18.5 times the weight of ranitidine hydrochloride The mixed solvent of base pyridine, the volume ratio of acetone to 2,4-lutidine is 3:2. After culturing the crystal for 1.5 hours, the temperature is lowered to -5°C at a rate of 12.5°C / hour, and then the stirring speed is maintained at 135 rpm. Stirring for crystallization and crystallization for 2.5 hours; filtering, 45°C, and drying under reduced pressure to obtain ranitidine ...
Embodiment 3
[0030] Example 3: The preparation method of ranitidine hydrochloride compound, the steps are as follows:
[0031] Dissolve the solid ranitidine hydrochloride in a methanol solution with a volume of 10 times the weight of ranitidine hydrochloride at 25°C; first add a mixed solvent of ethanol and acetone with a total volume of 12 times the weight of ranitidine hydrochloride, ethanol The volume ratio to acetone is 1.5:1. Stir while adding, control the temperature at 25°C, and cultivate the crystal for 1 hour; then add acetone and 2,4-dimethyl in a total volume of 25 times the weight of ranitidine hydrochloride The mixed solvent of base pyridine, the volume ratio of acetone to 2,4-lutidine is 3:2. After culturing the crystals for 2 hours, the temperature is reduced to -5°C at a rate of 15°C / hour, and then the stirring speed is maintained at 150 rpm. Stirring for crystallization and crystallization for 3 hours; filtering, drying at 50°C under reduced pressure to obtain ranitidine hyd...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com