Method for determining ranitidine hydrochloride related substances through high performance liquid chromatography
A technique of ranitidine hydrochloride and high performance liquid chromatography, which is applied in the field of determination of related substances of ranitidine hydrochloride by high performance liquid chromatography, can solve the problem of undisclosed ranitidine hydrochloride determination method, reduce risk, and fail to assess hazards To achieve the effect of facilitating quality inspection and monitoring, improving sensitivity and accuracy, and facilitating safe promotion and application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The present embodiment provides a method for the determination of related substances of ranitidine hydrochloride by high performance liquid chromatography, which is determined using the following conditions:
[0048] Chromatographic column: Octadecylsilane bonded silica gel is used as a filler; the specification of the chromatographic column is Welch-Xtimate-C18, 4.6mm×250mm, 5μm; a Yuexu peak suppressor is added in front of the column;
[0049] Mobile phase A: modified phosphate buffer solution with a volume ratio of 98:2 (dissolve 6.8ml phosphoric acid in 1900ml water, add 1ml triethylamine and 8.6ml 50% sodium hydroxide solution, add water to 2000ml; use phosphoric acid or 50 % sodium hydroxide solution to adjust the pH value to 6.70 ± 0.05) and the mixed solution of acetonitrile;
[0050] Mobile phase B: a mixture of modified phosphate buffer and acetonitrile with a volume ratio of 78:22;
[0051] Flow rate: 1.2ml / min;
[0052] Column temperature: 25°C;
[0053] ...
experiment example 1
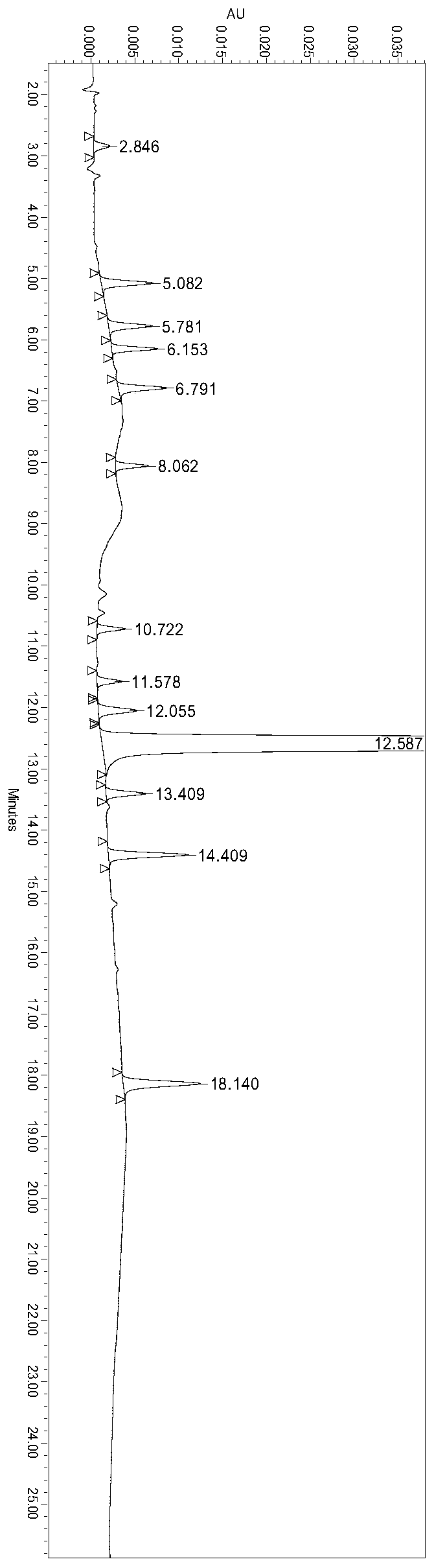
[0064] Experimental Example 1 System Suitability Test
[0065] The preparation of need testing solution: get about 100mg of ranitidine hydrochloride, accurately weigh, put in 100ml measuring bottle, add water appropriate amount and make dissolving, dilute with water to scale, shake up, as need testing solution.
[0066] Preparation of contrast solution: accurately measure the appropriate amount of the test solution, and quantitatively dilute it with a solvent to make a solution containing 1 μg ranitidine hydrochloride in every 1ml, as a contrast solution.
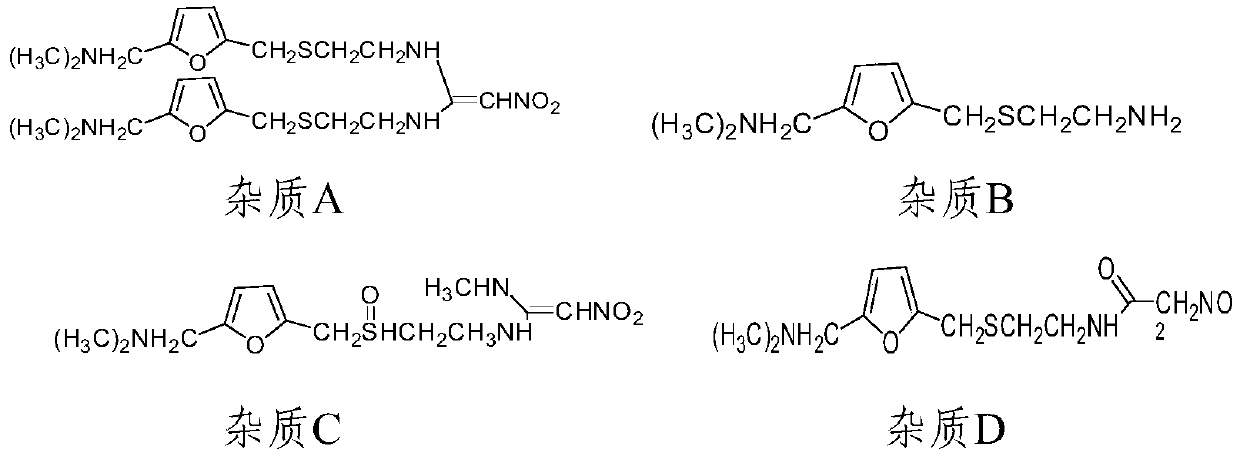
[0067] Preparation of system suitability solution: ranitidine hydrochloride impurity A reference substance, impurity B reference substance, impurity C reference substance, impurity D reference substance, impurity E reference substance, impurity F reference substance, impurity G reference substance, impurity H control substance standard substance, impurity I reference substance, impurity J reference substance, impurity K ref...
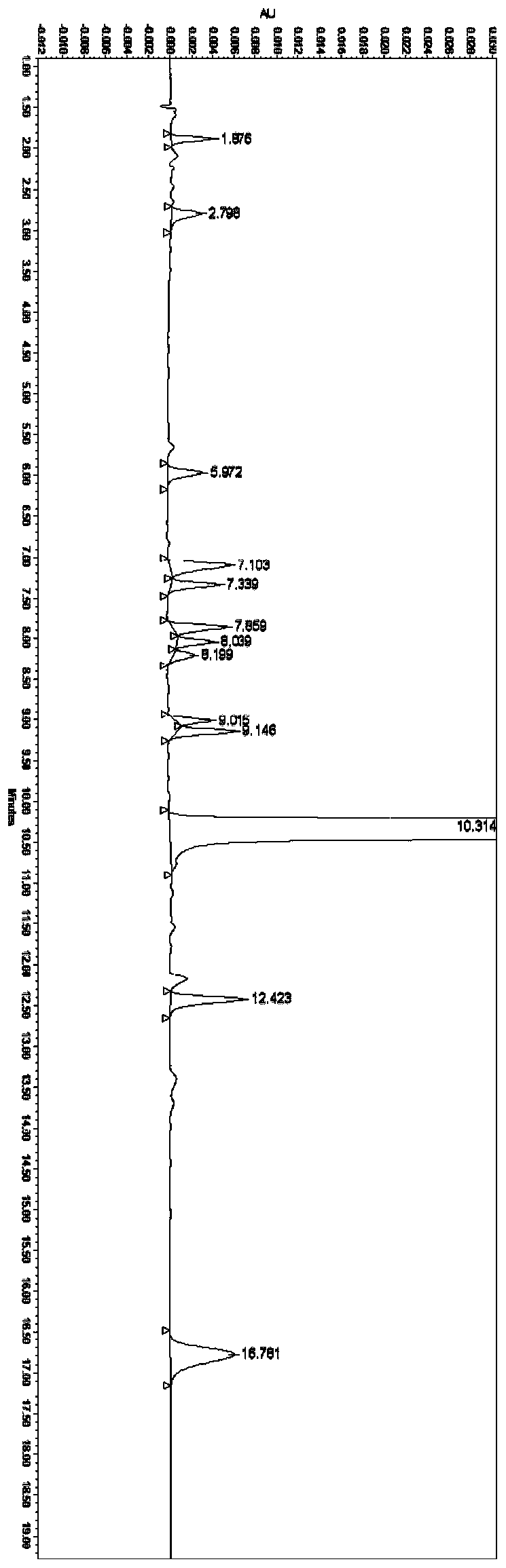
experiment example 2
[0074] Experimental example 2 linearity and range test
[0075] Solvent: water
[0076] Linear stock solution: take ranitidine hydrochloride impurity A reference substance, impurity B reference substance, impurity C reference substance, impurity D reference substance, impurity E reference substance, impurity F reference substance, impurity G reference substance, impurity H reference substance, Impurity I reference substance, impurity J reference substance, impurity K reference substance, impurity L reference substance and ranitidine hydrochloride reference substance are each about 10 mg, put them in 100ml measuring bottles respectively, add a proper amount of solvent to dissolve and dilute to the mark, shake Evenly, as each stock solution, accurately measure an appropriate amount, and dilute to make a series of linear solutions.
[0077] Precisely measure 10 μl of the above-mentioned solutions, inject them into a liquid chromatograph, and record the chromatograms; the results...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com