Ranitidine pharmaceutical composition and preparation method thereof
A technology of ranitidine and ranitidine hydrochloride, which is applied in the direction of drug combinations, pharmaceutical formulas, and medical preparations of non-active ingredients, and can solve the problems of inability to meet drug release requirements, large gaps in bioavailability, and long-term stability of drugs Poor drug resistance and other problems, to achieve the effect of ensuring bioavailability, good stability, and improving drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
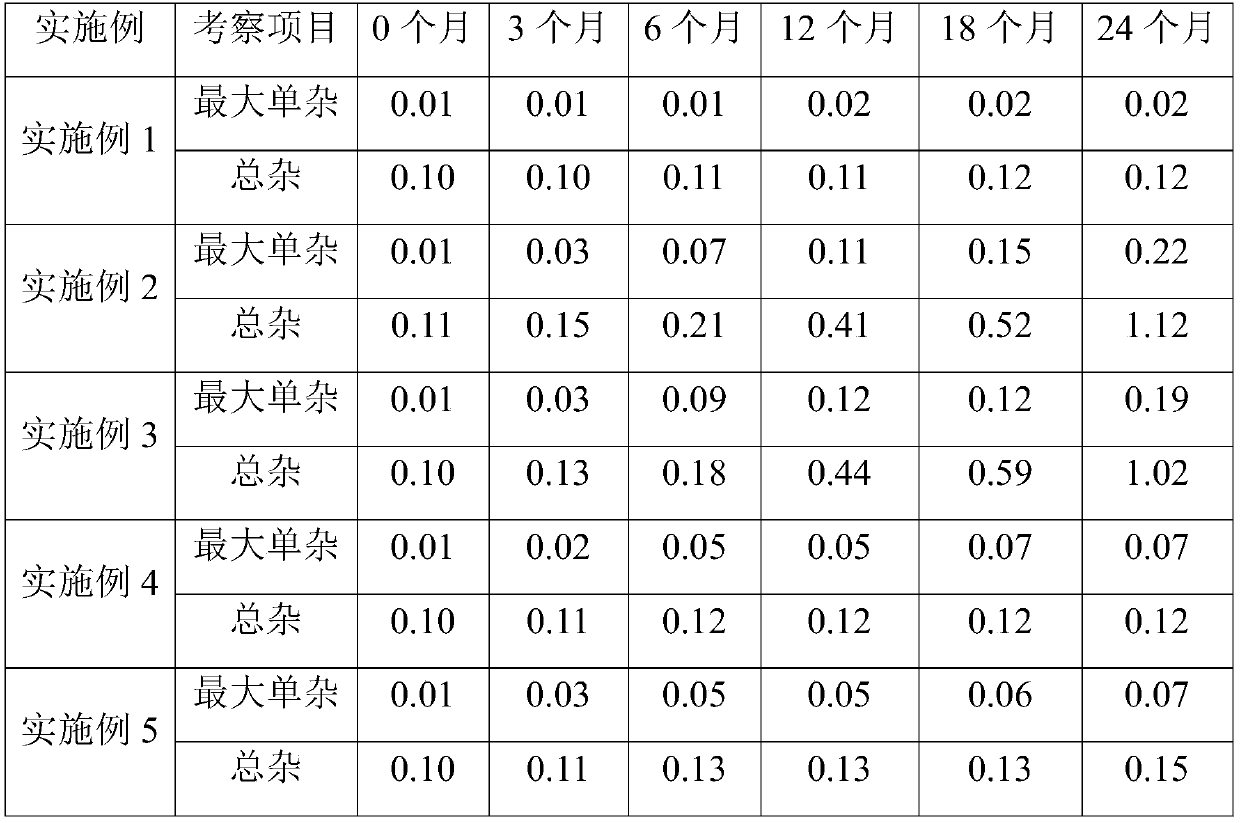
Examples
Embodiment 1
[0021] Embodiment 1 (specification 150mg)
[0022] Preparation prescription:
[0023] Ranitidine Hydrochloride 150mg
[0024] Lactose 90mg
[0025] Polyethylene glycol 30mg
[0026] Benzoic acid 24.9mg
[0027] Magnesium stearate 5.1mg
[0028] Preparation Process:
[0029] 1. Put ranitidine hydrochloride in a grinder and grind for about 30 minutes to obtain ranitidine hydrochloride powder with a particle size of 20 μm.
[0030] 2. Mix ranitidine hydrochloride powder and lactose evenly, add polyethylene glycol and benzoic acid, make a soft material with 5% PVP (50% ethanol aqueous solution), pass through a 20-mesh sieve for granulation, and dry at 50°C for 1 hour.
[0031] 3. Add magnesium stearate to the dried granules, sieve the granules with 20 mesh, and press into tablets (pressure 3.0Kg / cm 2 ).
Embodiment 2
[0032] Embodiment 2 (specification 150mg)
[0033] Preparation prescription:
[0034] Ranitidine Hydrochloride 150mg
[0035] Lactose 60mg
[0036] Polyethylene glycol 60mg
[0037] Benzoic acid 24.9mg
[0038] Magnesium stearate 5.1mg
[0039] Preparation Process:
[0040] With embodiment 1, difference is that charging amount is different.
Embodiment 3
[0041] Embodiment 3 (specification 150mg)
[0042] Preparation prescription:
[0043] Ranitidine Hydrochloride 150mg
[0044] Lactose 80mg
[0045] Polyethylene glycol 40mg
[0046] Benzoic acid 24.9mg
[0047] Magnesium stearate 5.1mg
[0048] Preparation Process:
[0049] With embodiment 1, difference is that charging amount is different.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com