Preparation method of ranitidine hydrochloride tablets
A technology of ranitidine hydrochloride tablets and ranitidine hydrochloride, which is applied to medical preparations with no active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc., which can solve the problem of low production efficiency and moisture absorption of tablet cores and other problems to achieve the effect of avoiding the drying process, solving moisture absorption, improving product quality and production efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
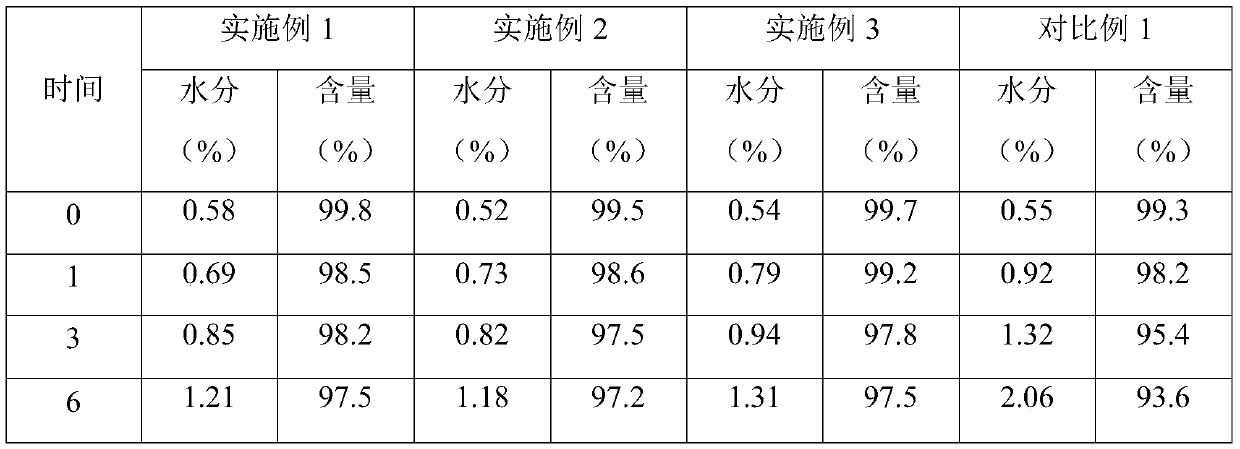
Embodiment 1
[0021] The prescription composition of every 10,000 ranitidine hydrochloride tablets is: ranitidine hydrochloride 1674g, PH101 type microcrystalline cellulose 700g, pharmaceutical direct pressure anhydrous calcium hydrogen phosphate 500g, magnesium stearate 20g, silicon dioxide 20g.
[0022] Preparation:
[0023] (1) First, add ranitidine hydrochloride, microcrystalline cellulose and anhydrous calcium hydrogen phosphate into the mixer, set the mixer speed to 8 rpm, and mix for 20 minutes;
[0024] (2) Then add the prescribed amount of magnesium stearate and silicon dioxide into the mixer, set the mixer speed to 8rpm, mix for 5min and then compress the tablet, and the hardness of the tablet is controlled at 100-120N, to obtain dynamite hydrochloride Nitidine tablets.
Embodiment 2
[0026] The prescription composition of every 10,000 ranitidine hydrochloride tablets is: ranitidine hydrochloride 1674g, PH102 type microcrystalline cellulose 800g, pharmaceutical direct pressure anhydrous calcium hydrogen phosphate 600g, magnesium stearate 30g, silicon dioxide 30g.
[0027] Preparation:
[0028] (1) First, add ranitidine hydrochloride, microcrystalline cellulose and anhydrous calcium hydrogen phosphate into the mixer, set the mixer speed to 8 rpm, and mix for 20 minutes;
[0029] (2) Then add the prescribed amount of magnesium stearate and silicon dioxide into the mixer, set the mixer speed to 8rpm, mix for 5min and then compress the tablet, and the hardness of the tablet is controlled at 100-120N, to obtain dynamite hydrochloride Nitidine tablets.
Embodiment 3
[0031] The prescription composition of every 10,000 ranitidine hydrochloride tablets is: ranitidine hydrochloride 1674g, PH101 type microcrystalline cellulose 300g, PH102 type microcrystalline cellulose 300g, PH200 type microcrystalline cellulose 300g, pharmaceutical direct pressure type Anhydrous calcium hydrogen phosphate 900g, magnesium stearate 40g, silicon dioxide 40g.
[0032] Preparation:
[0033] (1) First, add ranitidine hydrochloride, microcrystalline cellulose and anhydrous calcium hydrogen phosphate into the mixer, set the mixer speed to 8 rpm, and mix for 20 minutes;
[0034] (2) Then add the prescribed amount of magnesium stearate and silicon dioxide into the mixer, set the mixer speed to 8rpm, mix for 5min and then compress the tablet, and the hardness of the tablet is controlled at 100-120N, to obtain dynamite hydrochloride Nitidine tablets.
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com