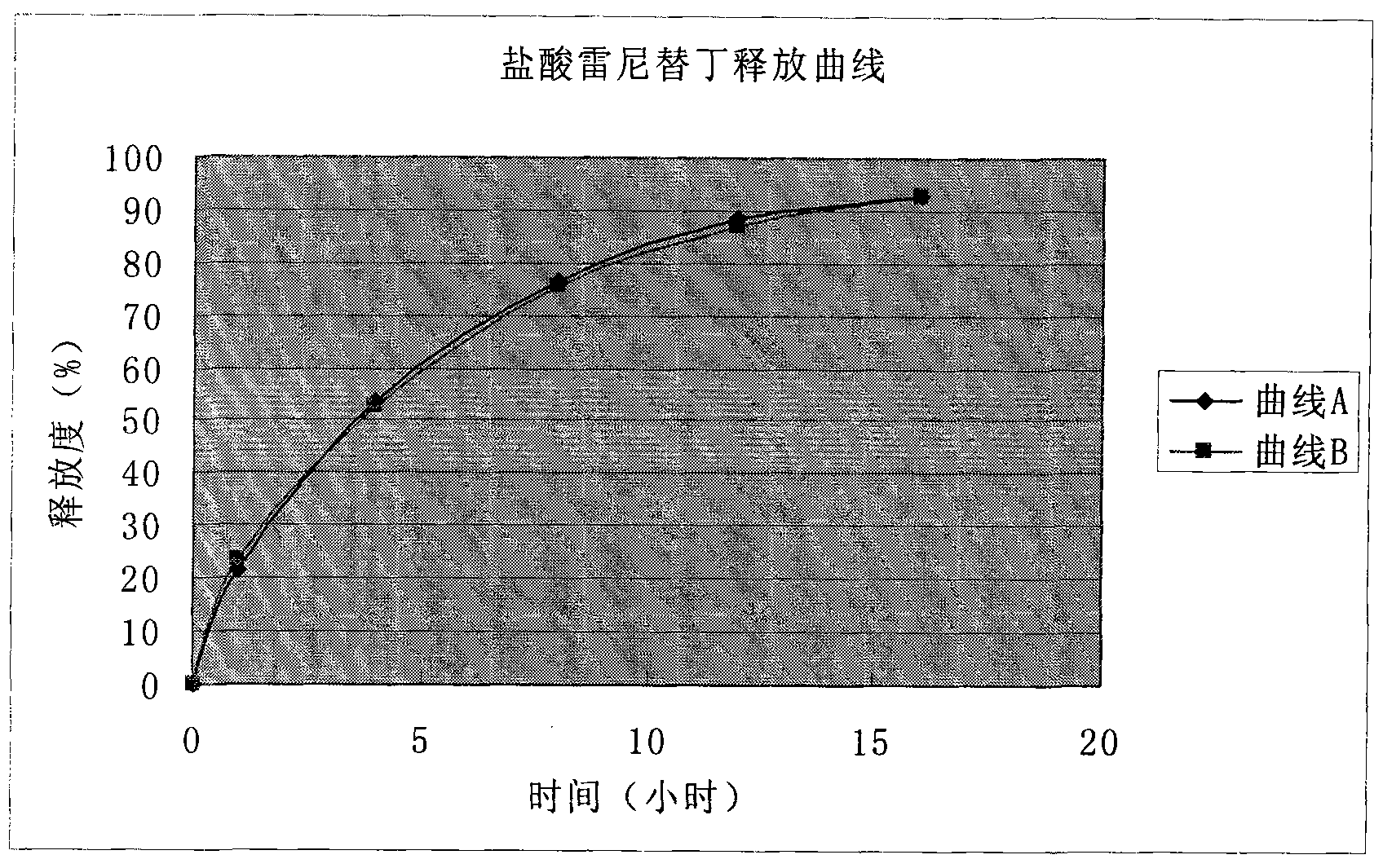
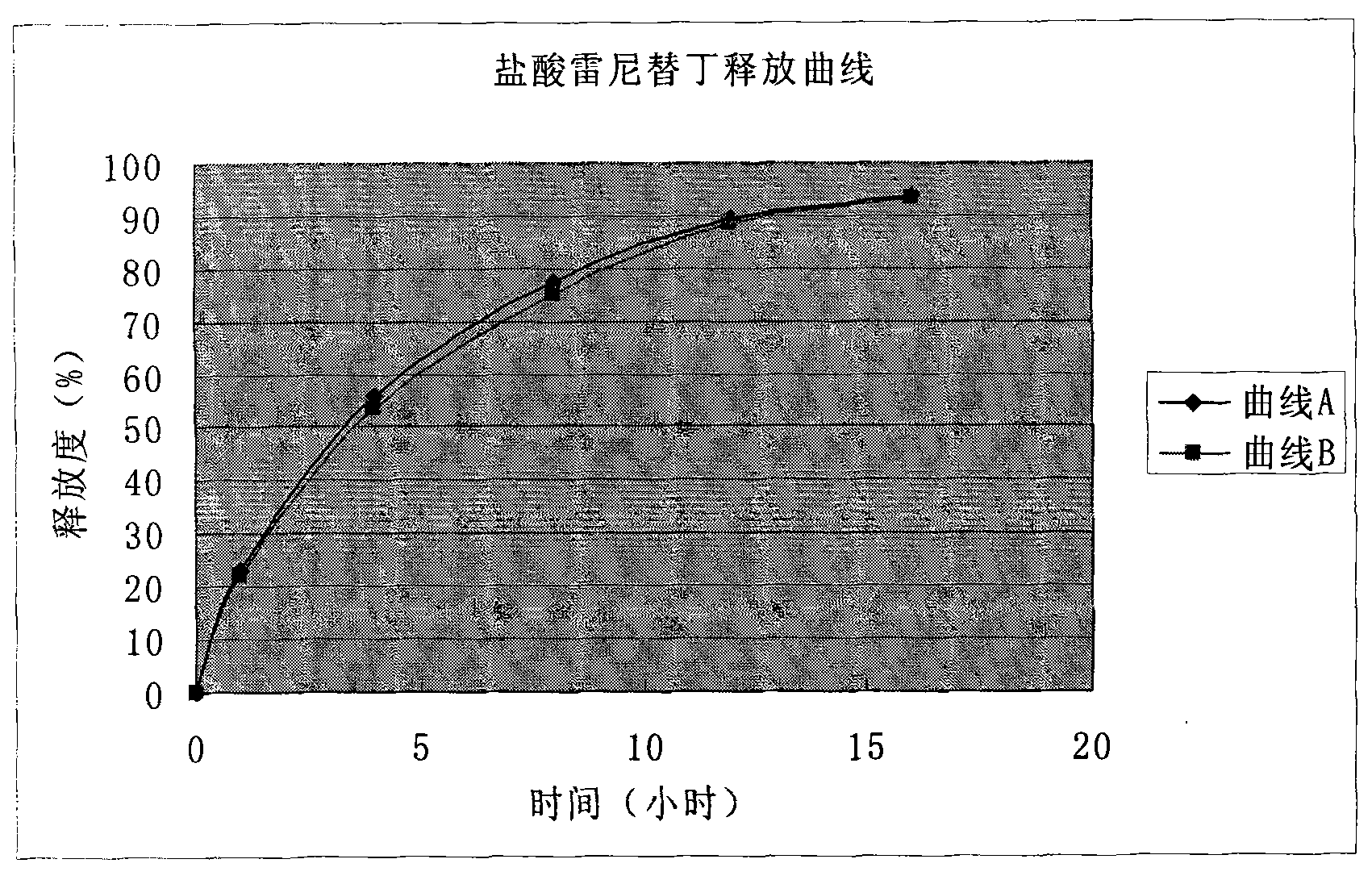
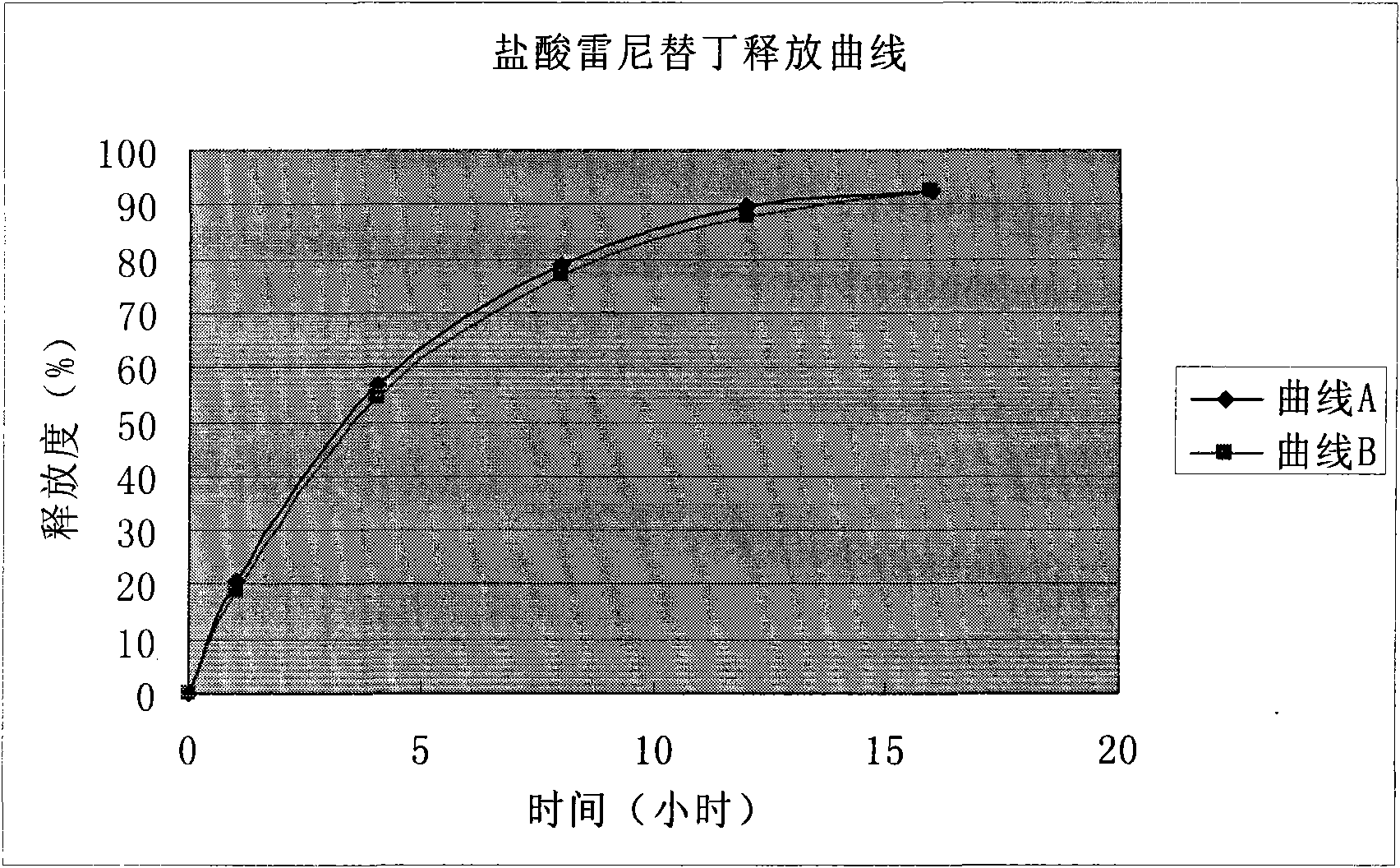
Solid preparation of ranitidine hydrochloride/bismuth potassium citrate medicinal composition
A technology of ranitidine hydrochloride and potassium bismuth citrate, which is applied in the field of medicine, can solve the problems of low bioavailability, poor stability of ranitidine hydrochloride, peaks and troughs of release, etc., and achieves high bioavailability and reduces drugs. Side effects, release smooth and long-lasting effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] The preparation prescription (1000) of embodiment 1 ranitidine hydrochloride / bismuth potassium citrate tablet
[0071] Ranitidine Hydrochloride 100g
[0072] Bismuth Potassium Citrate 110g
[0073] Chitosan 400g
[0074] Sodium Alginate 160g
[0075] Lactose 100g
[0076] 150g pregelatinized starch
[0077] Carboxymethyl Starch Sodium 60g
[0078] Povidone K30 15g
[0081] Preparation Process
[0082] (1) 100g ranitidine hydrochloride, 110g bismuth potassium citrate, 400g chitosan, and 160g sodium alginate were dissolved in 2000ml of purified water to obtain an aqueous phase;
[0083] (2) Mix 1000ml peanut oil and 4000ml chloroform evenly to obtain an oil phase;
[0084] (3) Slowly drip the water phase obtained above into the oil phase under stirring conditions, stir and emulsify for 10 minutes after dripping, then add 50g of sodium lauryl sulfate to make the gel completely, then add 500ml of n-hexane an...
Embodiment 2
[0089] The preparation prescription (1000) of embodiment 2 ranitidine hydrochloride / bismuth potassium citrate capsules
[0090] Ranitidine Hydrochloride 150g
[0091] Bismuth Potassium Citrate 110g
[0092] Chitosan 300g
[0093] Sodium Alginate 80g
[0094] Microcrystalline Cellulose 100g
[0095] Low-substituted hydroxypropyl cellulose 65g
[0096] Sodium Carboxymethyl Cellulose 3g
[0098] Preparation Process
[0099] (1) 150g ranitidine hydrochloride, 110g bismuth potassium citrate, 300g chitosan, and 80g sodium alginate were dissolved in 2000ml of purified water to obtain an aqueous phase;
[0100] (2) Mix 1000ml peanut oil and 4000ml chloroform evenly to obtain an oil phase;
[0101] (3) Slowly drip the water phase obtained above into the oil phase under stirring conditions, stir and emulsify for 30min after dripping, then add 40g of sodium lauryl sulfate to make the gel completely, then add 500ml of n-hexane and 500ml of water , the two...
Embodiment 3
[0106] The preparation prescription (1000 bags) of embodiment 3 ranitidine hydrochloride / bismuth potassium citrate granules
[0107] Ranitidine Hydrochloride 100g
[0108] Bismuth Potassium Citrate 110g
[0109] Chitosan 500g
[0110] Sodium Alginate 240g
[0111] Sucrose 280g
[0112] Aspartame 30g
[0113] Mannitol 70g
[0114] Povidone K30 20g
[0115] Preparation Process
[0116] (1) 100g ranitidine hydrochloride, 110g bismuth potassium citrate, 500g chitosan, and 240g sodium alginate were dissolved in 2500ml of purified water to obtain an aqueous phase;
[0117] (2) Mix 1000ml peanut oil and 4000ml chloroform evenly to obtain an oil phase;
[0118] (3) Slowly drip the water phase obtained above into the oil phase under stirring conditions, stir and emulsify for 20min after dripping, then add 50g of sodium lauryl sulfate to make the gel completely, then add 500ml of n-hexane and 500ml of water , the two phases are separated, and the microcapsules sink in the water...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com