Patents
Literature
34 results about "Lafutidine" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
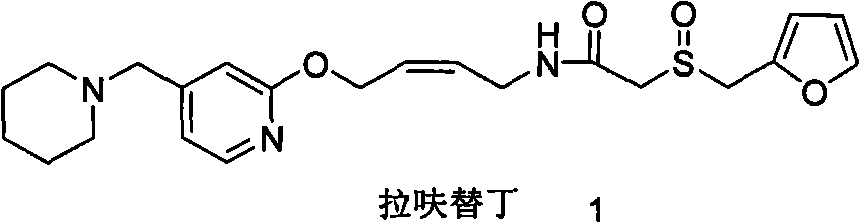
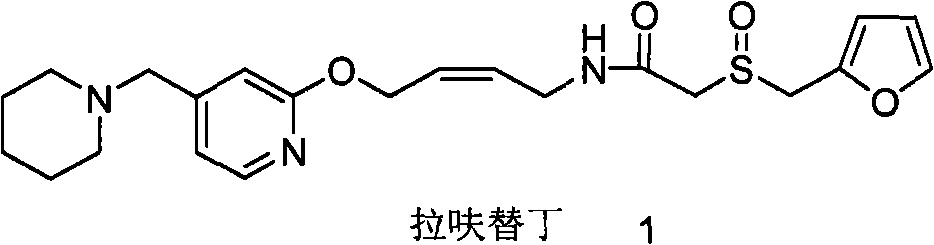
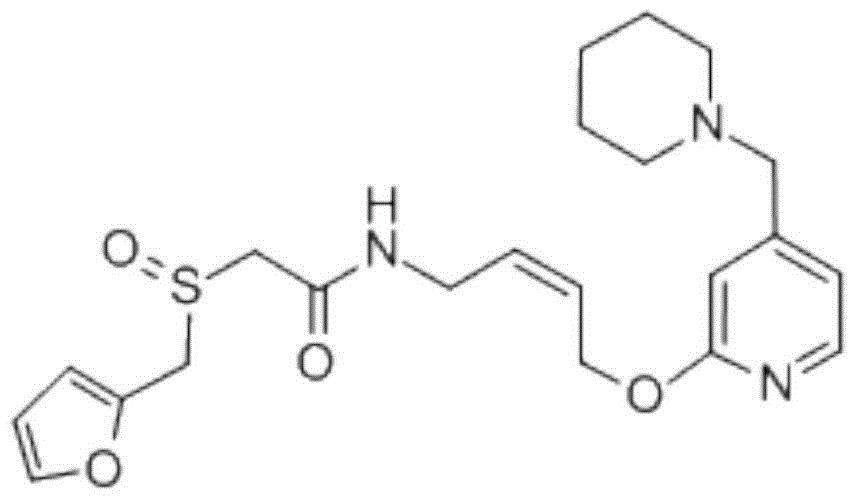
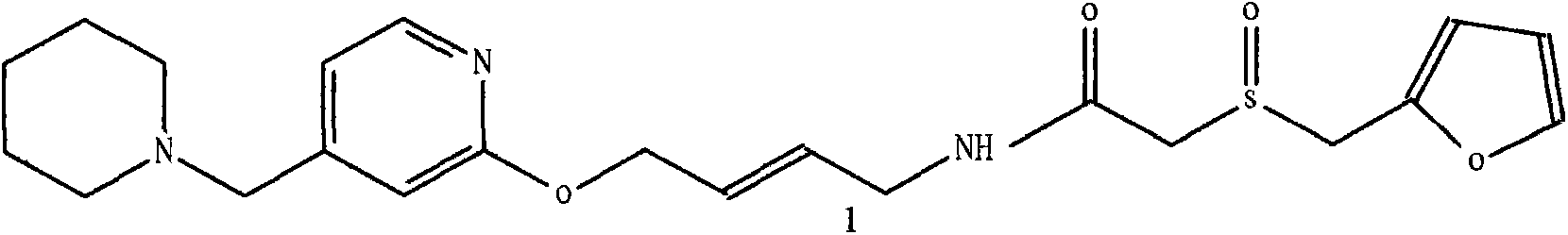
Lafutidine (INN) is a second generation histamine H₂ receptor antagonist having multimodal mechanism of action and used to treat gastrointestinal disorders. It is marketed in Japan and India.
Lafutidine gastric-retention controlled-release composite
ActiveCN101919817AFacilitated releaseRegulatory releaseOrganic active ingredientsDigestive systemUse medicationControl release
The invention relates to a lafutidine gastric-retention controlled-release composite belonging to the field of pharmaceutic preparations. The lafutidine gastric-retention controlled-release composite is characterized by comprising the following components in percentage by weight: 5%-20% of the lafutidine, 10%-40% of framework materials, 10%-30% of assistant bleaching agents, 5%-15% of foaming agents, 5%-15% of filling agents and 0.5%-10% of lubricating agents. The composite has reasonability and simple preparation process; compared with same pharmaceuticals, the lafutidine gastric-retention controlled-release composite has little dose, good tolerance, little side effect, and the like; and in addition, compared with the conventional tablets, the lafutidine gastric-retention controlled-release composite enhances the solubility of lafutidine, prolongs the action time (prolonged from 2-3 hours to 5-6 hours) on the upper parts of a stomach and a small intestine, promotes the absorption, enhances the bioavailability, reduces the pharmaceutical usage times, achieves the maximum treatment effect through minimum doses, reduces the concentration change of peaks and valleys and has good patient compliance.
Owner:SHANDONG QIDU PHARMA
Lafutidine compound and novel preparation method of lafutidine compound
InactiveCN102702181AChange the status quo of low puritySolve puzzlesNervous disorderOrganic chemistryAlcoholOrganosolv
The invention relates to a lafutidine compound and a novel preparation method of the lafutidine compound. The preparation method comprises the following steps: 1) dissolving a lafutidine crude product into an organic solvent, filtering and removing insoluble impurities, obtaining the first filtrate; 2) adding adsorptive inorganic substances to the first filtrate, sharply agitating, filtering and removing the adsorptive inorganic substances after standing, obtaining the second filtrate, and compressing and concentrating the second filtrate; (3) heating the second filtrate to 75 DEG C or less, keeping the temperature for a period of time, concentrating the second filtrate, slowly adding a mixing solvent, of which the volume ratio of alcohol to water is 0.2-1, under agitation, reducing the temperature to 2 DEG C at most in a gradient way, recrystallizing, separating, washing and drying the separated crystal to obtain the refined lafutidine; (4) returning any separated crystallized crystallizing mother solution to the step 3). The preparation method disclosed by the invention helps to solve the problems of low purity in rough preparation of lafutidine and lafutidine crude drug, has the characteristics of simplicity, easiness in control and industrial production, the quality of the preparation product is improved, and toxic and side effects are reduced.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
Method for preparing lafutidine by virtue of aminolysis
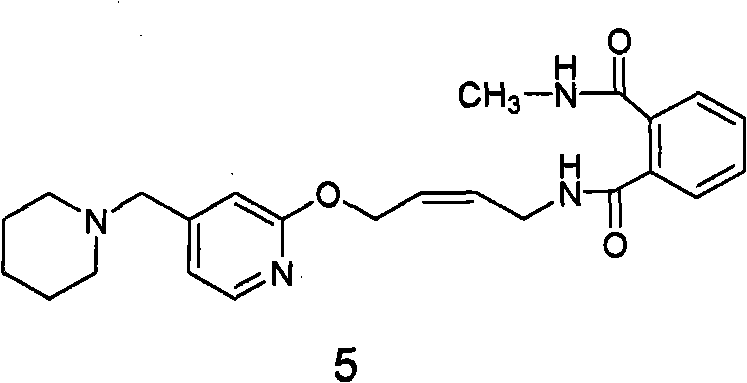
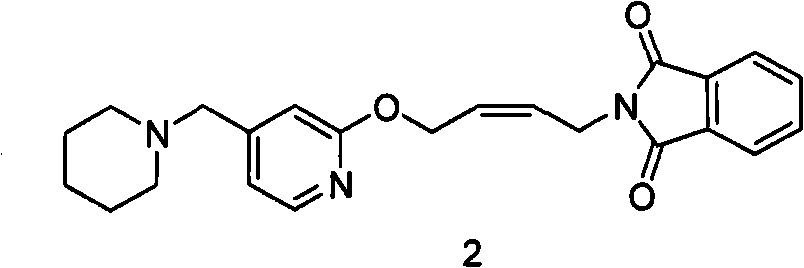
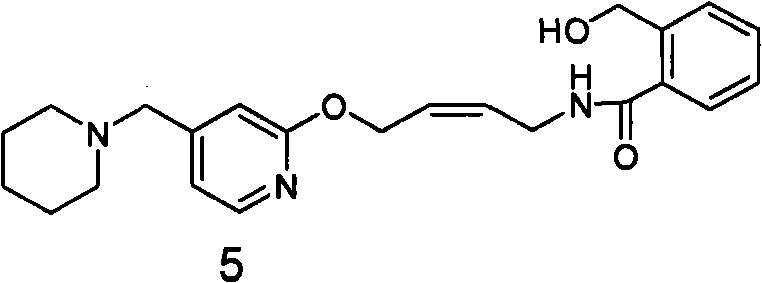
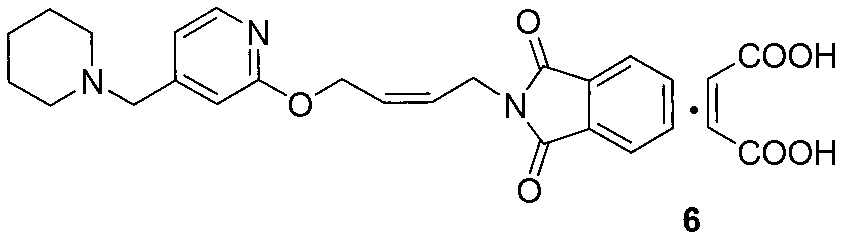
The invention relates to a method for preparing lafutidine by virtue of aminolysis. The method comprises the following steps: reacting a compound shown in a formula 2 or a pharmaceutically acceptable salt thereof with amine RNH2 so as to generate a compound shown in a formula 5, wherein R represents hydrogen, linear-chain or branched C1-4 alkyl, phenyl or C1-4 alkyl substituted phenyl; reacting the compound shown in the formula 5 with the amine RNH2 so as to obtain a compound shown in a formula 3, wherein R represents hydrogen, linear-chain or branched C1-4 alkyl, phenyl or C1-4 alkyl substituted phenyl; condensing the compound shown in the formula 3 and a compound shown in a formula 4 so as to obtain the lafutidine; and optionally, converting the obtained lafutidine into a pharmaceutically acceptable salt of the lafutidine.
Owner:CHINA RESOURCES DOUBLE CRANE PHARMA COMPANY
Lafutidine lyophilized powder injection and preparing method thereof
InactiveCN101199527AAvoid degradationPrevent precipitationOrganic active ingredientsPowder deliveryMANNITOL/SORBITOLCITRATE ESTER
The invention relates to lafutidine freeze-dried injection, which can treat gastric ulcer, duodenal ulcer and ulcer on the anastomosed part. The lafutidine freeze-dried injection is composed of lafutidine and mannitol, wherein, the mass ratio of the lafutidine and the mannitol is 1 : 10 to 100; the preferable mass ratio is 1 : 20 to 50; and one or the mixture of sodium chloride and citrate can be added into the lafutidine freeze-dried injection so as to prevent the medicine bottle from exploding in the process of freeze-drying the lafutidine freeze-dried injection. The preparation processes are as following: the lafutidine is taken to be placed inside the aseptic container, and then the water for injection is added to be heated until the temperature reaches 70 DEG C so that the lafutidine can be dissolved in the water for injection; and then the mannitol is added to be mixed so that the mannitol can be dissolved and is mixed evenly; the content of the intermediate is measured; after the content of the intermediate is qualified, in the aseptic condition, the solution is filtered until to be clear by microporous membrane of 0.22 microns; the filtrate is filled inside the aseptic silin bottle; and part of the silin bottle is plugged by butyl rubber closure, with the posterior steps of tray filling, freeze-drying in the freeze dryer, tamponing, unpacking, mouth rolling, quality inspecting and packaging.
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
Lafutidine injecta and its prepn
InactiveCN1421204AImprove stabilityImprove bioavailabilityOrganic active ingredientsDigestive systemVeinTreatment effect
The present invention is Lafutidine injection for intravenous injection and intramuscular injection. The recipe of the Lafutidine injection includes Lafutidine, organic solvent, surfactant, antioxidant, osmotic pressure regulator and water for injection. Compared with Lafutidine tablet, the Lafutidine injection has fast effect, short peak reaching time in the blood high medicine concentration andhigh treating effect; and compared with Famotidine or Ranitidine injection, the Lafutidine injection has less dosage and higher curative effect in treating gastric ulcer.
Owner:黄振华
Synthesis method of 2-chloro-4-(piperidylmethyl)pyridine
The invention relates to a synthesis method of 2-chloro-4-(piperidylmethyl)pyridine, belonging to the field of medicine synthesis. The method comprises the following steps: dropwisely adding a nitrite water solution into acid and 2-amino-4-methylpyridine to react, thereby obtaining a white solid product; adding POCl3 into the white solid to obtain a compound 2-chloro-4-methylpyridine; dropwisely adding SO2Cl2 into the compound 2-chloro-4-methylpyridine while adding a free-radical initiator batch by batch, and distilling under reduced pressure to obtain a compound 2-chloro-4-chloromethylpyridine; and condensing the 2-chloro-4-chloromethylpyridine and piperidine to obtain the 2-chloro-4-(piperidylmethyl)pyridine. Compared with the traditional synthesis method of 2-chloro-4-(piperidylmethyl)pyridine, the synthesis method provided by the invention has the advantages of simpler steps (only three steps) and lower cost of raw materials, and the total yield is higher than 32%. Generally, the invention can greatly lower the synthesis cost of lafutidine.
Owner:常州市赛努尔高分子科技有限公司
A new process for preparing lafutidine
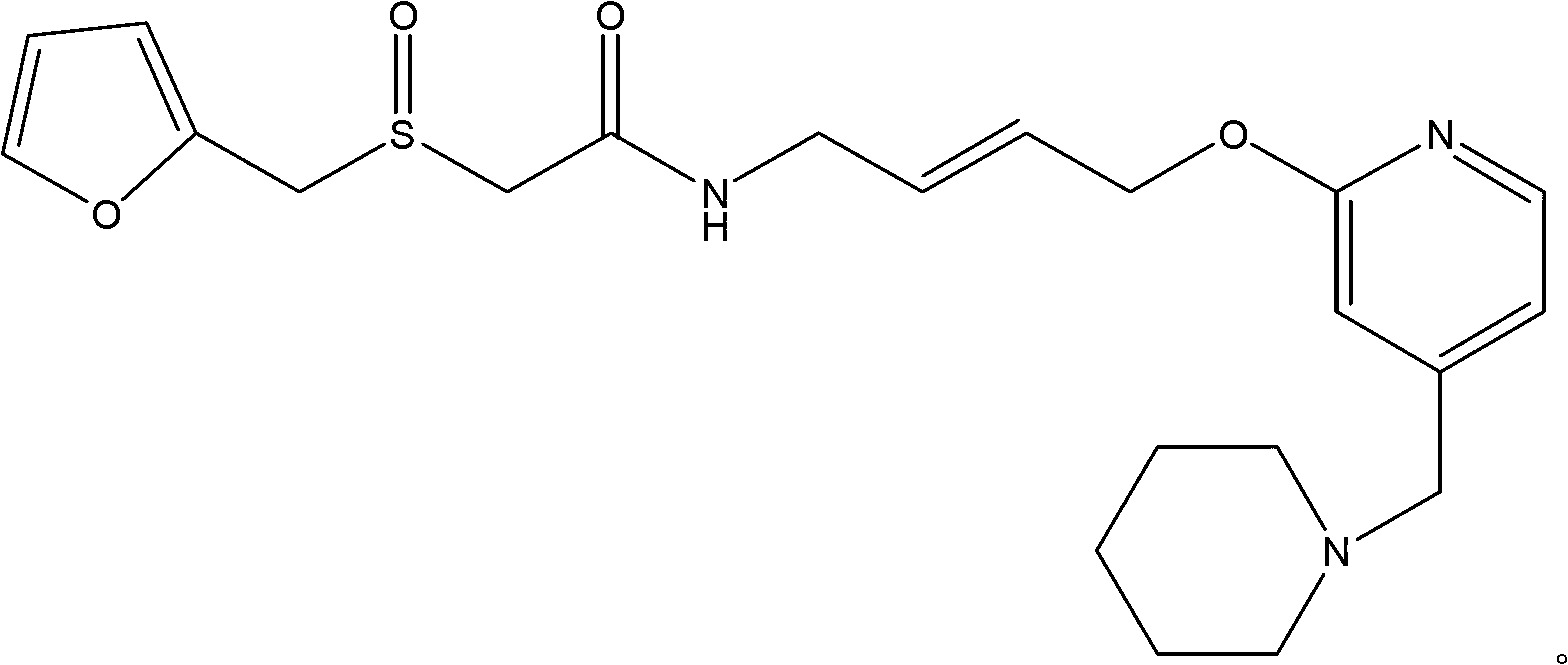
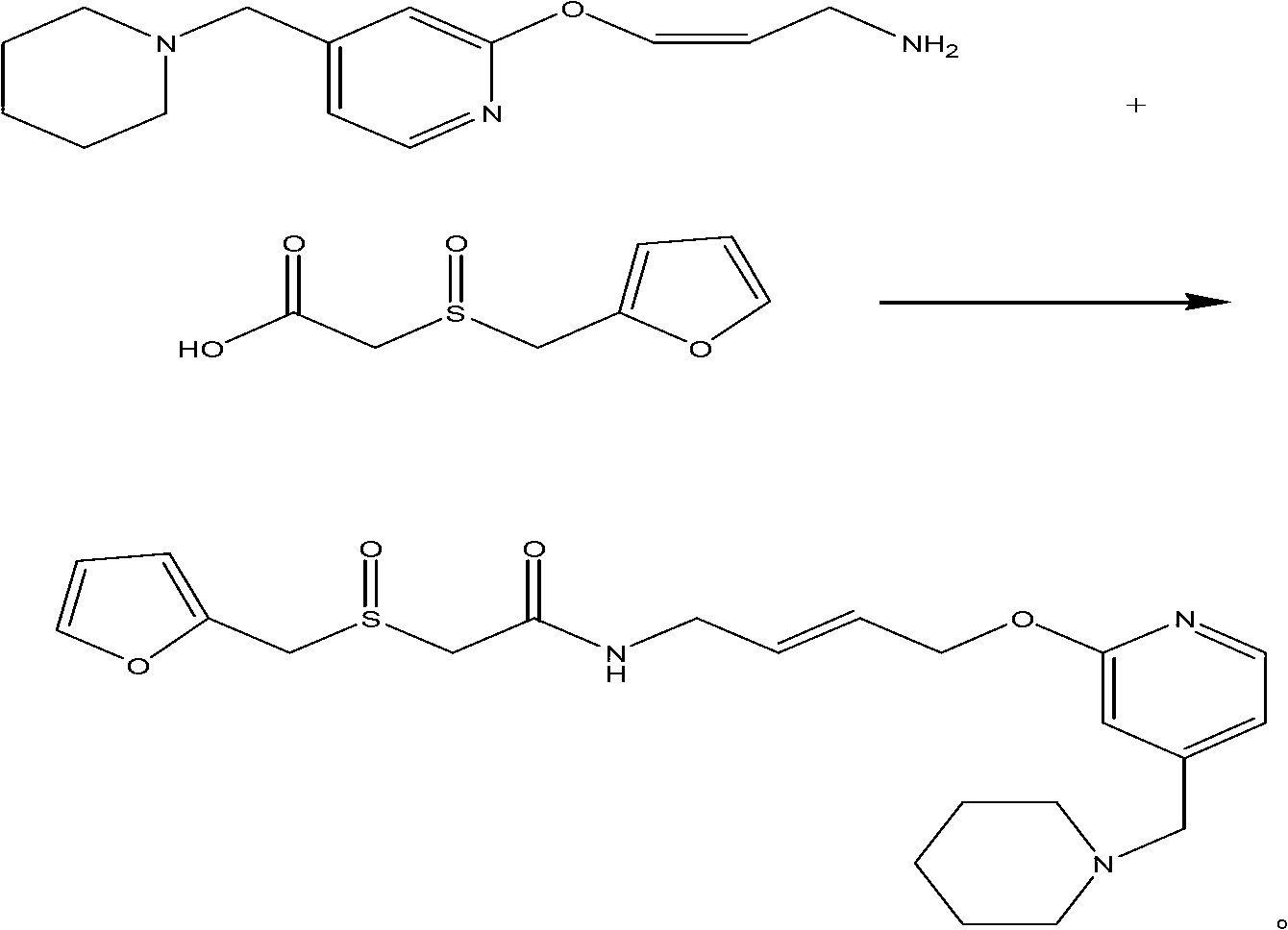
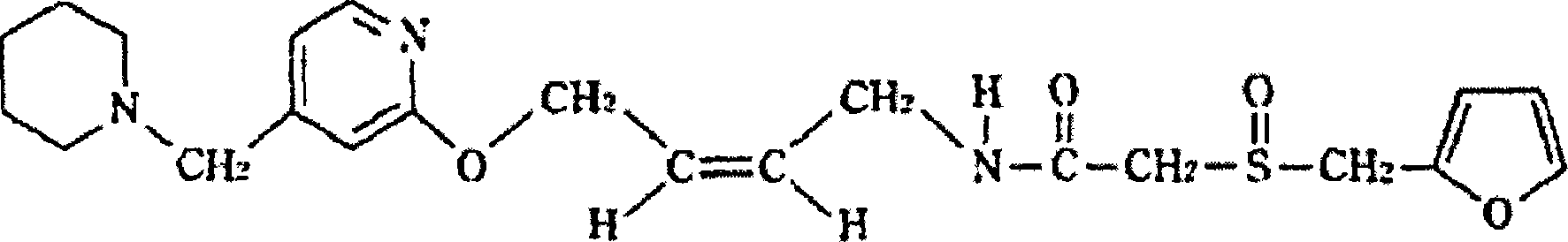
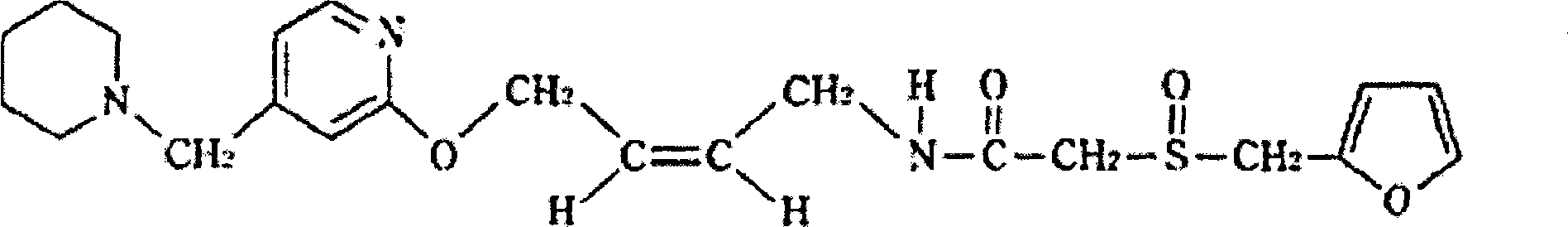
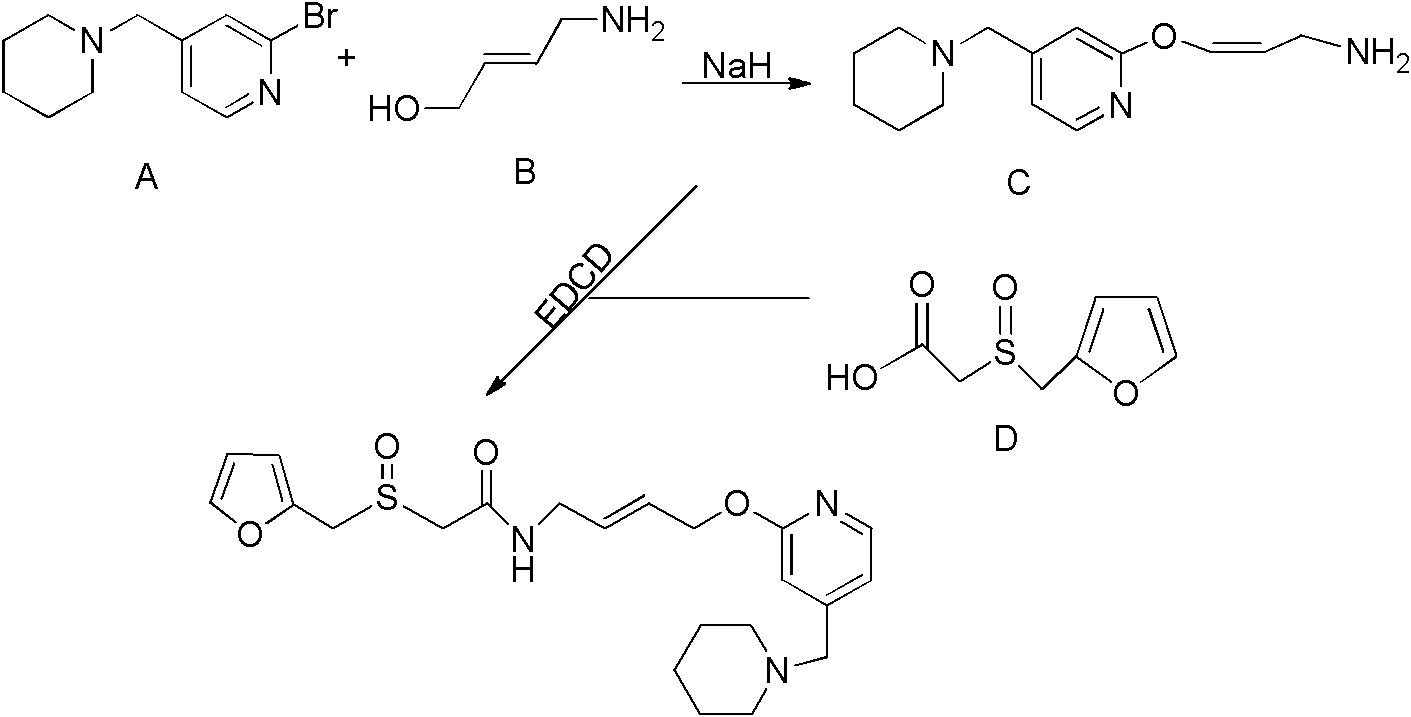
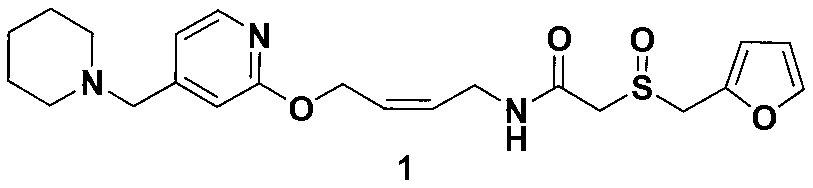
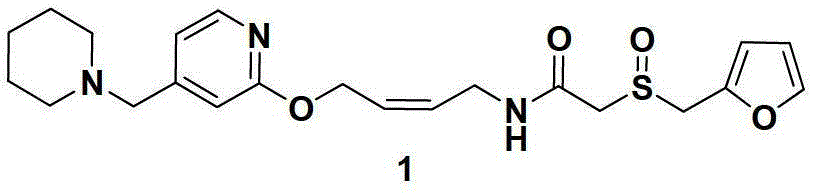
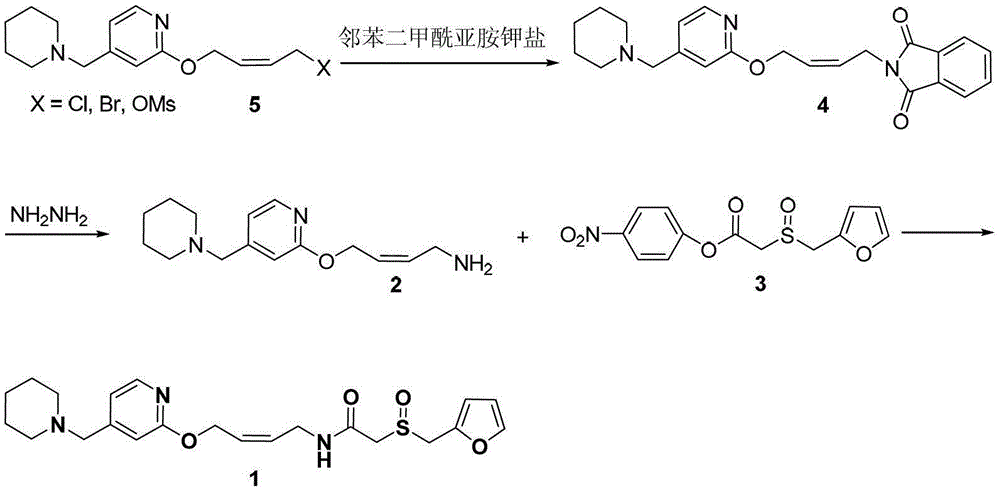
The invention relates to a preparation method for lafutidine, and the technology comprises the following steps: preparing 4-[4-(piperidine-1-group-methylene)pyridine-2-group-oxygen]-2(Z)-butylene-1-amine and preparing 2-(2-furans methyl sulfoxide group)acetate, then dissolving 2-(2-furans methyl sulfoxide group)acetate into methylene chloride at the room temperature, and then adding a methylene chloride solution possessing 4-[4-(piperidine-1-group-methylene)pyridine-2-group-oxygen]-2(Z)-butylene-1-amine at the room temperature, stirring and separating to obtain lafutidine. The preparation method of lafutidine of the invention modifies the original route, wherein the reaction is milder and easy to operate without using sodium hydride, the intermediate fragment employs a nontoxic and innocuous molybdenum tungsten metal for catalyzing, the reaction condition is milder, the environmental pollution can be reduced and the reaction cost can be decreased.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
Reducing preparation method for lafutidine
The invention relates to a reducing preparation method for lafutidine, which comprises the following steps: reducing a compound shown in formula 2 or a pharmaceutically acceptable salt thereof with a reducing agent to obtain a compound shown in formula 5; heating to decompose the compound shown in formula 5 to obtain a compound shown in formula 3; carrying out condensation reaction on the compound shown in formula 3 and a compound shown in formula 4 to obtain lafutidine; and optionally converting the obtained lafutidine into a pharmaceutically acceptable salt thereof.
Owner:CHINA RESOURCES DOUBLE CRANE PHARMA COMPANY
Method for preparing lafutidine from hydroxylamine hydrochloride
The invention relates to a novel chemical synthetic method for lafutidine, and in particular relates to a method for preparing the lafutidine from hydroxylamine hydrochloride serving as aminolysis reagent. The method comprises the following steps of: (1) reacting a compound in a formula 4 with the hydroxylamine hydrochloride and sodium hydroxide so as to obtain a compound in a formula 2; (2) carrying out condensation on the compound in the formula 2 and a compound in a formula 3 so as to obtain the lafutidine in a formula 1. The whole preparation process is as shown in the specification. When in preparation of the key intermediate the compound in the formula 2, the hydroxylamine hydrochloride is chosen for substituting other reagents for aminolysis reaction, and the prepared lafutidine product has higher purity and can reach 99.88%; compared with the condition that the aminolysis reagent is removed from the intermediate, the hydroxylamine is easier to remove; and furthermore, the hydroxylamine hydrochloride is a solid reagent, has high purity and high stability, and industrial production is more easily realized.
Owner:北京国联诚辉医药技术有限公司
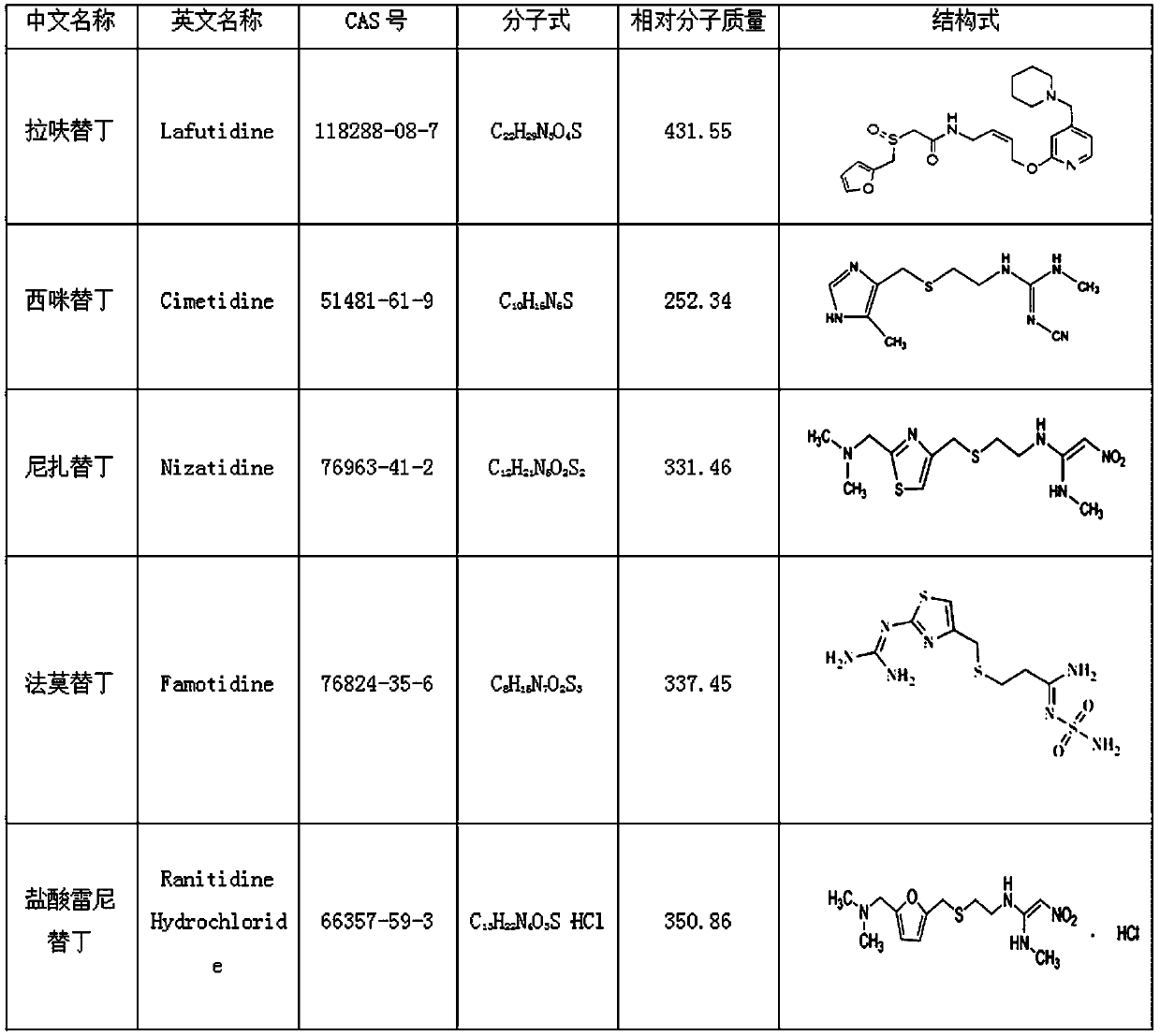
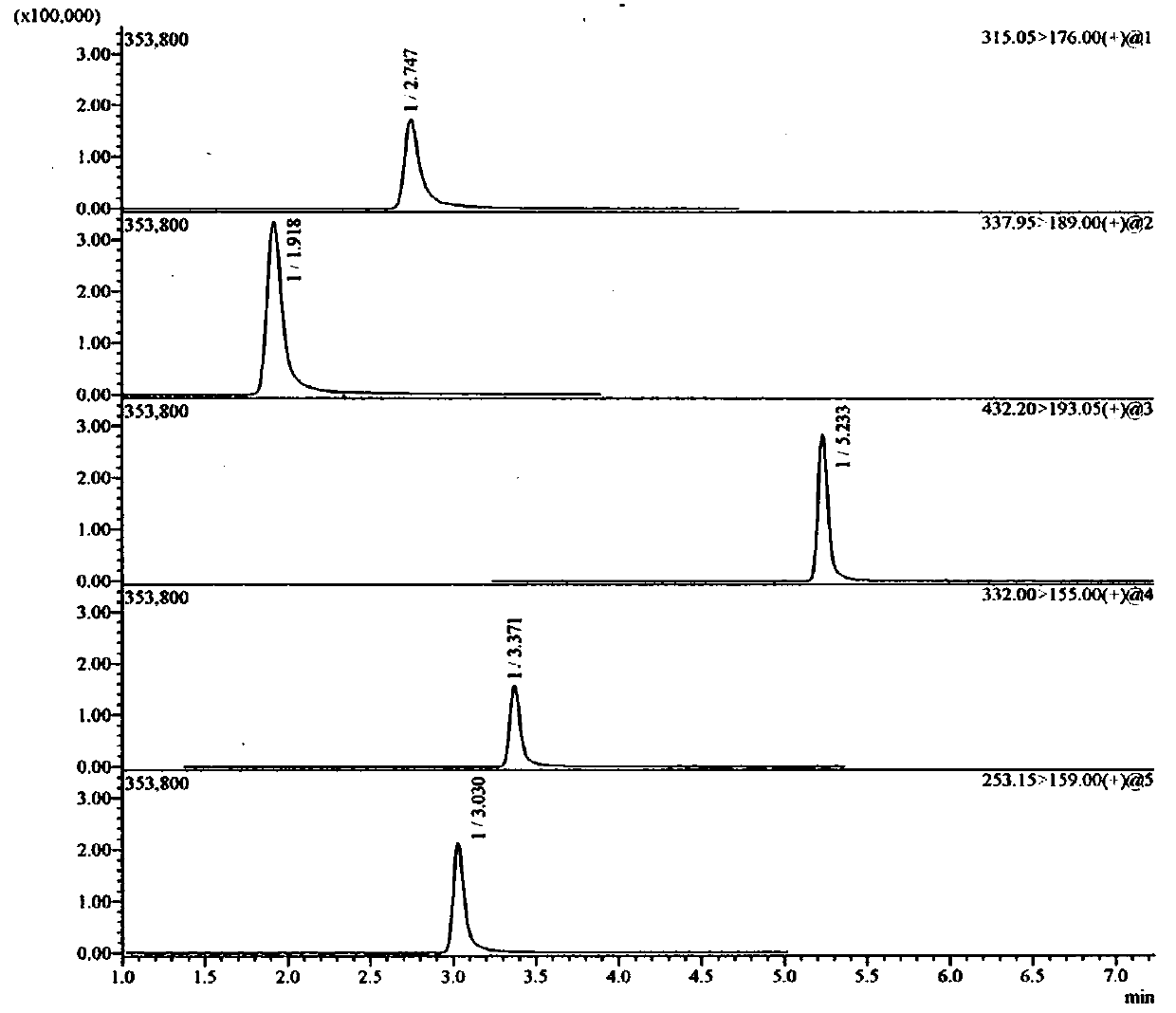
Method for detecting ranitidine hydrochloride, cimetidine, famotidine, nizatidine and lafutidine
The invention discloses a method for detecting ranitidine hydrochloride, cimetidine, famotidine, nizatidine and lafutidine. The method comprises the following steps: (1) selecting chromatographic conditions; (2) selecting mass spectrometric conditions; (3) preparing a standard solution; (4), preparing samples; (5), detecting and calculating. Tests prove that the method is rapid and high in specificity; the method is suitable for detecting the illegally added ranitidine hydrochloride, famotidine, lafutidine, nizatidine, cimetidine and other chemical medicines in Chinese patent medicines and health-care foods which have the effects of invigorating the stomach, improving the gastrointestinal function (having an auxiliary protection effect on gastric mucosal injury) and having an auxiliary protection effect on gastric mucosal injury or in other foods illegally claimed to have said functions.
Owner:太原市食品药品检验所
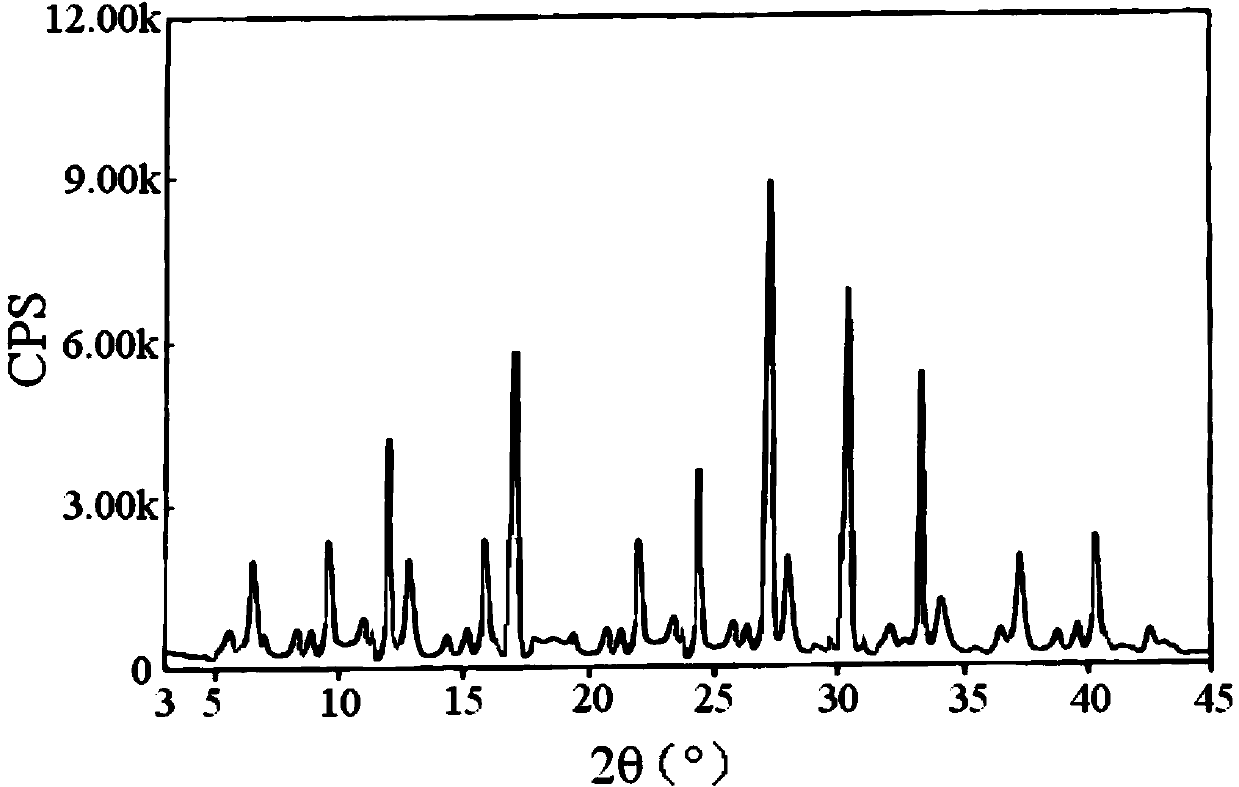
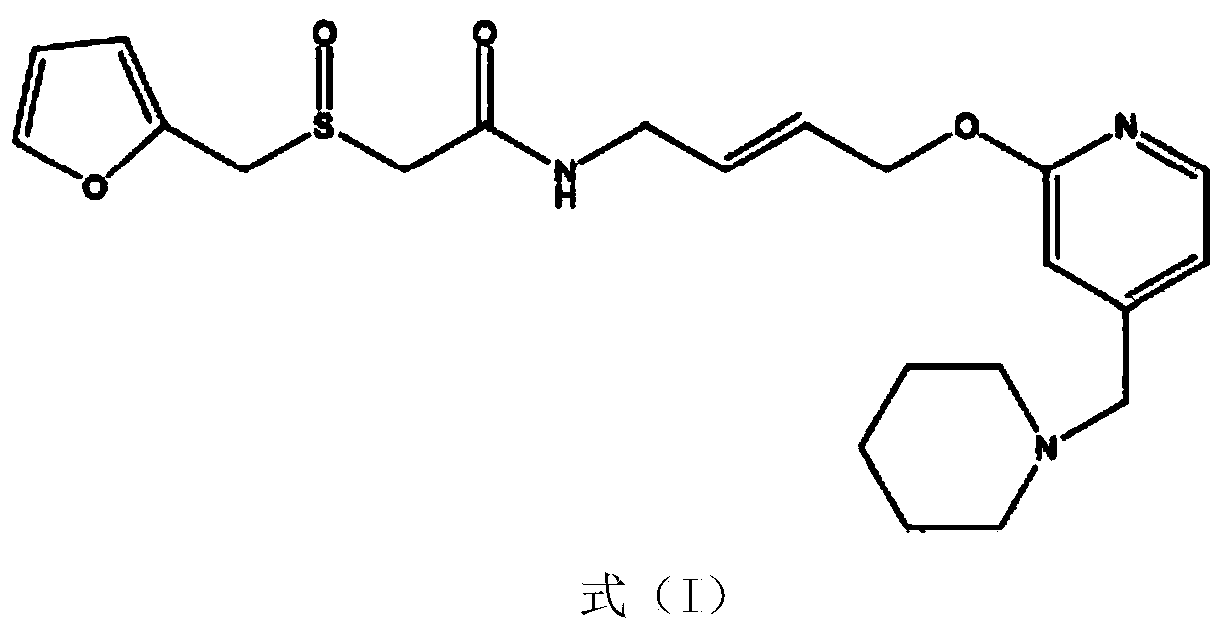
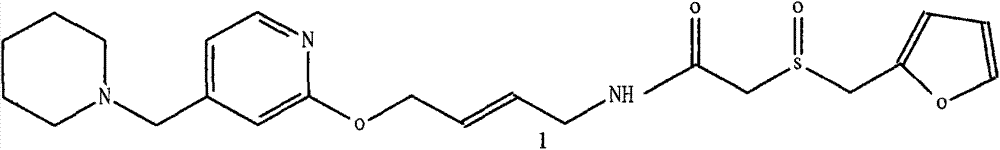
Lafutidine crystal compound
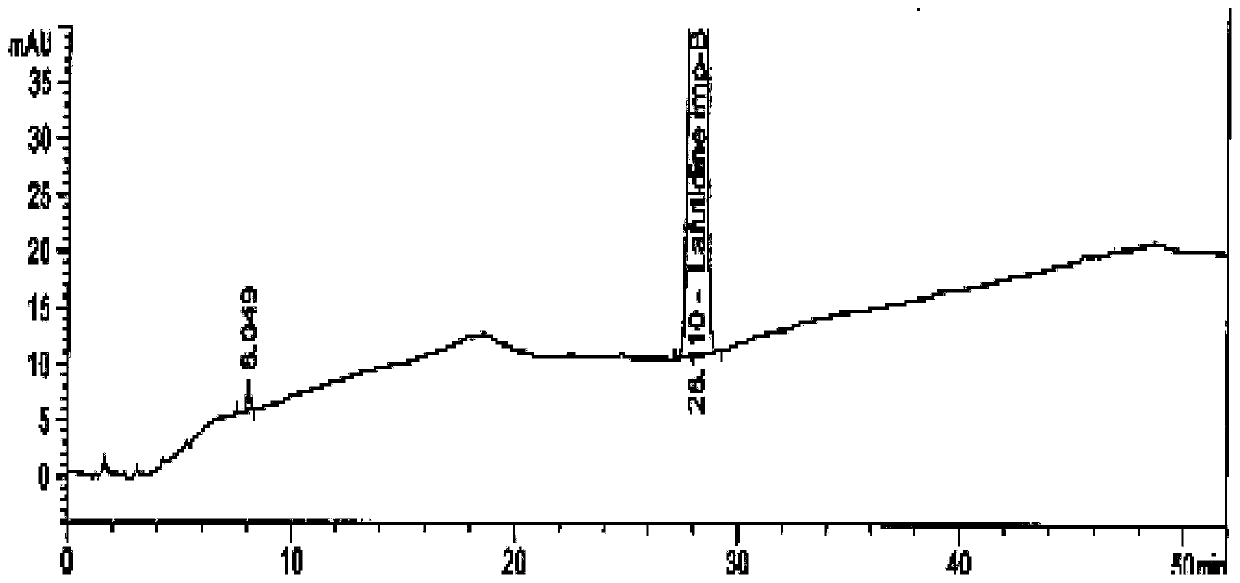
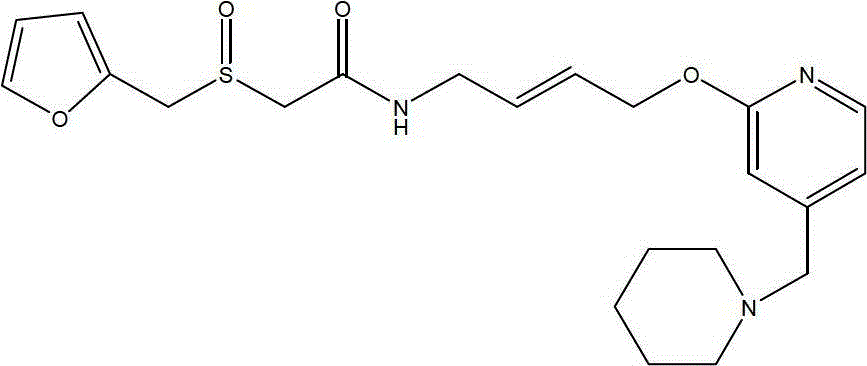
The invention belongs to the technical field of a medicine, and particularly relates to a lafutidine crystal compound shown in a formula (I). An X-ray powder diffraction spectrum obtained by using a Cu-K alpha ray is shown in a figure 1. The lafutidine crystal compound provided by the invention is a novel lafutidine crystal compound different from that in the prior art, the novel lafutidine crystal compound has improved solubility in water while the preparation prepared by adopting the novel lafutidine crystal compound disclosed by the invention has good bioavailability in comparison with the prior art.
Owner:YOUCARE PHARMA GROUP
Lafutidine composition freeze-dried powder injection for injection
InactiveCN103550170AReduce dosageReduce adverse reactionsPowder deliveryOrganic active ingredientsSolubilityIntestinal structure
The invention provides a lafutidine composition freeze-dried powder injection for injection and relates to the field of medicines and medicine manufacturing technique. The lafutidine composition freeze-dried powder injection includes the following raw materials: by weight, 8.34-9.21 parts of lafutidine, 3.75-5.56 parts of chitosan nanoparticles and 87.23-88.13 parts of water for injection. The invention has the following advantages: 1) the composition composed of lafutidine and chitosan nanoparticles according to the ratio of 1:0.5 can raise water solubility of lafutidine and shorten dissolution time of lafutidine, has good stability, has few insoluble particles, and is beneficial to be used in clinic; 2) the composition can promote reproduction of beneficial bacteria in the intestine, inhibit growth of harmful bacteria and achieve the effect of absorbing nutrients, has antiacid and anti-ulcer activities, can prevent medicinal stimulation of the stomach, has better anti-Hp performance, promotes ulcer healing, minimizes recurrence, has high healing rate of mice, and can be used to reduce dosage of lafutidine in clinic and decrease adverse reaction of lafutidine; and 3) the chitosan nanoparticles can replace mannitol to be used as a freeze-drying skeleton agent of the freeze-dried powder injection so as to eliminate the active effect of mannitol on human body.
Owner:HAINAN WEI KANG PHARMA QIANSHAN
Lafutidine chewing tablet and preparation method thereof
InactiveCN105687147ABlocking effect is effectiveLong-lasting blocking effectOrganic active ingredientsDigestive systemUlcer healingLafutidine
The present invention discloses a lafutidine chewing tablet and a preparation method thereof, the lafutidine chewing tablet is prepared from lafutidine, a filler, a flavoring agent, a binder, a coating agent and a lubricant; the lafutidine chewing tablet is a highly-efficient long-acting H2 receptor antagonist, has obvious inhibitory effect on gastric acid secretion, can inhibit gastric acid secretion caused by histamine, pentagastrin, food and the like, has gastric mucosal protective effect, has dose-dependent inhibition effect on the formation of a variety of experimental animal ulcer models, promotes ulcer healing, relieves symptoms and prevents ulcer recurrence. The lafutidine chewing tablet is easy to take, can promote drug dissolution and absorption in the body, even in a water deficiency state, can ensure medication on time, is especially suitable for the elderly, children, stroke patients, and patients with poor gastrointestinal functions and swallowing difficulties, and may reduce gastrointestinal tract drug burdens.
Owner:HEILONGJIANG ZHICHENG MEDICAL TECH
Lafutidine lyophilized powder injection and preparing method thereof
InactiveCN101199527BAvoid degradationPrevent precipitationOrganic active ingredientsPowder deliveryMANNITOL/SORBITOLCITRATE ESTER
Owner:JIANGSU AOSAIKANG PHARMA CO LTD
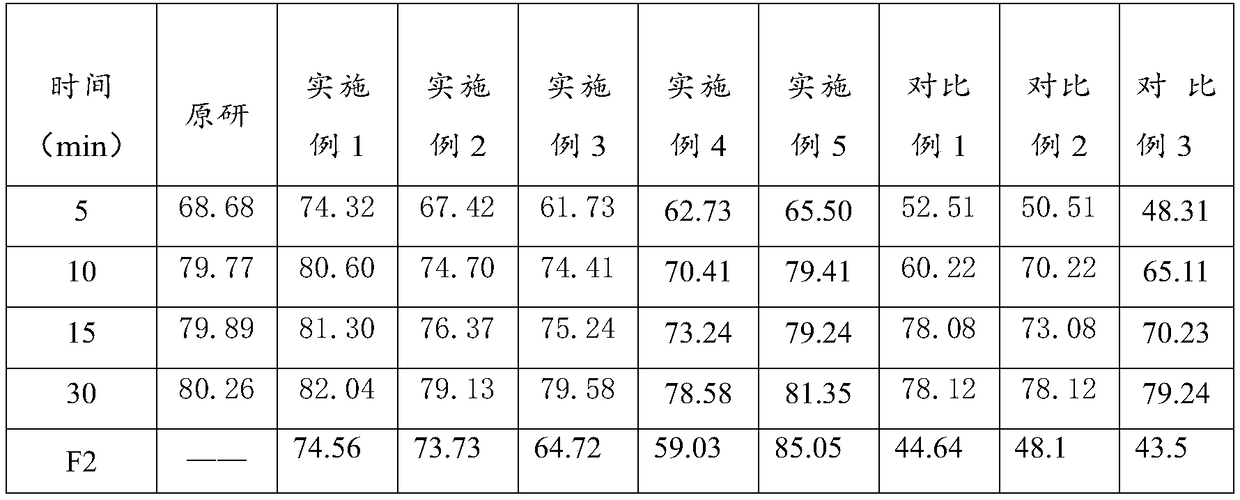
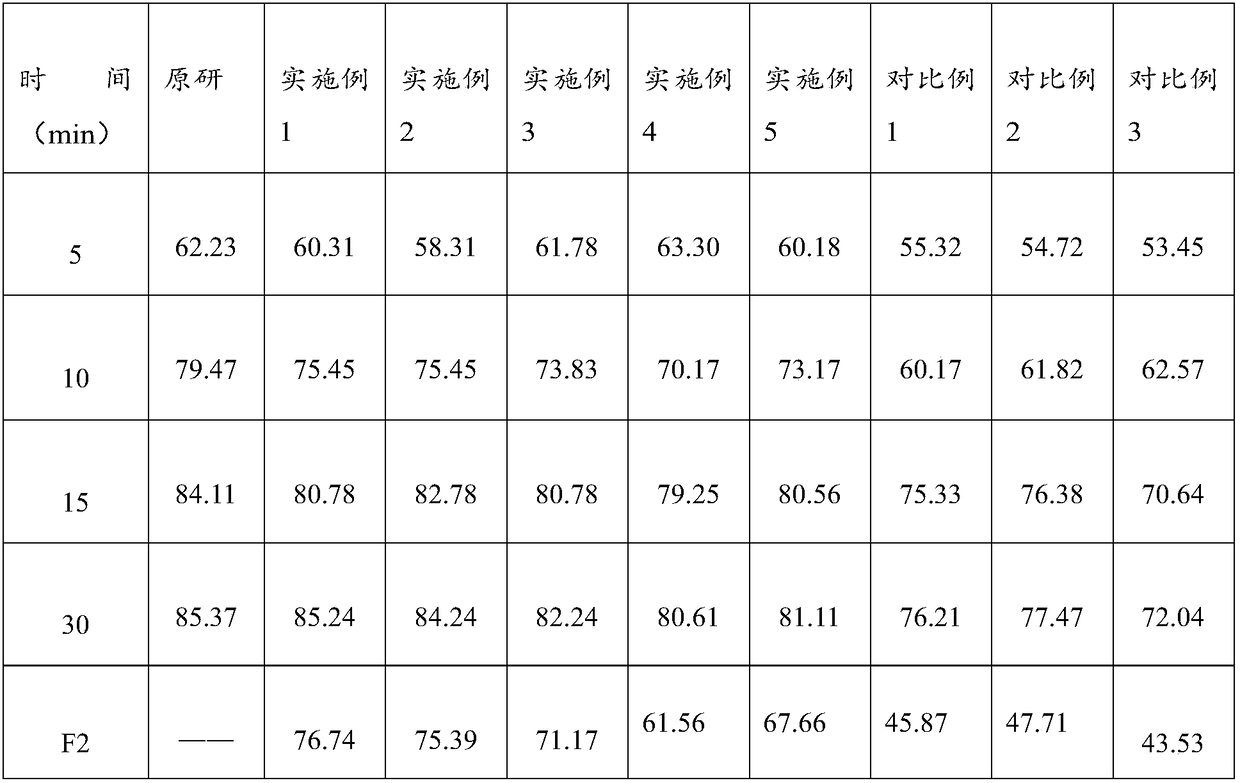
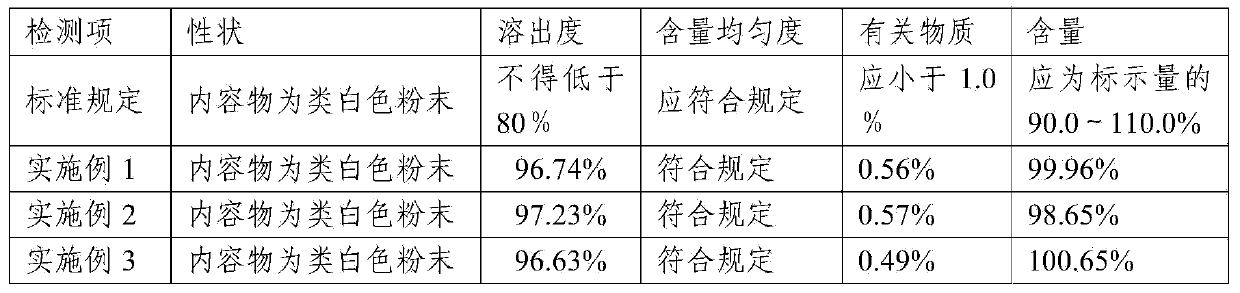
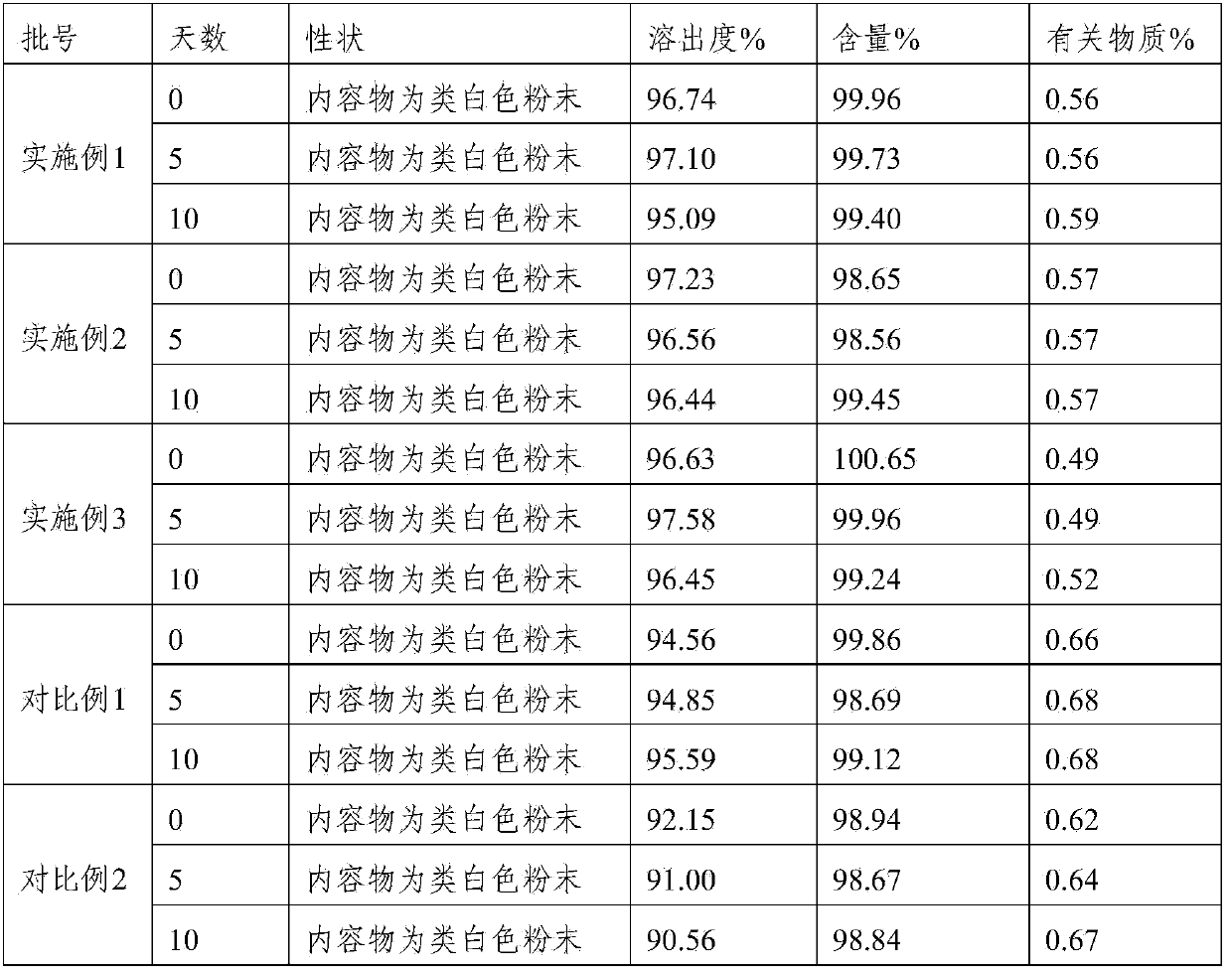
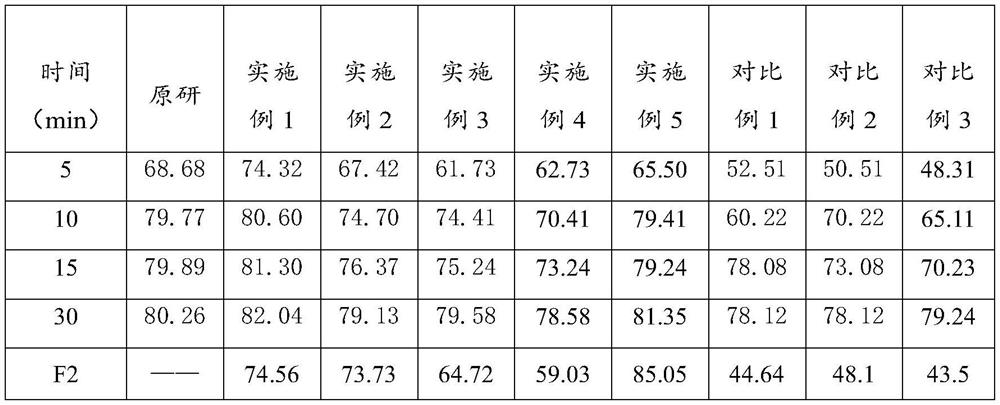
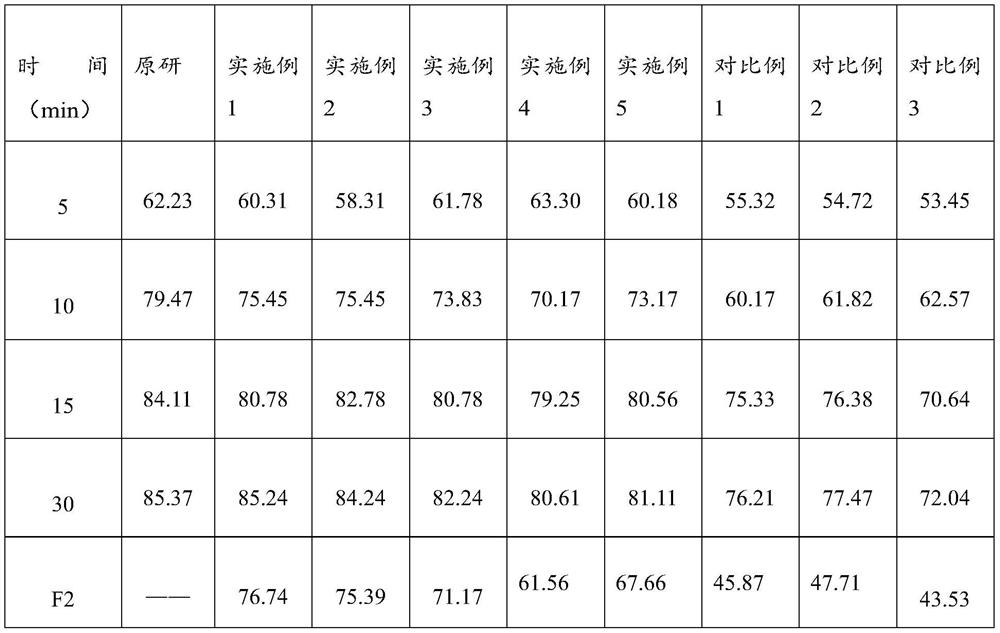
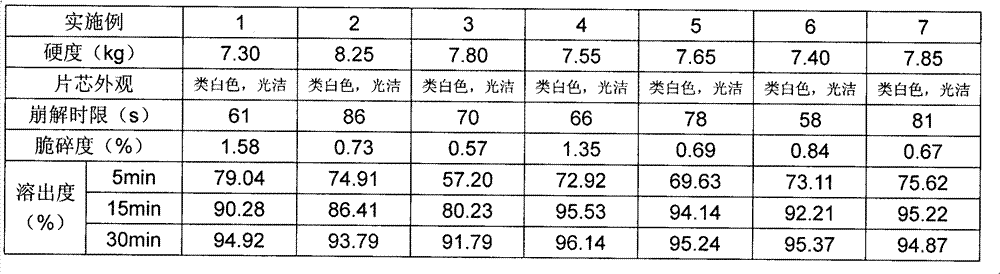
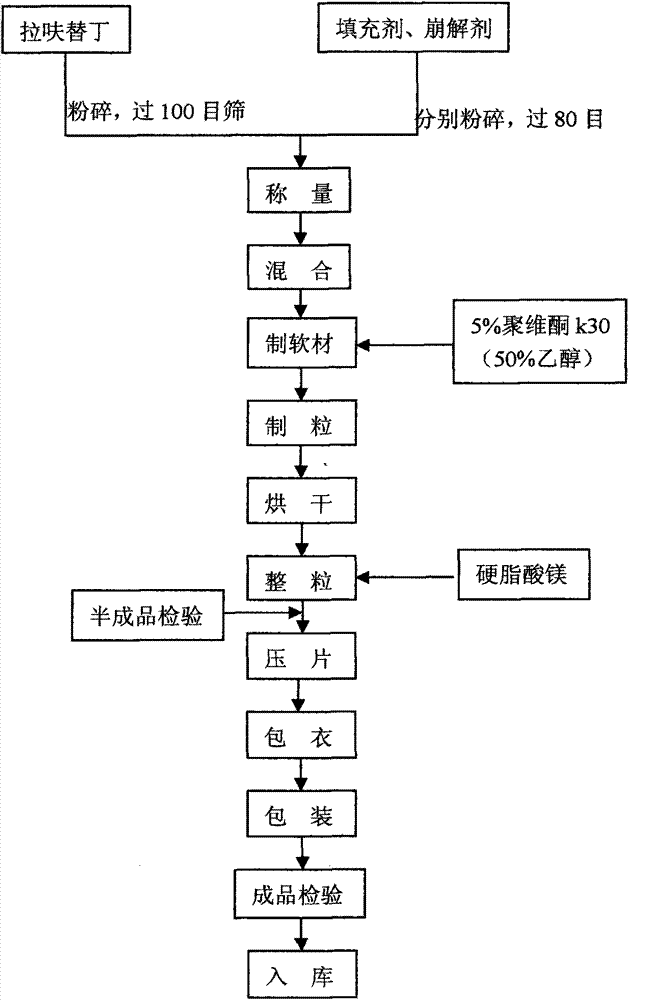
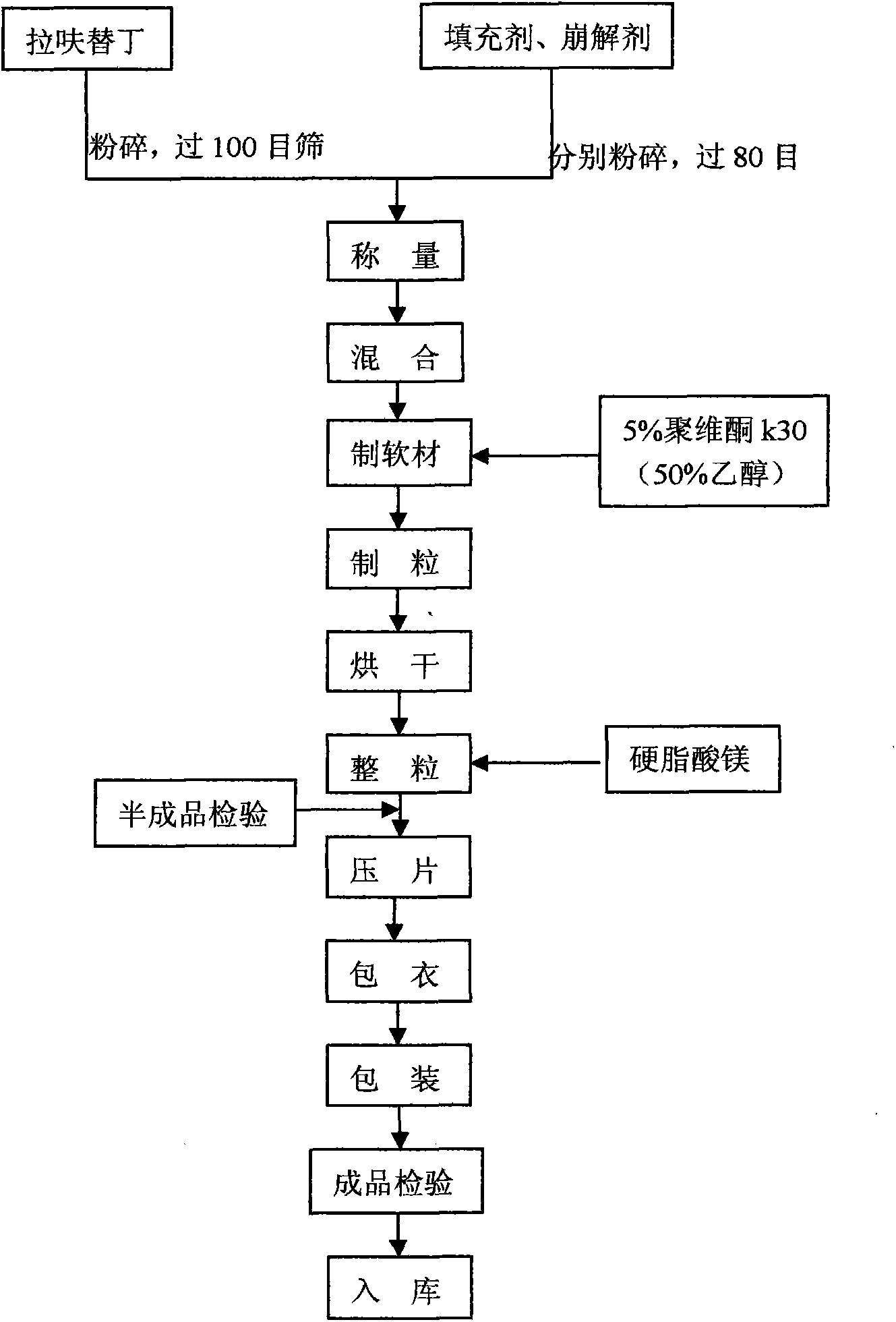
Lafutidine tablet and preparation method thereof
ActiveCN109364037AImprove stabilityThe content of the main drug is uniformOrganic active ingredientsDigestive systemLafutidineEnergy consumption
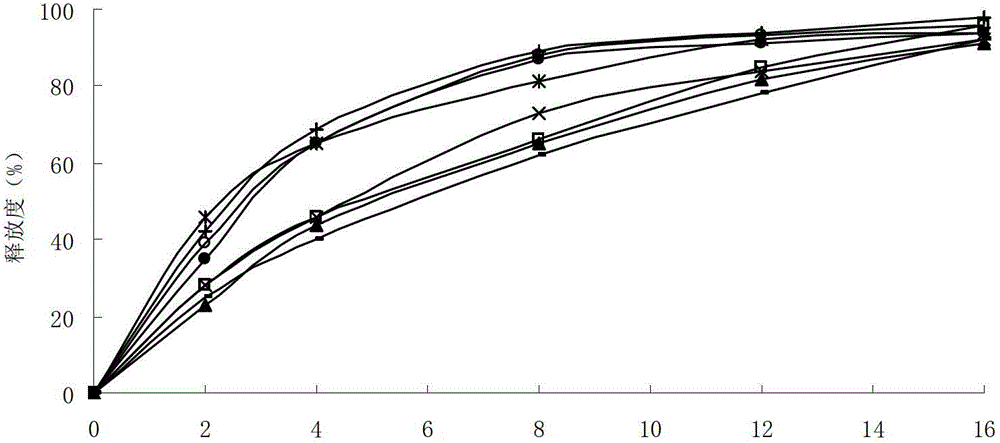
The invention relates to the field of medicine preparation, in particular to a lafutidine tablet and a preparation method thereof. The preparation method of the lafutidine tablet comprises the following steps: enabling lafutidine raw materials subjected to pretreatment to be mixed with a binding agent, an internal disintegrant and a filling agent and then carrying out fluidized drying; and then mixing with an external disintegrant and a lubricant to carry out tabletting and coating. The particle size range D10 of the lafutidine raw materials subjected to the pretreatment is 1.015-2.16 mu m, D50 is 2.684-7.367 mu m, and D90 is 4.325-52.8 mu m. By the preparation method, the prepared lafutidine tablet can be guaranteed to have good stability, the dissolution curve is similar to that researched originally, and the preparation process is low in energy consumption and pollution, and is green and environmentally friendly.
Owner:湖北舒邦药业有限公司
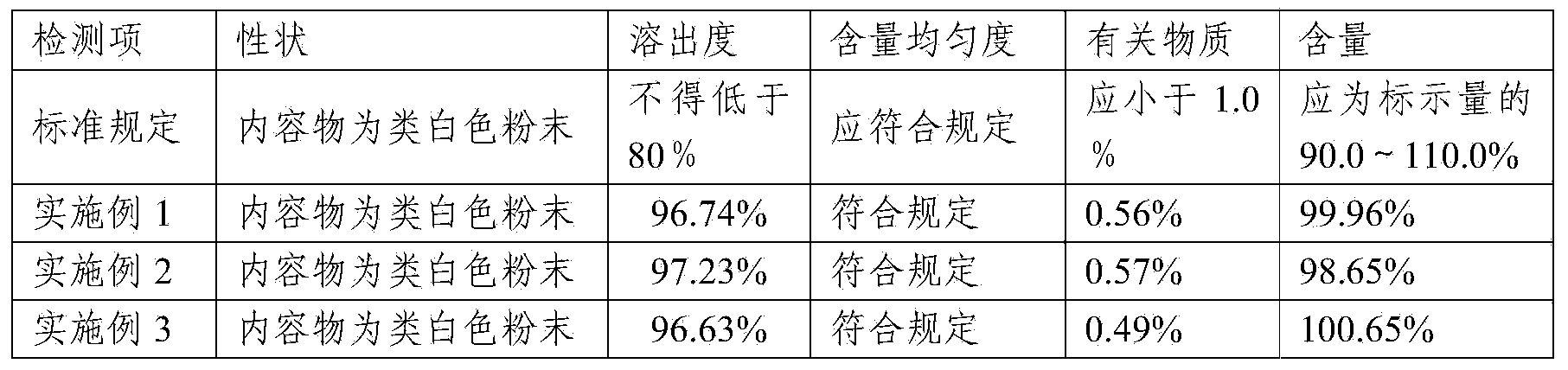
Compound containing Lafutidine and preparation containing Lafutidine
ActiveCN103417513AFast dissolutionLow impurity contentOrganic active ingredientsDigestive systemLactoseDissolution
The invention relates to a compound containing Lafutidine and a preparation containing Lafutidine. The compound comprises Lafutidine, lactose, tween-80, hydroxypropyl cellulose and talcum powder. Compared with the prior art, the compound is evidently faster in capsule dissolution rate and low in impurity content. In addition, the results of various detection of the compound meet the specification.
Owner:YOUCARE PHARMA GROUP +1
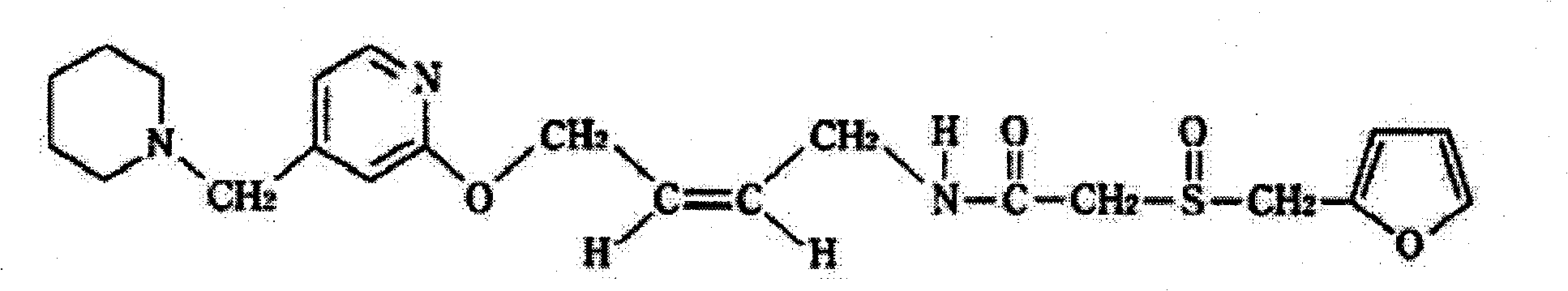
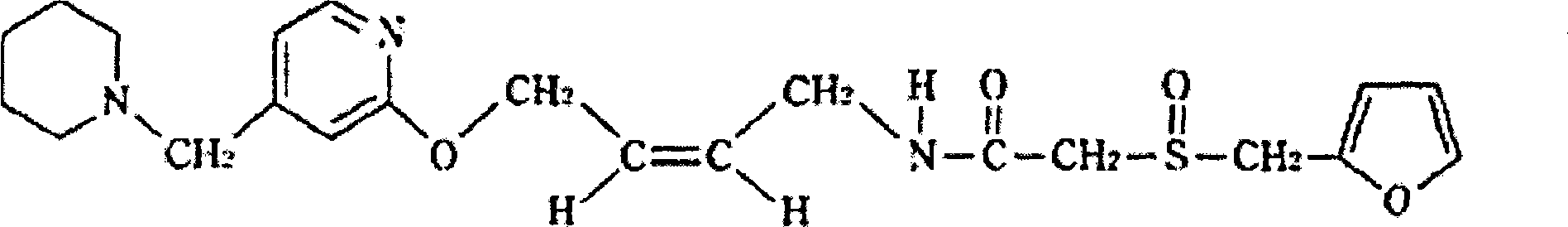
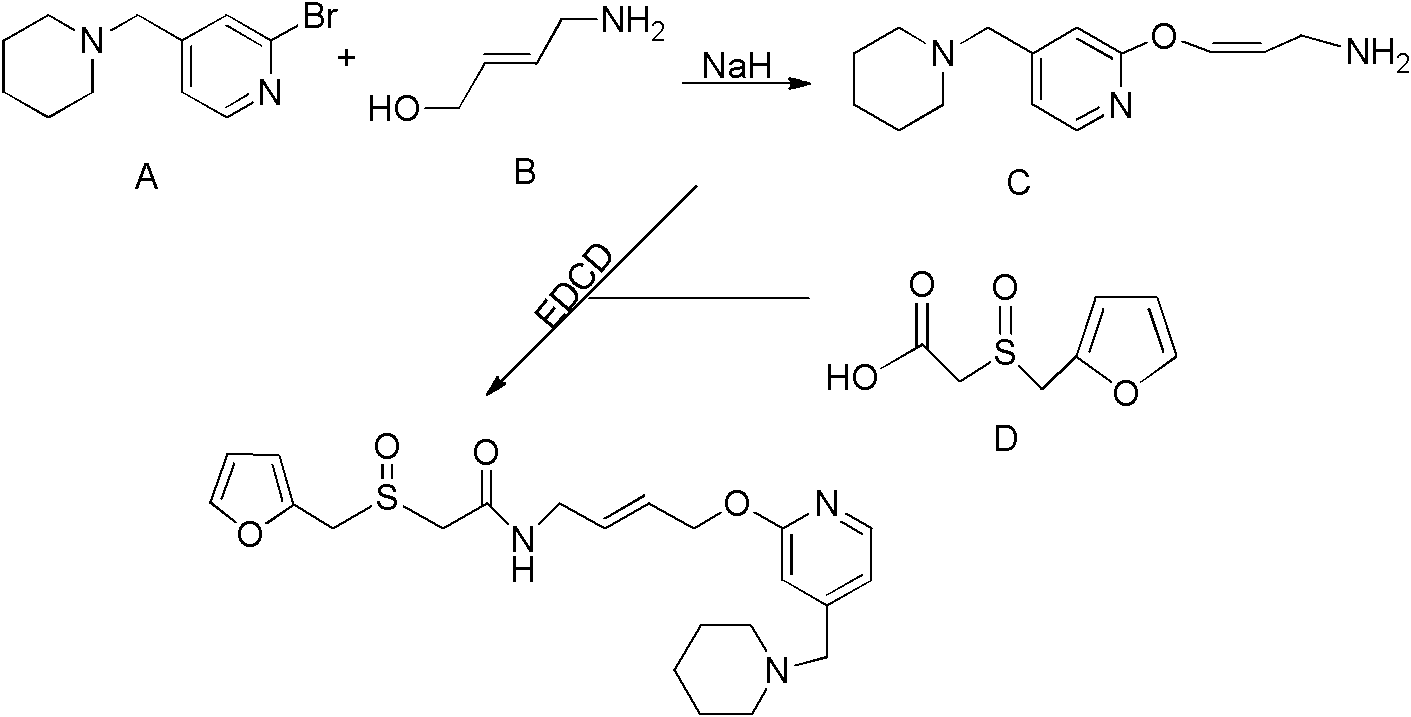
A kind of synthetic method of lafutidine oxidation impurity
The invention belongs to the technical field of chemistry and in particular relates to a synthetic method of Lafutidine oxide impurities, namely -2-[(2- furyl methyl) sulfonyl]-N-[4-[4-(1-piperidyl methyl)-2-pyridyl]oxygen-(Z)-2-butenyl] acetamide (formula I). The synthetic method mainly comprises the following steps: 2-(2-furfuryl sulfydryl)acetic acid-(4-nitrophenol) ester, namely the compound II is oxidized and then docked and refined with 4-[4-(N-piperidine methyl) pyridine-2-oxygen] cis-2-butene-1-amine, namely the compound IV to obtain oxide impurities of which the purity is more than 99.5%. The synthesized high-purity Lafutidine oxide impurities are taken as impurity standards for product inspection, thereby being beneficial to strengthening location and qualitativeness of the impurities so as to improve the quality control of Lafutidine active pharmaceutical ingredients.
Owner:YICHANG HEC CHANGJIANG PHARMA CO LTD
Novel technology for preparing lafutidine
The invention relates to a preparation method for lafutidine, and the technology comprises the following steps: preparing 4-[4-(piperidine-1-group-methylene)pyridine-2-group-oxygen]-2(Z)-butylene-1-amine and preparing 2-(2-furans methyl sulfoxide group)acetate, then dissolving 2-(2-furans methyl sulfoxide group)acetate into methylene chloride at the room temperature, and then adding a methylene chloride solution possessing 4-[4-(piperidine-1-group-methylene)pyridine-2-group-oxygen]-2(Z)-butylene-1-amine at the room temperature, stirring and separating to obtain lafutidine. The preparation method of lafutidine of the invention modifies the original route, wherein the reaction is milder and easy to operate without using sodium hydride, the intermediate fragment employs a nontoxic and innocuous molybdenum tungsten metal for catalyzing, the reaction condition is milder, the environmental pollution can be reduced and the reaction cost can be decreased.
Owner:SICHUAN KELUN PHARMA RES INST CO LTD
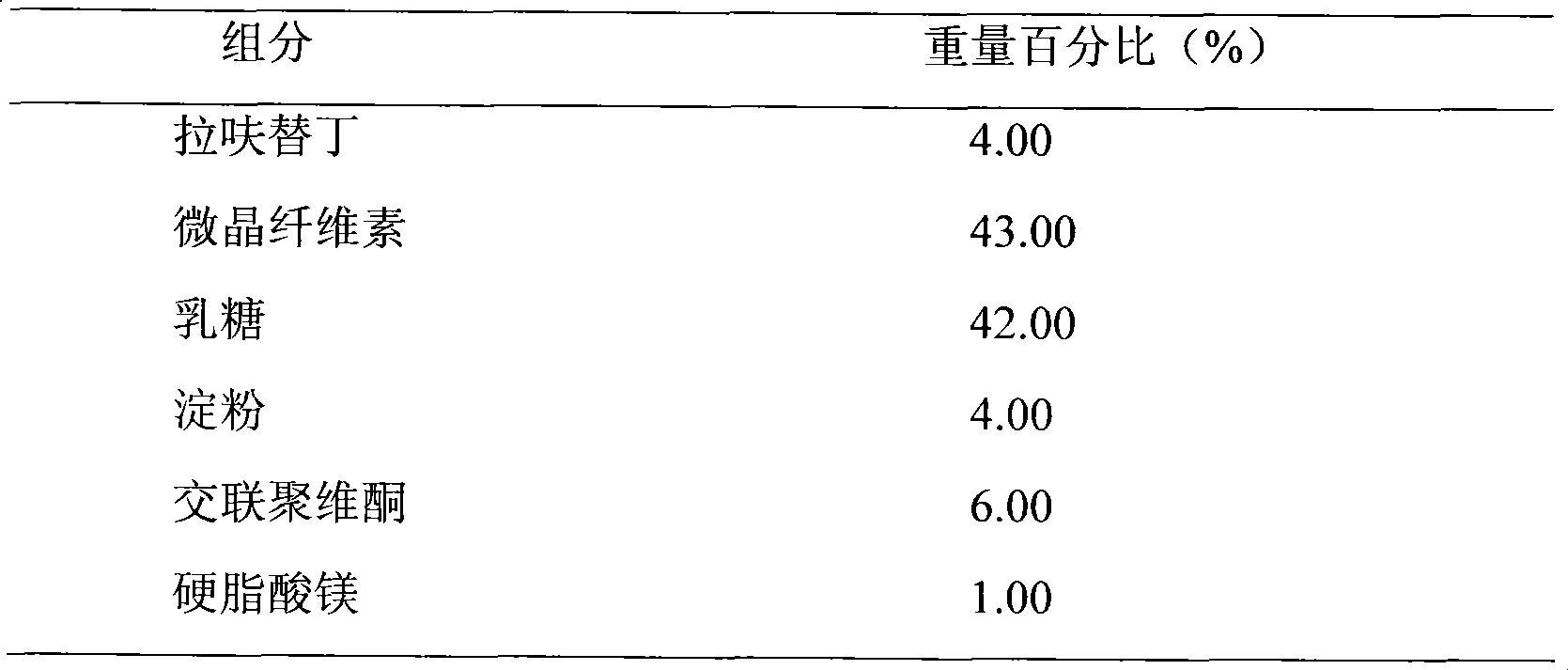
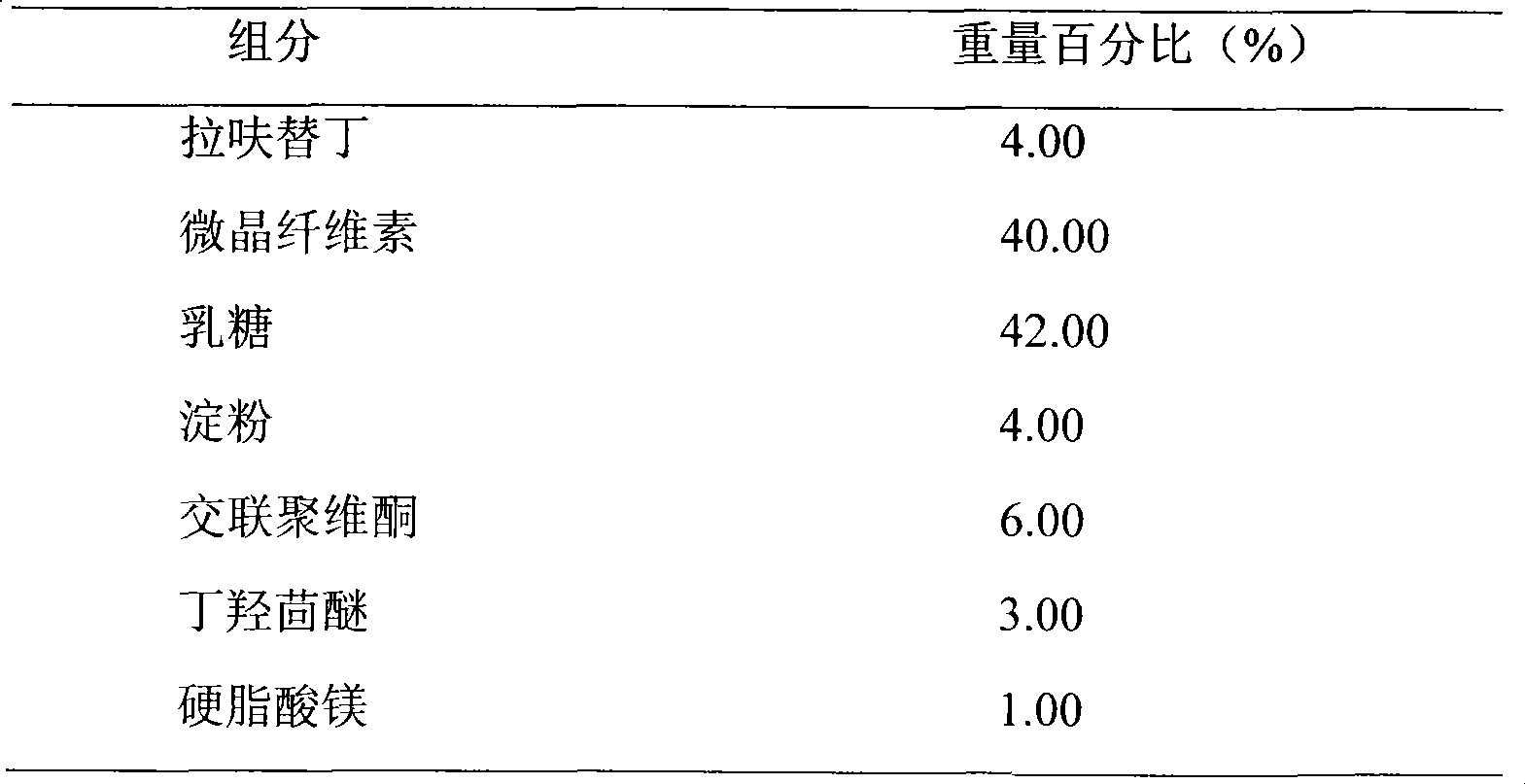
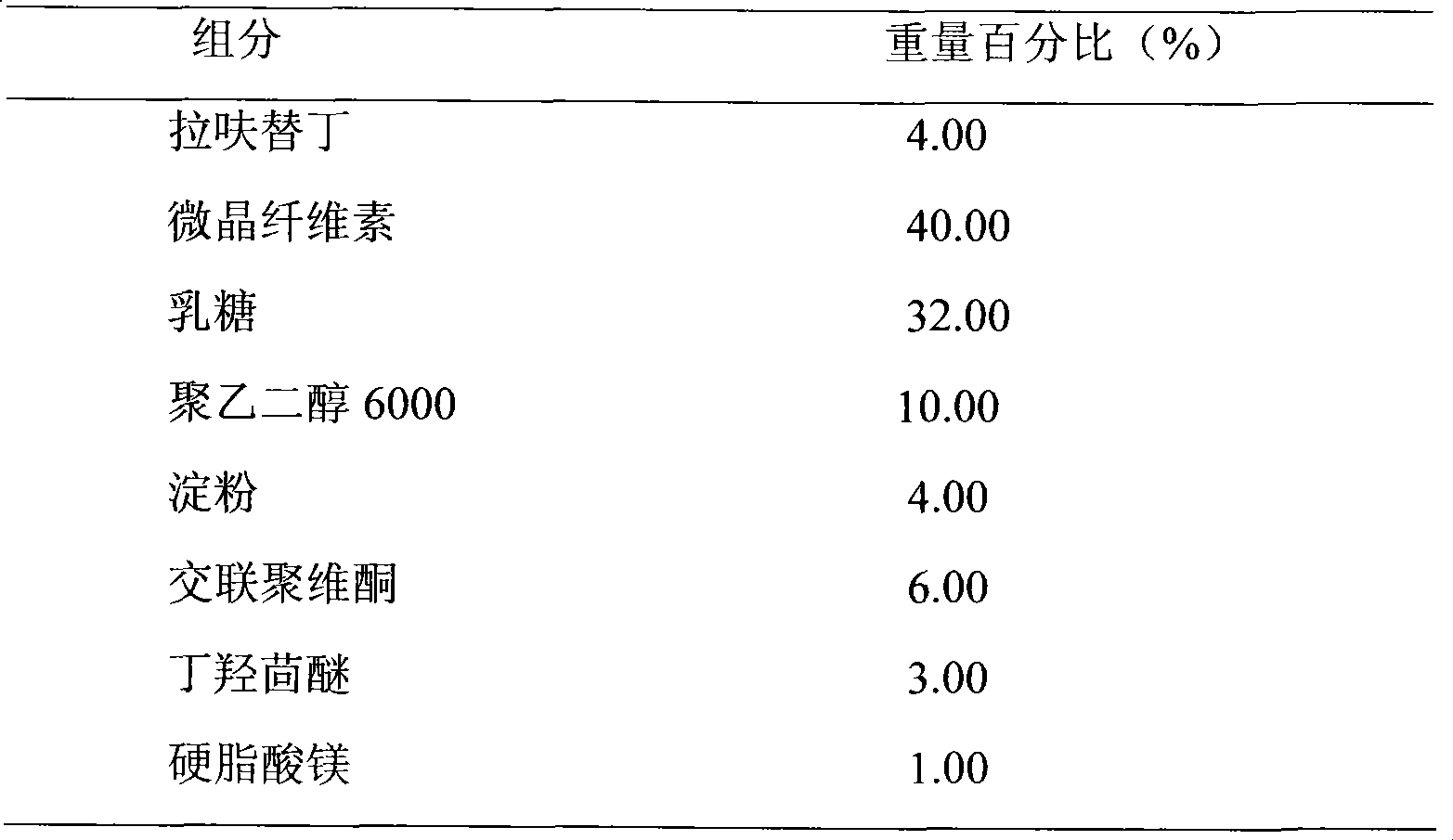
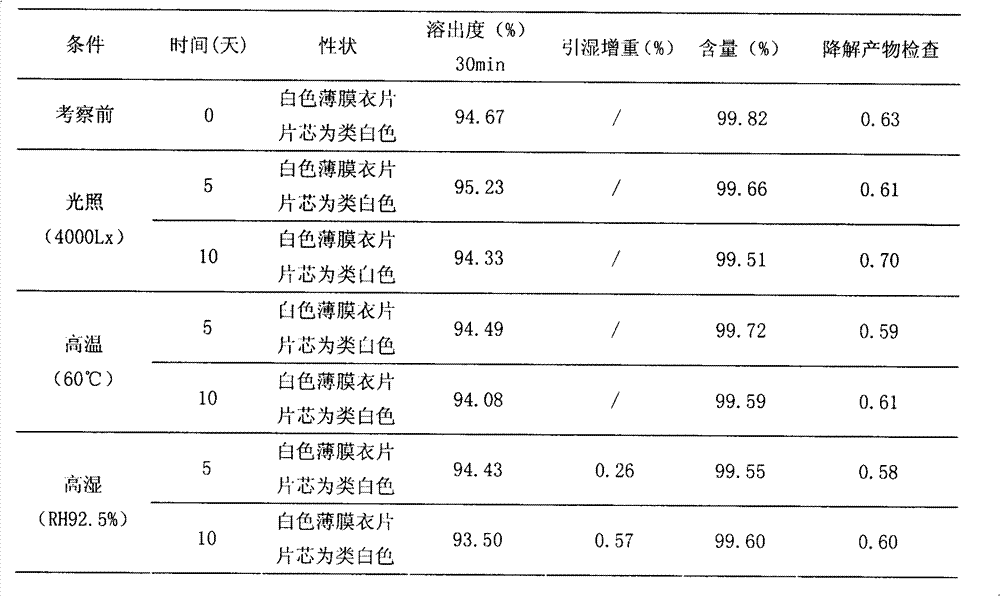
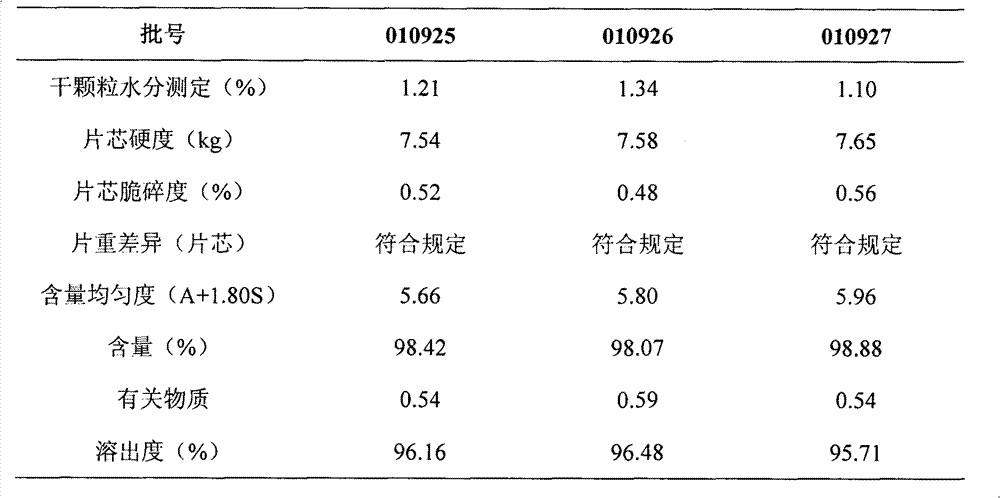
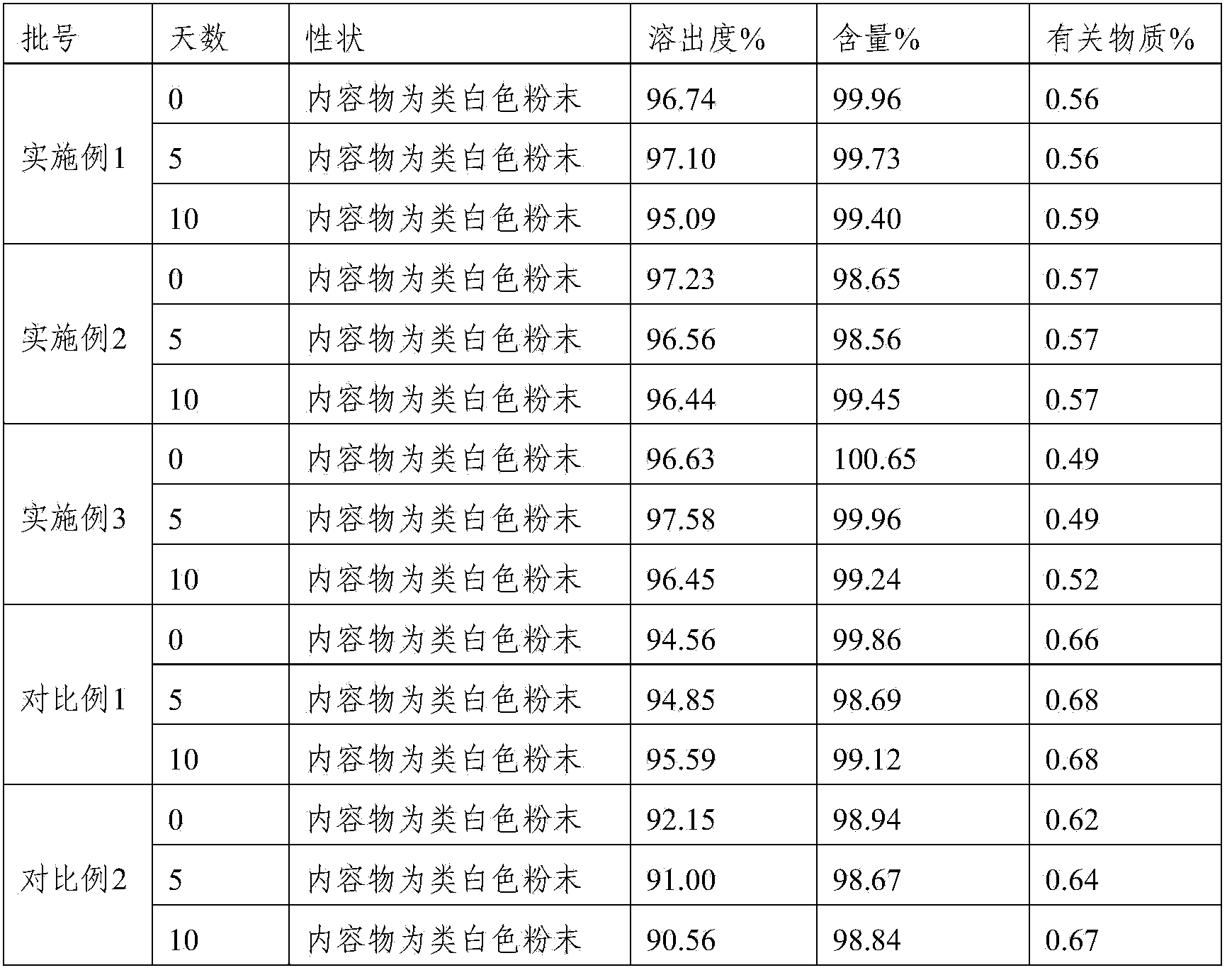
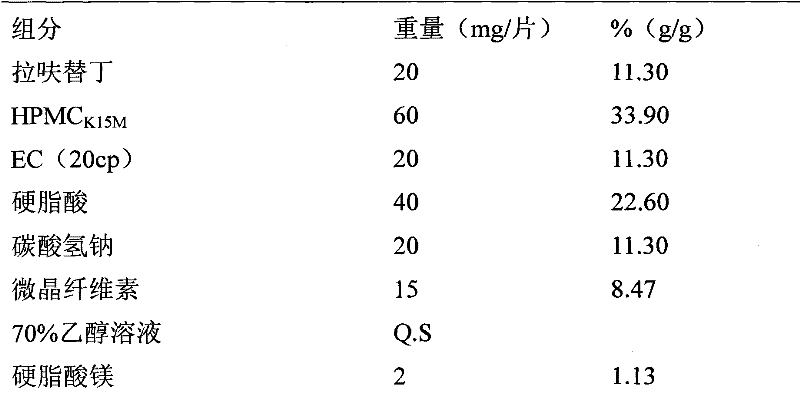
A kind of highly stable lafutidine tablet and preparation technology thereof
ActiveCN103768029BHigh dissolution rateImprove stabilityOrganic active ingredientsDigestive systemCross-linkDissolution
The present invention discloses high stability lafutidine tablets and a preparation process thereof, and belongs to the field of pharmaceutics. The high stability lafutidine tablets comprise lafutidine with the specific weight percentage, mannitol, cross-linked sodium carboxymethyl cellulose, povidone K30, magnesium stearate, and micro-powder silica gel. Compared with the lafutidine tablets of the traditional formula, the lafutidine tablets of the present invention have characteristics of good dissolution rate, low related substance content, high stability and effective assurance of drug quality and patient medication safety.
Owner:ANHUI BIOCHEM BIO PHARMA
The method that hydroxylamine hydrochloride prepares lafutidine
Owner:北京国联诚辉医药技术有限公司
Oral solid medicament composition containing lafutidine
The invention relates to an oral solid medicine composition containing lafutidine, which comprises an active ingredient, namely lafutidine and salt thereof, a solid dispersant, an antioxidant and other pharmaceutical excipients, and can be used for treating peptic ulcer.
Owner:BEIJING D VENTUREPHARM TECH DEV
Lafutidine tablet and preparation method thereof
ActiveCN109364037BImprove stabilityGuaranteed therapeutic effectOrganic active ingredientsDigestive systemLafutidinePharmaceutical Substances
The invention relates to the field of medicine preparation, in particular to a lafutidine tablet and a preparation method thereof. The preparation method of the lafutidine tablet comprises the following steps: enabling lafutidine raw materials subjected to pretreatment to be mixed with a binding agent, an internal disintegrant and a filling agent and then carrying out fluidized drying; and then mixing with an external disintegrant and a lubricant to carry out tabletting and coating. The particle size range D10 of the lafutidine raw materials subjected to the pretreatment is 1.015-2.16 mu m, D50 is 2.684-7.367 mu m, and D90 is 4.325-52.8 mu m. By the preparation method, the prepared lafutidine tablet can be guaranteed to have good stability, the dissolution curve is similar to that researched originally, and the preparation process is low in energy consumption and pollution, and is green and environmentally friendly.
Owner:湖北舒邦药业有限公司
Lafutidine coated tablet and preparation method thereof
The invention relates to the field of a medicinal preparation, in particular to a lafutidine-containing coated tablet and a preparation method thereof.. The preparation provided by the invention is an oral solid preparation consisting of lafutidine, lactose, microcrystalline cellulose, starch, sodium starch glycolate and magnesium stearate. In order to ensure the main dissolution rate, most of the auxiliaries in the prescription are hydrophilic. In the invention, the technology of the preparation is simple and adopts a few auxiliaries; and the product has stable quality and good dissolution rate, takes an effect quickly and acts for a long time.
Owner:JIANGSU RUNBANG PHARMA
Compound containing Lafutidine and preparation containing Lafutidine
ActiveCN103417513BFast dissolutionLow impurity contentOrganic active ingredientsDigestive systemCelluloseCombinatorial chemistry
The invention relates to a compound containing Lafutidine and a preparation containing Lafutidine. The compound comprises Lafutidine, lactose, tween-80, hydroxypropyl cellulose and talcum powder. Compared with the prior art, the compound is evidently faster in capsule dissolution rate and low in impurity content. In addition, the results of various detection of the compound meet the specification.
Owner:YOUCARE PHARMA GROUP +1
Synthesis method of 2-chloro-4-(piperidylmethyl)pyridine
The invention relates to a synthesis method of 2-chloro-4-(piperidylmethyl)pyridine, belonging to the field of medicine synthesis. The method comprises the following steps: dropwisely adding a nitrite water solution into acid and 2-amino-4-methylpyridine to react, thereby obtaining a white solid product; adding POCl3 into the white solid to obtain a compound 2-chloro-4-methylpyridine; dropwisely adding SO2Cl2 into the compound 2-chloro-4-methylpyridine while adding a free-radical initiator batch by batch, and distilling under reduced pressure to obtain a compound 2-chloro-4-chloromethylpyridine; and condensing the 2-chloro-4-chloromethylpyridine and piperidine to obtain the 2-chloro-4-(piperidylmethyl)pyridine. Compared with the traditional synthesis method of 2-chloro-4-(piperidylmethyl)pyridine, the synthesis method provided by the invention has the advantages of simpler steps (only three steps) and lower cost of raw materials, and the total yield is higher than 32%. Generally, the invention can greatly lower the synthesis cost of lafutidine.
Owner:常州市赛努尔高分子科技有限公司
Lafutidine gastric-retention controlled-release composition
ActiveCN101919817BExtended stayIntegrity guaranteedOrganic active ingredientsDigestive systemUse medicationControl release
The invention relates to a lafutidine gastric-retention controlled-release composite belonging to the field of pharmaceutic preparations. The lafutidine gastric-retention controlled-release composite is characterized by comprising the following components in percentage by weight: 5%-20% of the lafutidine, 10%-40% of framework materials, 10%-30% of assistant bleaching agents, 5%-15% of foaming agents, 5%-15% of filling agents and 0.5%-10% of lubricating agents. The composite has reasonability and simple preparation process; compared with same pharmaceuticals, the lafutidine gastric-retention controlled-release composite has little dose, good tolerance, little side effect, and the like; and in addition, compared with the conventional tablets, the lafutidine gastric-retention controlled-release composite enhances the solubility of lafutidine, prolongs the action time (prolonged from 2-3 hours to 5-6 hours) on the upper parts of a stomach and a small intestine, promotes the absorption, enhances the bioavailability, reduces the pharmaceutical usage times, achieves the maximum treatment effect through minimum doses, reduces the concentration change of peaks and valleys and has good patient compliance.
Owner:SHANDONG QIDU PHARMA
A kind of lafutidine composition and preparation method thereof
ActiveCN103349653BImprove stabilityImprove bioavailabilityOrganic active ingredientsDigestive systemPeptic ulcerLafutidine
Owner:YOUCARE PHARMA GROUP
Lafutidine liposome solid preparation and preparing method thereof
InactiveCN102716083BImprove product qualityUniform particle sizeOrganic active ingredientsDigestive systemSide effectCurative effect
The invention discloses a lafutidine liposome solid preparation and a preparing method thereof. Lafutidine liposome excellent in quality can be prepared through lafutidine, dipalmitoylglycero-phosphoglycerol, deoxysodium cholate and span 80, and then the lafutidine liposome can be prepared into solid preparation through a general preparation method. Compared with the existing preparation, the lafutidine liposome solid preparation greatly improves stability and bioavailability, improves quality of preparation products, reduces toxic and side effects and is remarkable in curing effect.
Owner:HAINAN MEILAN SMITH KLINE PHARMA
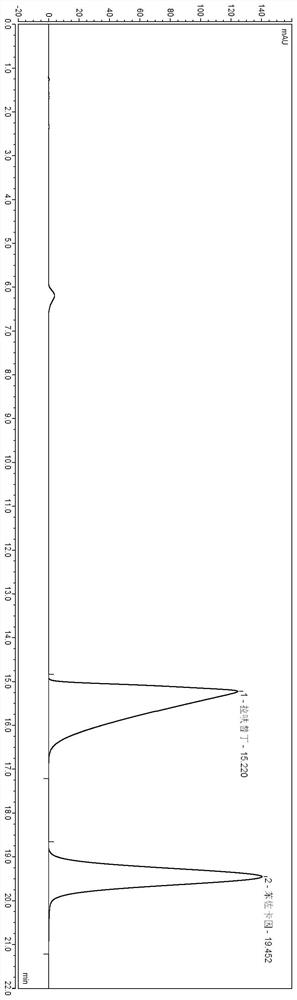
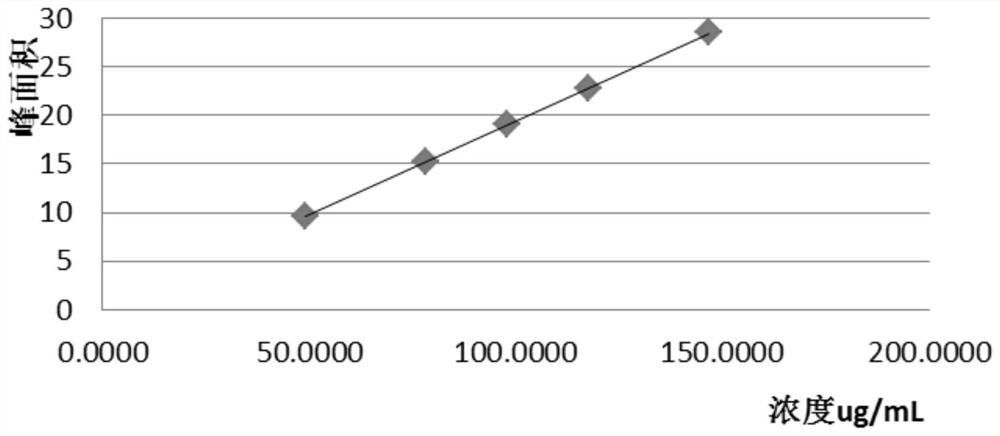
Lafutidine tablet content detection method
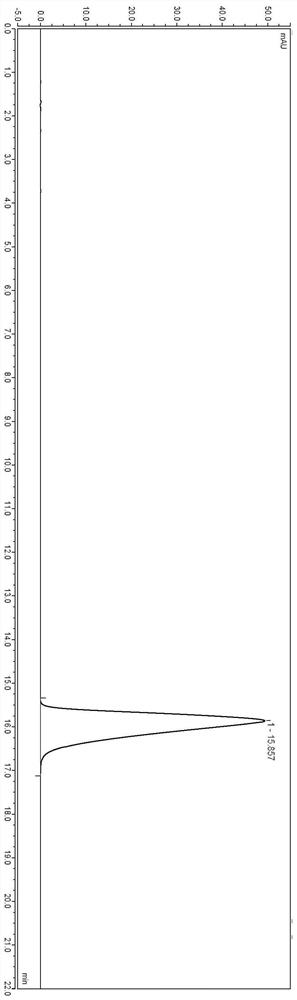
The invention relates to the technical field of oral solid preparation analysis, and provides a method for detecting the content of lafutidine tablets, which adopts a high performance liquid chromatography to detect the content of each component in the lafutidine tablets. Chromatographic conditions of the high performance liquid chromatography are as follows: a mobile phase is a mixed solution of acetonitrile and a phosphate buffer solution added with sodium pentanesulfonate in a volume ratio of (10: 90)-(20: 80); the column temperature is 35-45 DEG C; the detection wavelength is 250nm to 300nm, and the preparation range of detectable sample concentration is 50mu g / ml to 150mu g / ml. According to the method for detecting the content of the lafutidine tablets, the content of each substance in the lafutidine tablets is measured through a high performance liquid chromatography external standard method, chromatographic conditions are optimized, efficient detection of the content of each substance in the lafutidine tablets is achieved, the method is good in reproducibility, easy to operate, high in accuracy and low in requirement for a chromatographic column, and the method is suitable for industrial production. The separation degree, the durability and the reproducibility are good.
Owner:湖北舒邦药业有限公司
Lafutidine composition and preparation method thereof
ActiveCN103349653AImprove stabilityImprove bioavailabilityOrganic active ingredientsDigestive systemMedicinePeptic ulcer
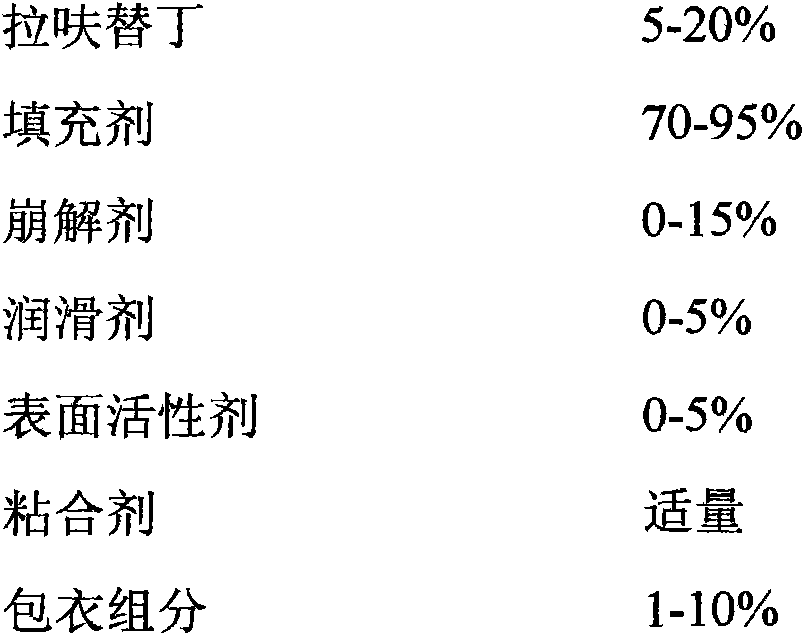
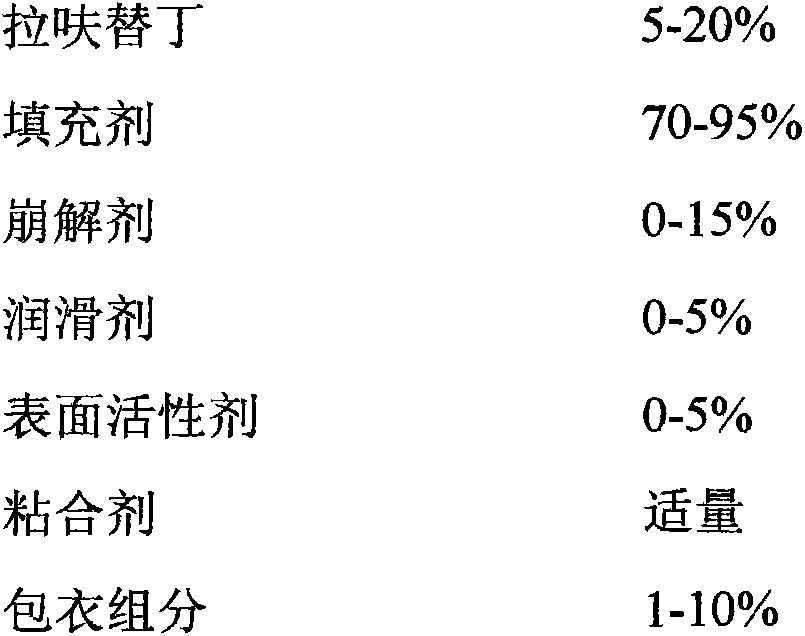
The present invention relates to an anti-peptic ulcer drug lafutidine composition and a preparation method thereof. The composition is prepared from the following components, by weight: 5-20% of lafutidine, 70-95% of a filler, 0-15% of a disintegrating agent, 0-5% of a lubricant, 0-5% of a surfactant, an appropriate amount of a binder, and 1-10% of a coating material. The preparation method is a wet granulation tableting method. The prepared lafutidine composition has characteristics of reliable quality, stable property, simple production process, meeting of requirements of large-scale preparation production, and low cost.
Owner:YOUCARE PHARMA GROUP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com