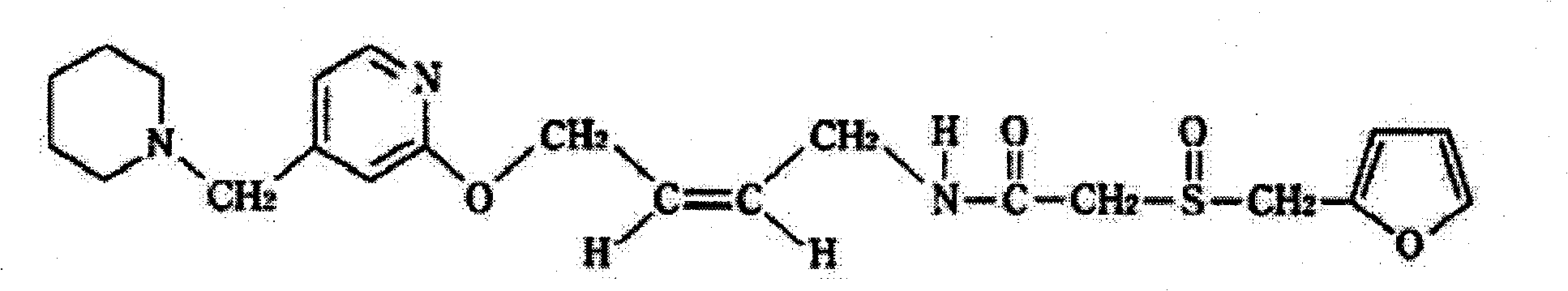
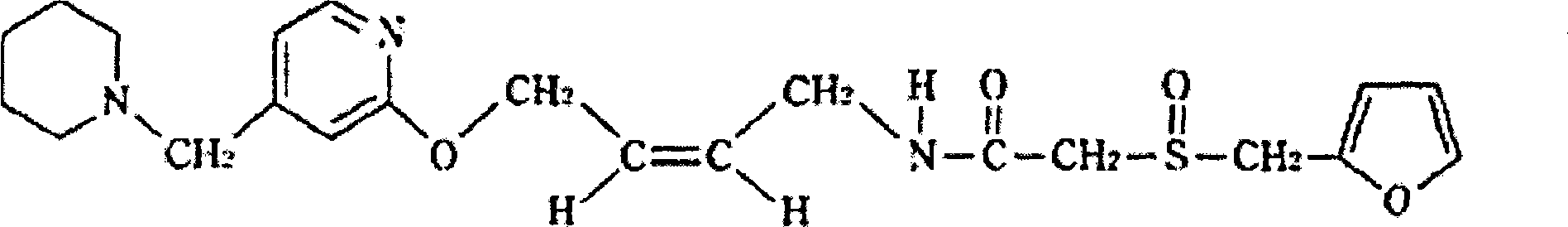
Lafutidine lyophilized powder injection and preparing method thereof
A technology for freeze-dried powder injection and lafutidine, which is applied in the field of lafutidine freeze-dried powder for injection, can solve the problems of increasing the irritation of injections, unable to prepare injections, and no improvement is proposed, and achieves low difficulty in rejecting waste, Excellent dissolving performance and manpower saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] prescription
[0022] Lafutidine 10g
[0023] Mannitol 100g
[0024] Add water for injection to 10000ml
[0025] A total of 1000 bottles were made
[0026] Take the prescribed amount of lafutidine and put it in a sterile container, add water for injection, heat to 70°C to dissolve, then add the prescribed amount of mannitol, stir to dissolve and mix evenly, measure the content of the intermediate, and test it under sterile conditions Next, filter with a 0.22 μm microporous membrane until clear, fill the filtrate in a sterile 25ml vial, partially plug it with a butyl rubber stopper, put it in a plate, turn on the freeze dryer in advance, and use heat transfer oil to cool the plate layer. Until the plate temperature reaches -40~-45°C, quickly send the lafutidine solution filled in the sterile cillin bottle into the freeze dryer, close the door, and keep the plate temperature at -40~-45°C for The temperature of the product drops. When the sample reaches -40°C, stop pla...
Embodiment 2
[0029] prescription
[0030] Lafutidine 10g
[0031] Mannitol 1000g
[0032] Sodium chloride 20g
[0033] Add water for injection to 10000ml
[0034] A total of 1000 bottles were made
[0035] Take the prescribed amount of lafutidine and put it in a sterile container, add water for injection, heat to 70°C to dissolve, then add the prescribed amount of mannitol and sodium chloride, stir to dissolve and mix evenly, measure the content of intermediates, after passing the Under sterile conditions, filter with a 0.22 μm microporous membrane until clear, fill the filtrate into a sterile 25ml vial, partially plug it with a butyl rubber stopper, put it in a plate, turn on the freeze dryer in advance, and use heat transfer oil to heat the plate. Layer refrigeration until the temperature of the layer reaches -40~-45℃, quickly send the lafutidine solution filled in the aseptic penicillin bottle into the freeze dryer, close the door, and keep the temperature of the layer at -40~- 45°...
Embodiment 3
[0038] prescription
[0039] Lafutidine 10g
[0040] Mannitol 200g
[0041] Sodium chloride 5g
[0042] Add water for injection to 10000ml
[0043] A total of 1000 bottles were made
[0044] Take the prescribed amount of lafutidine and put it in a sterile container, add water for injection, heat to 70°C to dissolve, then add the prescribed amount of mannitol and sodium chloride, stir to dissolve and mix evenly, measure the content of intermediates, and pass the test Under sterile conditions, filter with a 0.22 μm microporous membrane until clear, fill the filtrate in a sterile 25ml vial, partially plug it with a butyl rubber stopper, put it in a plate, turn on the lyophilizer in advance, and use heat transfer oil to Refrigerate the plate until the temperature of the plate reaches -40~-45℃, quickly send the lafutidine solution filled in the sterile pencil bottle into the freeze dryer, close the door, and keep the temperature of the plate at -40~ -45°C lowers the temperatur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com