Oral solid medicament composition containing lafutidine
A technology of lafutidine and composition, applied in the field of oral solid pharmaceutical compositions, can solve problems such as poor stability, patient compliance and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
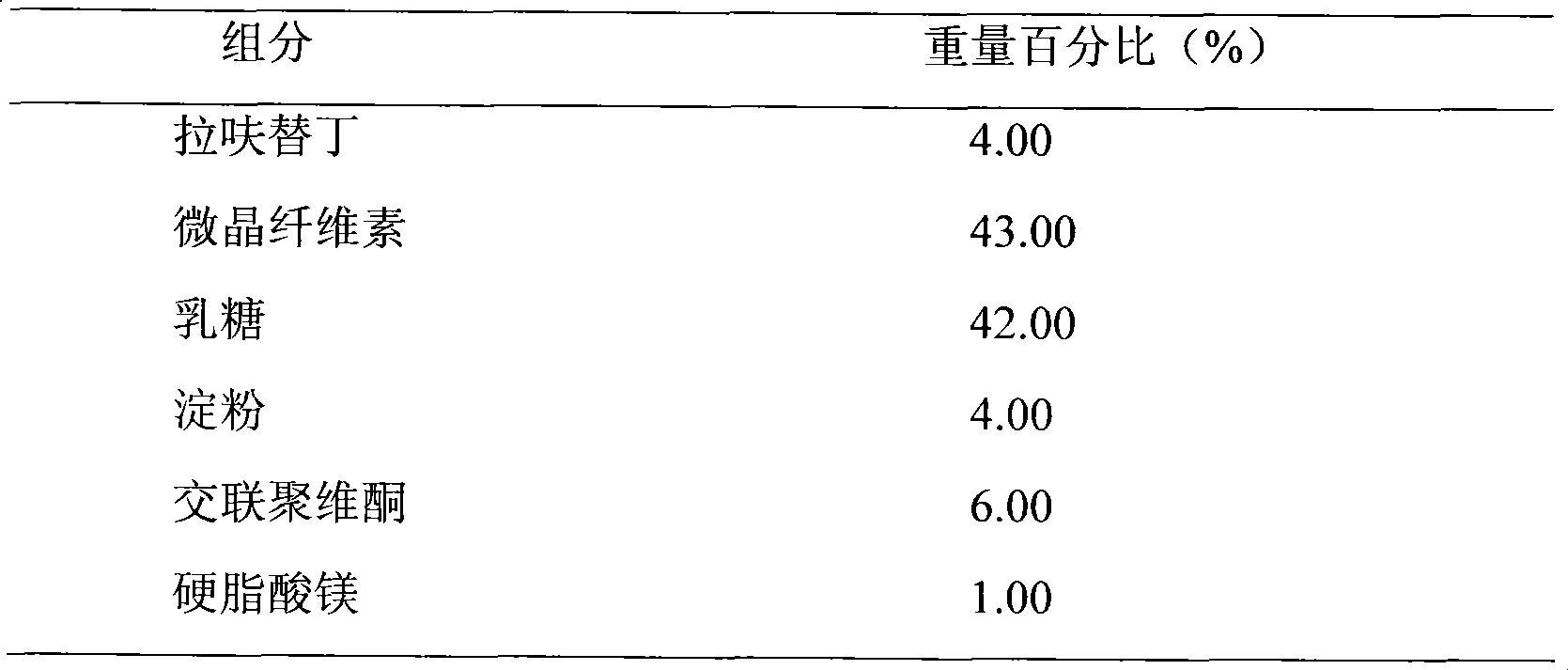
Embodiment 1
[0019]
[0020] Preparation Process:
[0021] Weigh the prescribed amount of lafutidine, lactose, and microcrystalline cellulose, mix them evenly, prepare soft materials with 10% starch slurry, make wet granules with 18 mesh, dry at 50°C-60°C, granulate, add magnesium stearate , mixed and pressed into tablets.
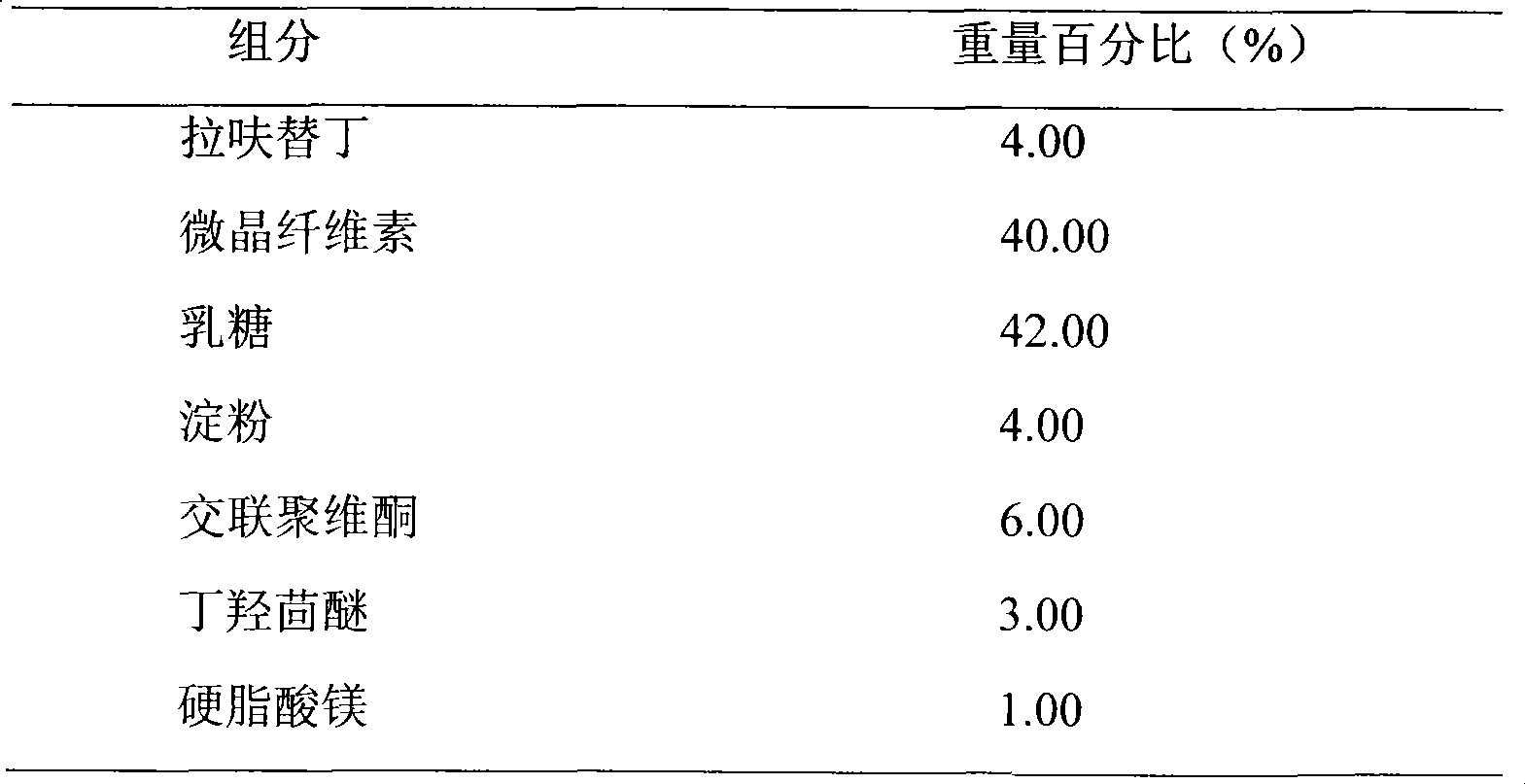
Embodiment 2
[0023]
[0024] Preparation Process:
[0025] Weigh the prescribed amount of lafutidine, lactose, and microcrystalline cellulose, mix them evenly, prepare soft materials with 10% starch slurry, make wet granules with 18 mesh, dry at 50°C-60°C, granulate, add butylated hydroxyanisole , magnesium stearate, mixed evenly and compressed into tablets.
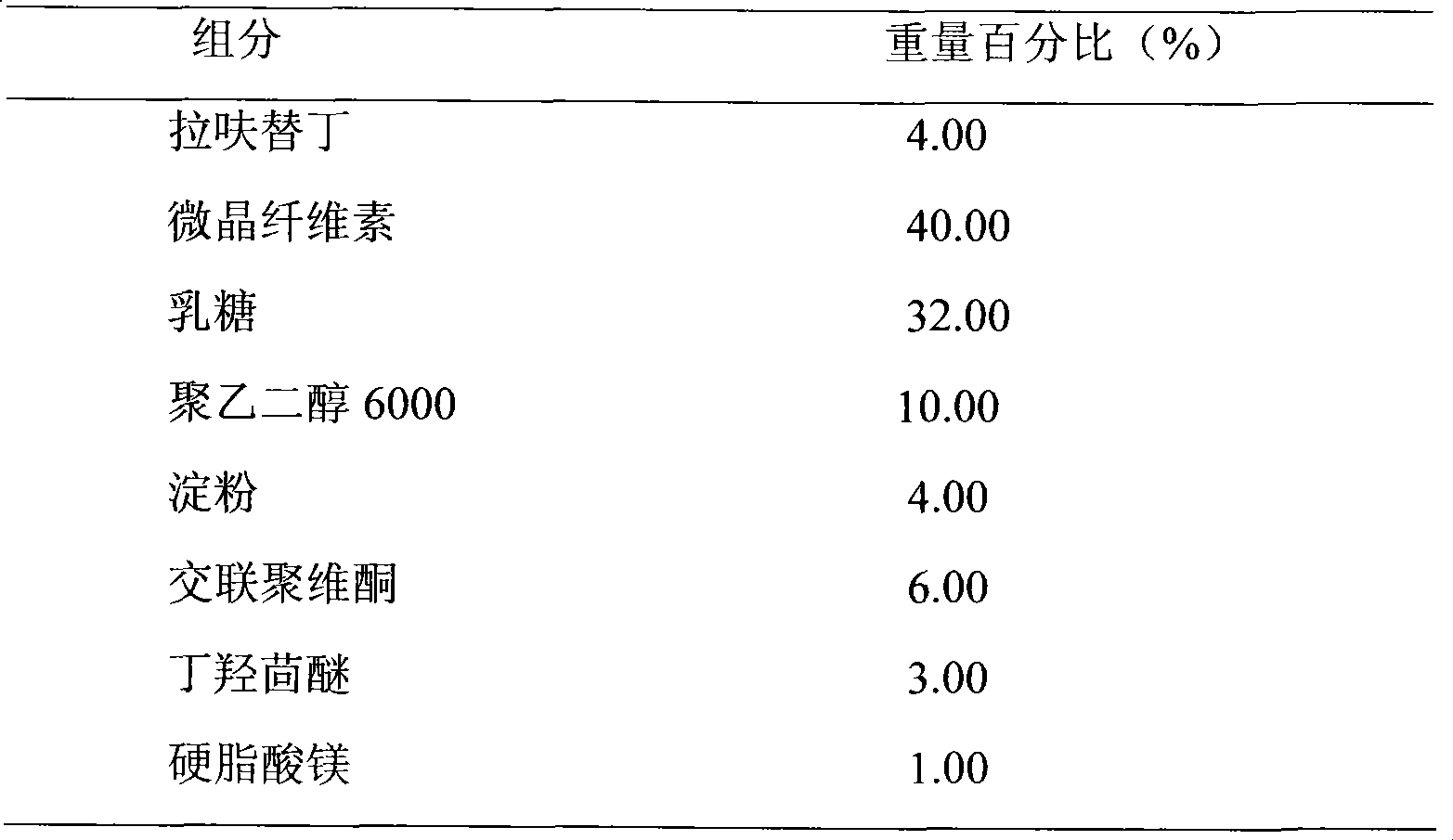
Embodiment 3
[0027]
[0028] Preparation Process:
[0029] Lafutidine and Polyethylene Glycol 6000 of prescription quantity are dissolved in 70% ethanol solution, after stirring and dissolving, spin evaporated to dryness to obtain solid mixture, powdered into fine powder, then mixed with lactose, microcrystalline cellulose Mix evenly, prepare soft materials with 10% starch slurry, make wet granules with 18 mesh, dry at 50°C-60°C, granulate, add BHA and magnesium stearate, mix evenly, and press into tablets.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com