MG53 (mitsugumin53) mutant as well as preparation method and application thereof
A mutant and site mutation technology, applied in the field of MG53 mutants, can solve the problems of unsolved channels, coexistence of positive and negative effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
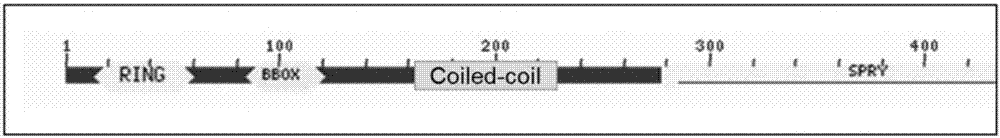
[0092] The coiled-coil domain of MG53 protein is a well-studied domain that mediates the interaction between proteins. It presents a unique repeating unit in sequence and is widely distributed. It participates in membrane fusion, membrane vesicle transport and many other important life process. Functionally, the coiled coil domain provides sufficient space for the head and tail structures of the protein during vesicle transport. In addition, the coiled coil domain can also serve as a backbone to recruit proteins to form complexes. Transfer the cell proliferation, differentiation and apoptosis signals into the MAP kinase module to form a signaling pathway.
[0093] The applicant believes that the coiled coil domain plays an important role in the vesicle transport and signal pathway transduction of MG53 protein. Therefore, the applicant chose the coiled coil domain of MG53 protein as the research object. Moreover, the applicant found that in the regulation of insulin signalin...
Embodiment 1
[0219] Embodiment 1: Construction of MG53 mutant plasmid MG53 S189A
[0220] 1. MG53 gene optimization, synthesis and MG53 S189A subcloning and plasmid preparation:
[0221] The following are the reagents and their sources used in the construction of the MG53 S189A plasmid:
[0222] Reagent: T4DNA Ligase Source: NEB Lot Number: 00309971,
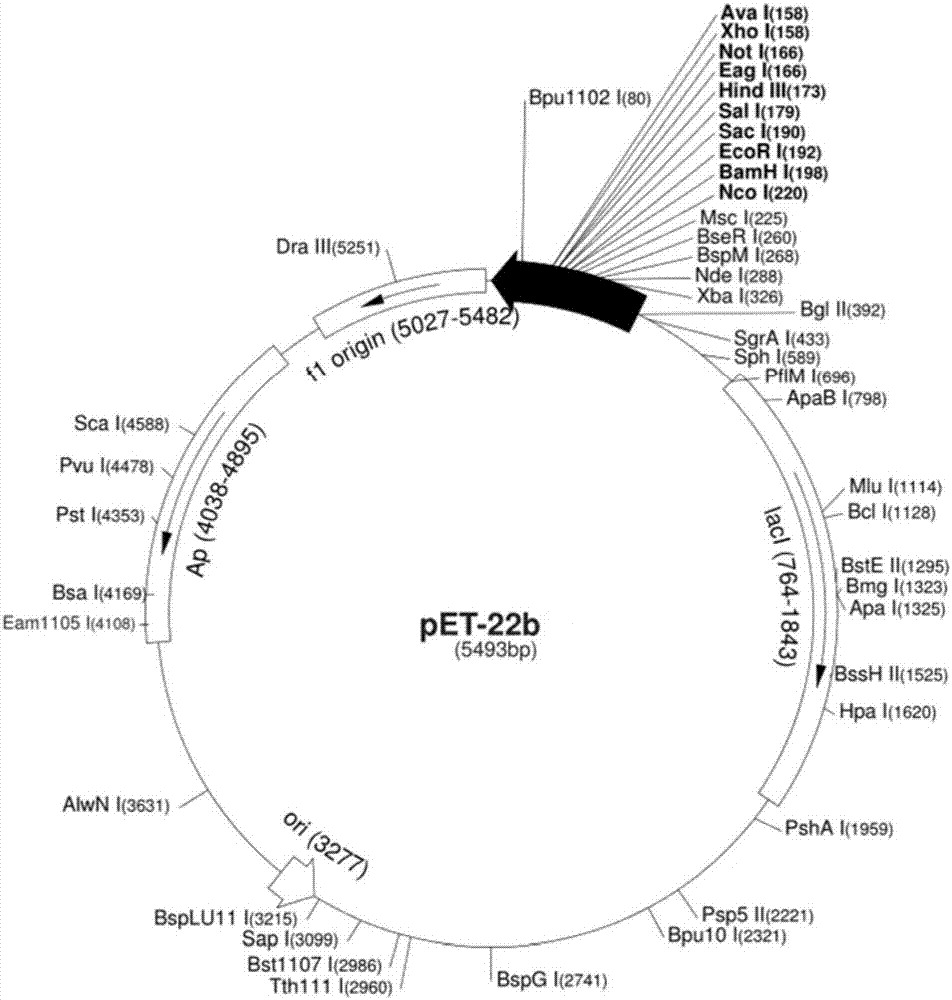
[0223] PET-22b plasmid, source: Novagen,
[0224] Competent cell source: Tiangen Biochemical Technology (Beijing) Co., Ltd. (CB105-02),
[0225] Experimental water source: ddH2O Batch number: 10 / 13 / 2015,
[0226] Endonuclease NdeI Source: NEB Cat. No.: R0111S,
[0227] Source of endonuclease XhoI: NEB Cat. No.: R5146S.
[0228] In addition, the equipment used in the construction of the MG53 S189A plasmid and its sources are listed in Table 1
[0229] Table 1, equipment used in constructing MG53 S189A plasmid
[0230] equipment name
model
manufacturers
MEGAFUGE 8R
Thermo
Electrophores...
Embodiment 2
[0291] Example 2: Expression and purification of MG53 mutant plasmid MG53 S189A
[0292] 2.1 Expression of MG53 mutant plasmid MG53 S189A
[0293] The medium used for expression is as follows:
[0294] 1) Shaker medium (400ml): LB medium (tryptone 2%; yeast powder 1%; sodium chloride 2%; 100 μg / ml Amp 1%);
[0295] 2) Fermentation medium (5 L): (tryptone 12%; yeast powder 24%; glycerol 4ml / L; dipotassium hydrogen phosphate 16.4%; potassium dihydrogen phosphate 2.32%; 100 μg / ml Amp 1%).
[0296] 3) Feed medium: 50% glycerol. The feed medium is used to supplement the carbon source and energy needed by the bacteria in the late stage of fermentation.
[0297] In the middle and late stages of the fermentation process, after the carbon source and energy in the fermentation medium are exhausted, the feed medium is used to supplement the carbon source and energy needed by the bacteria.
[0298] Express MG53 S189A according to the following steps:
[0299] 1) Activation of MG53 S189...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com