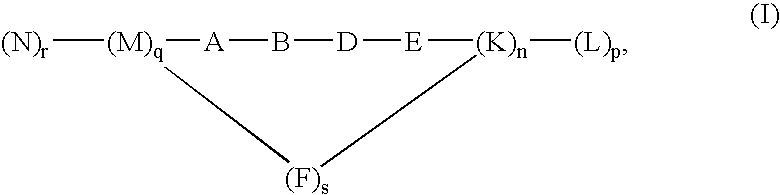
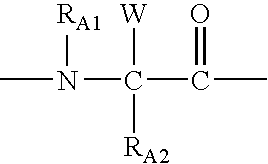
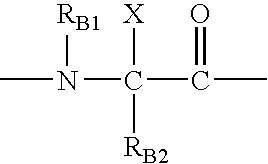Patents
Literature
172 results about "Coiled coil" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
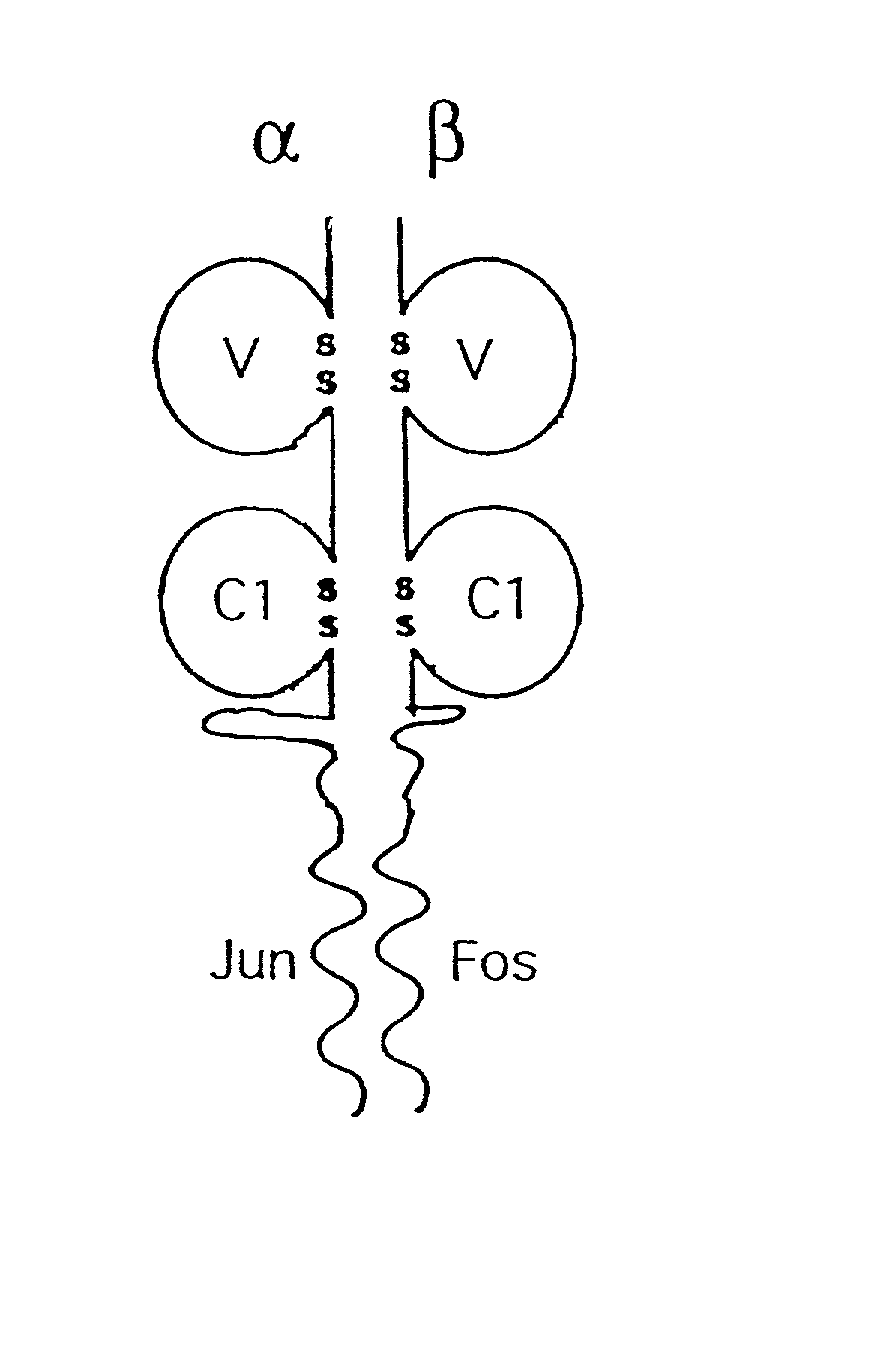
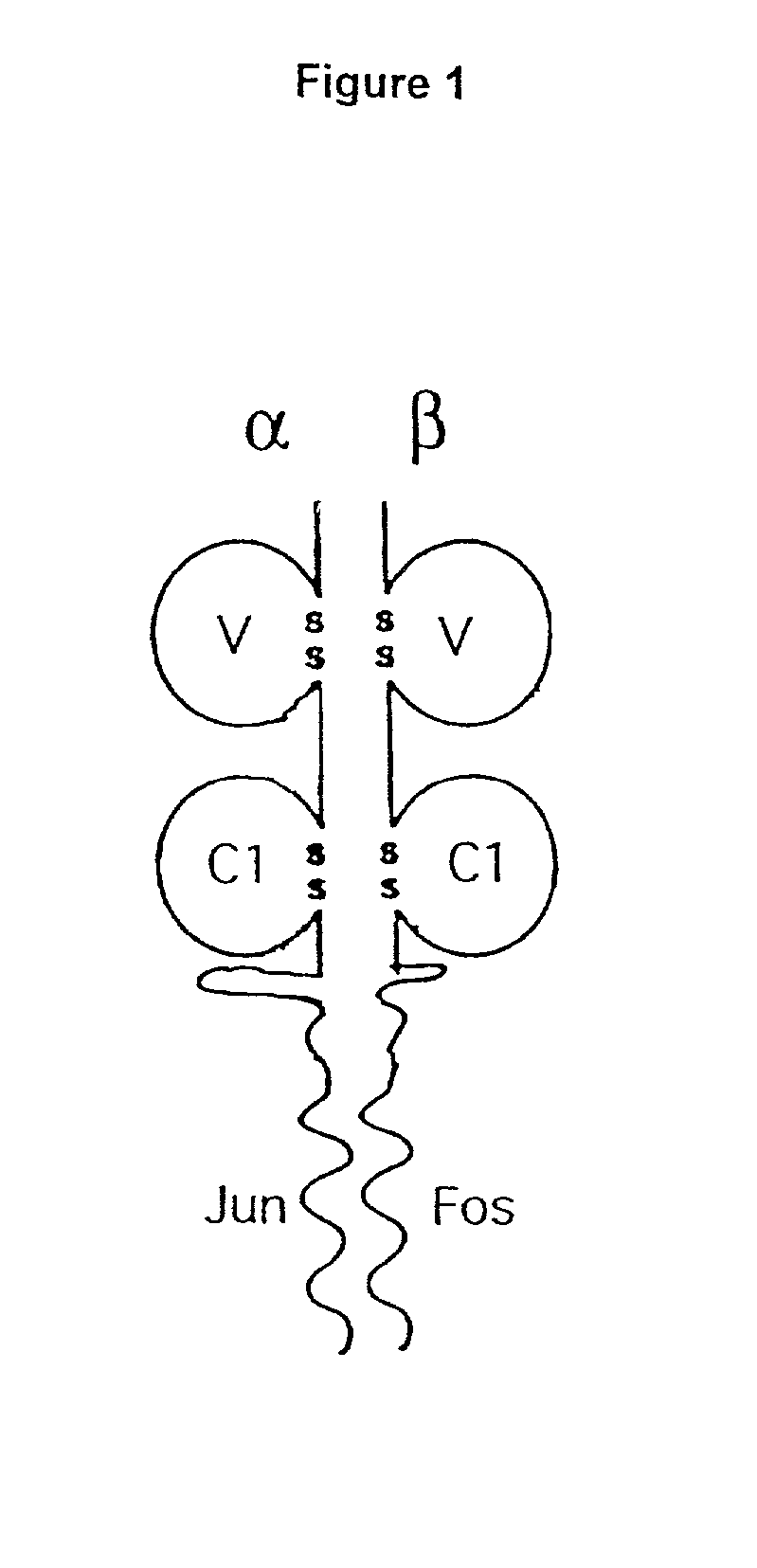
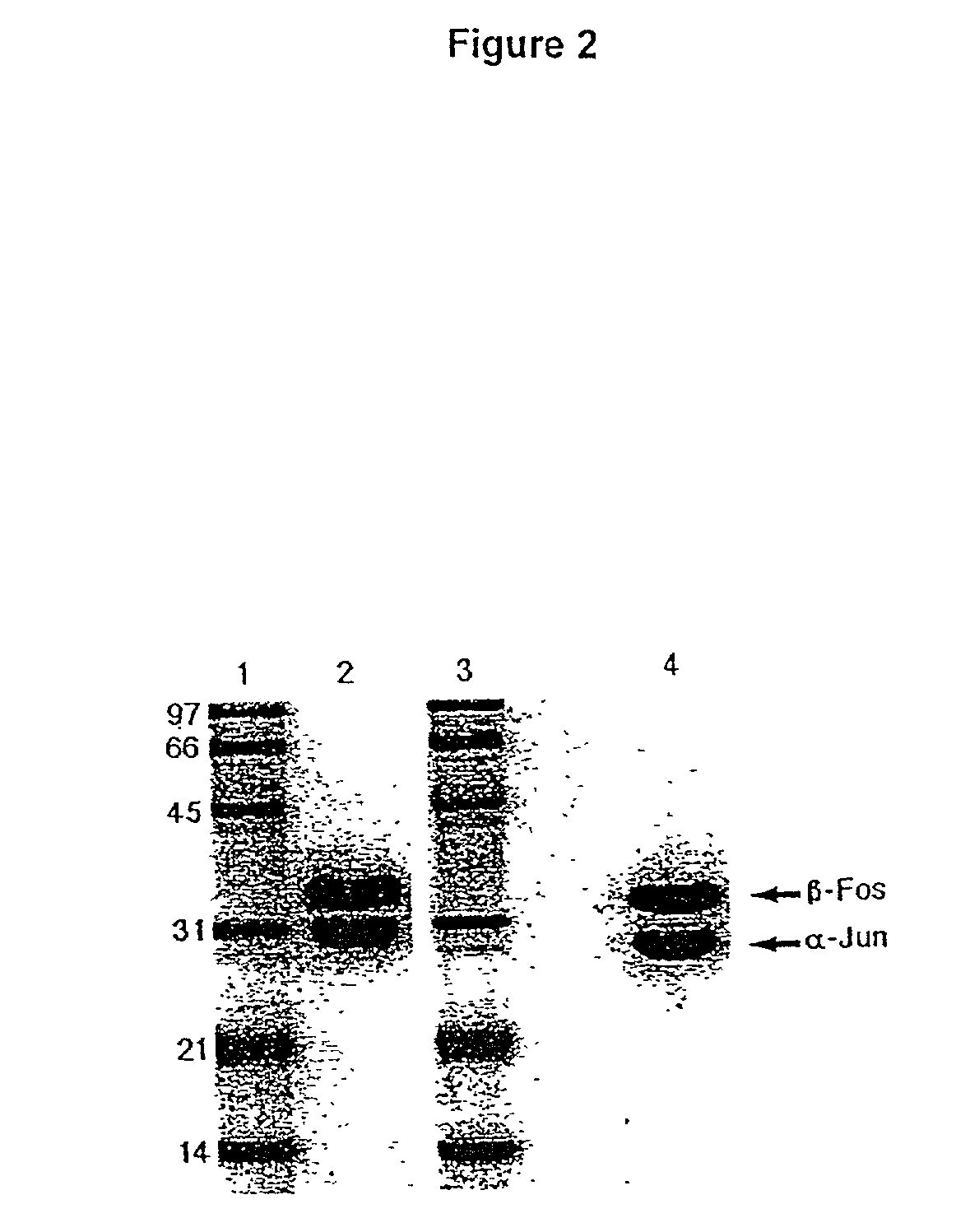
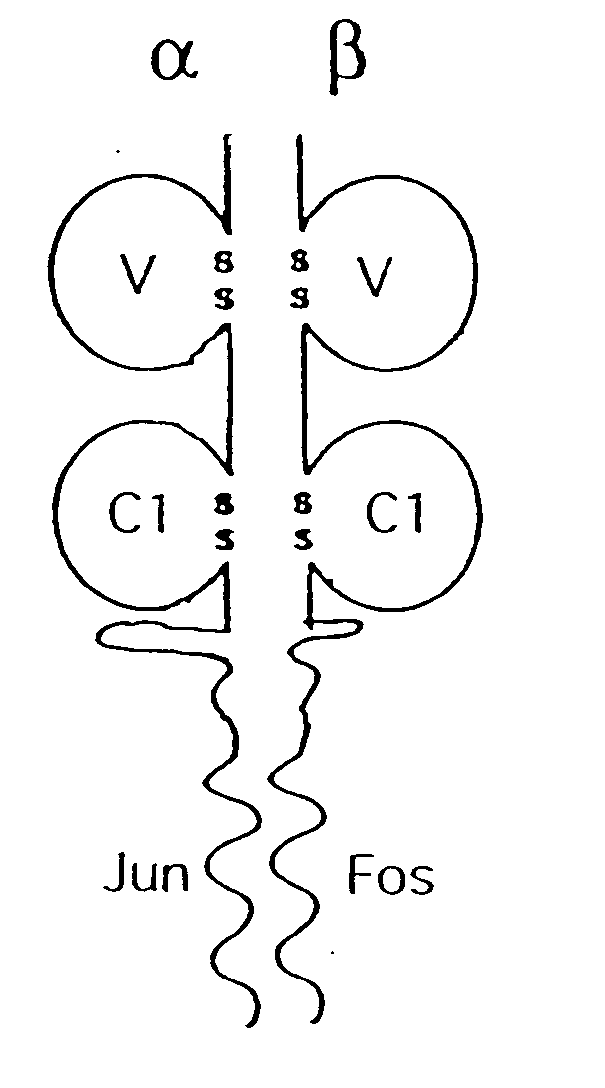
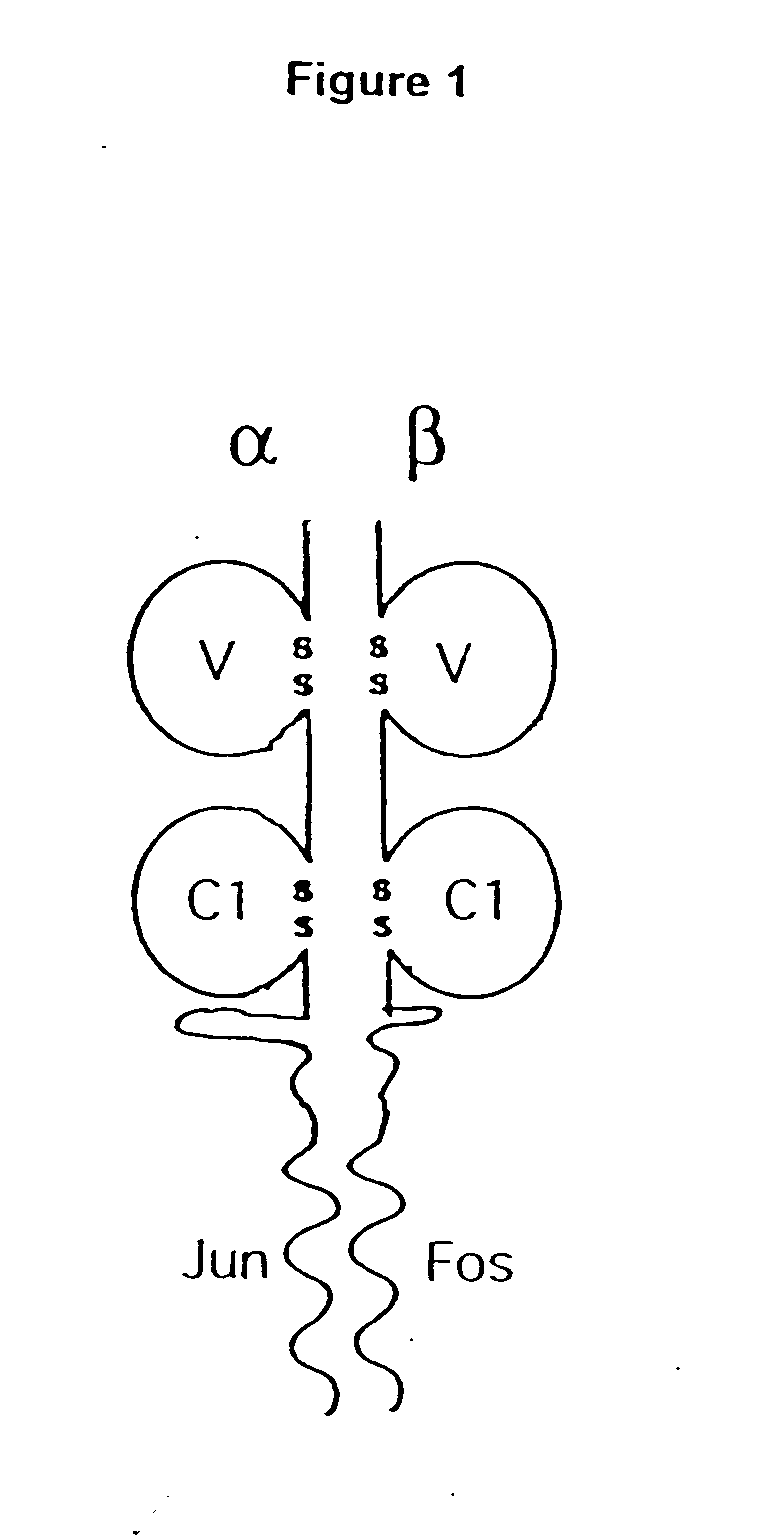
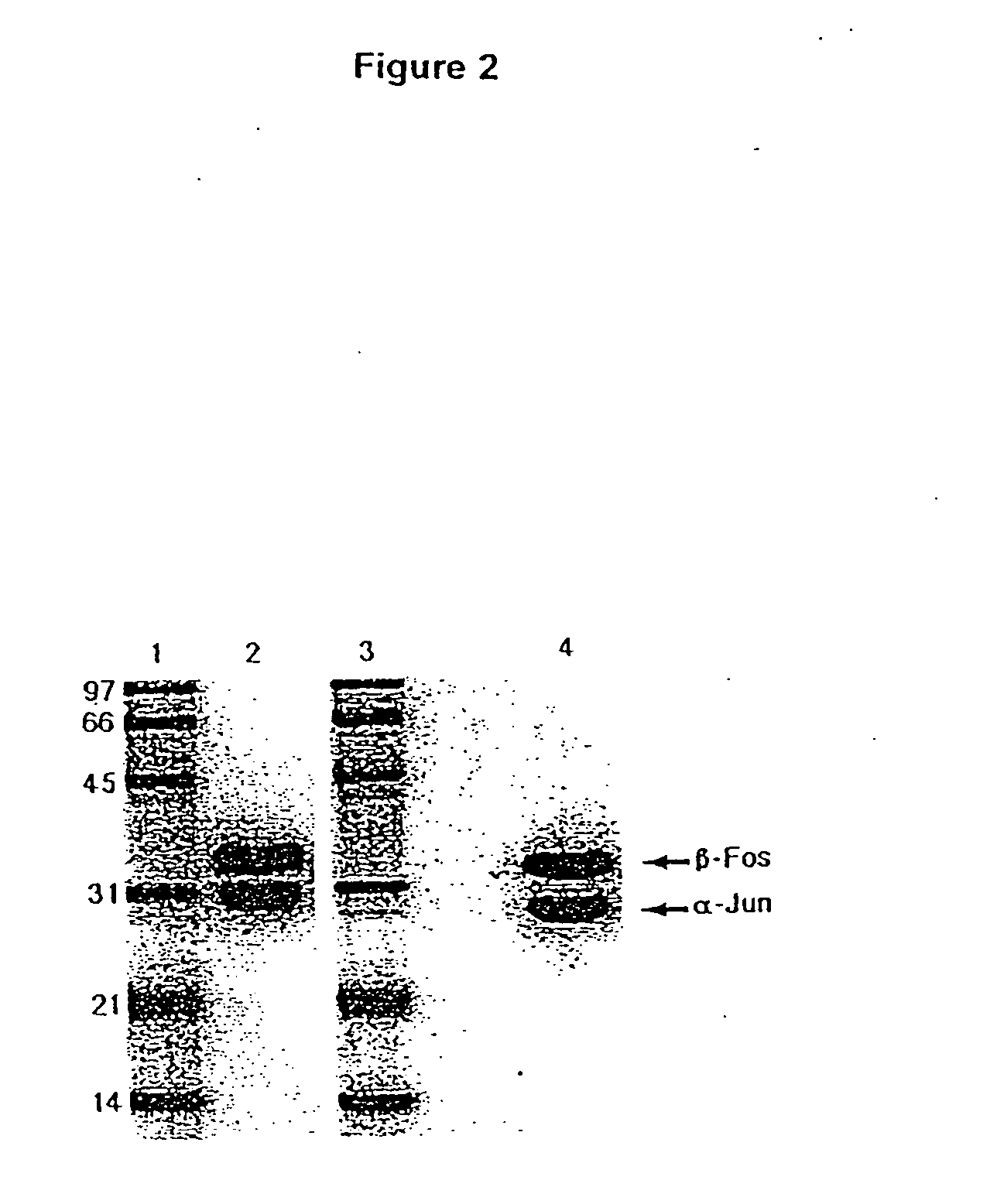
A coiled coil is a structural motif in proteins in which 2–7 alpha-helices are coiled together like the strands of a rope (dimers and trimers are the most common types). Many coiled coil-type proteins are involved in important biological functions such as the regulation of gene expression, e.g. transcription factors. Notable examples are the oncoproteins c-Fos and c-jun, as well as the muscle protein tropomyosin.
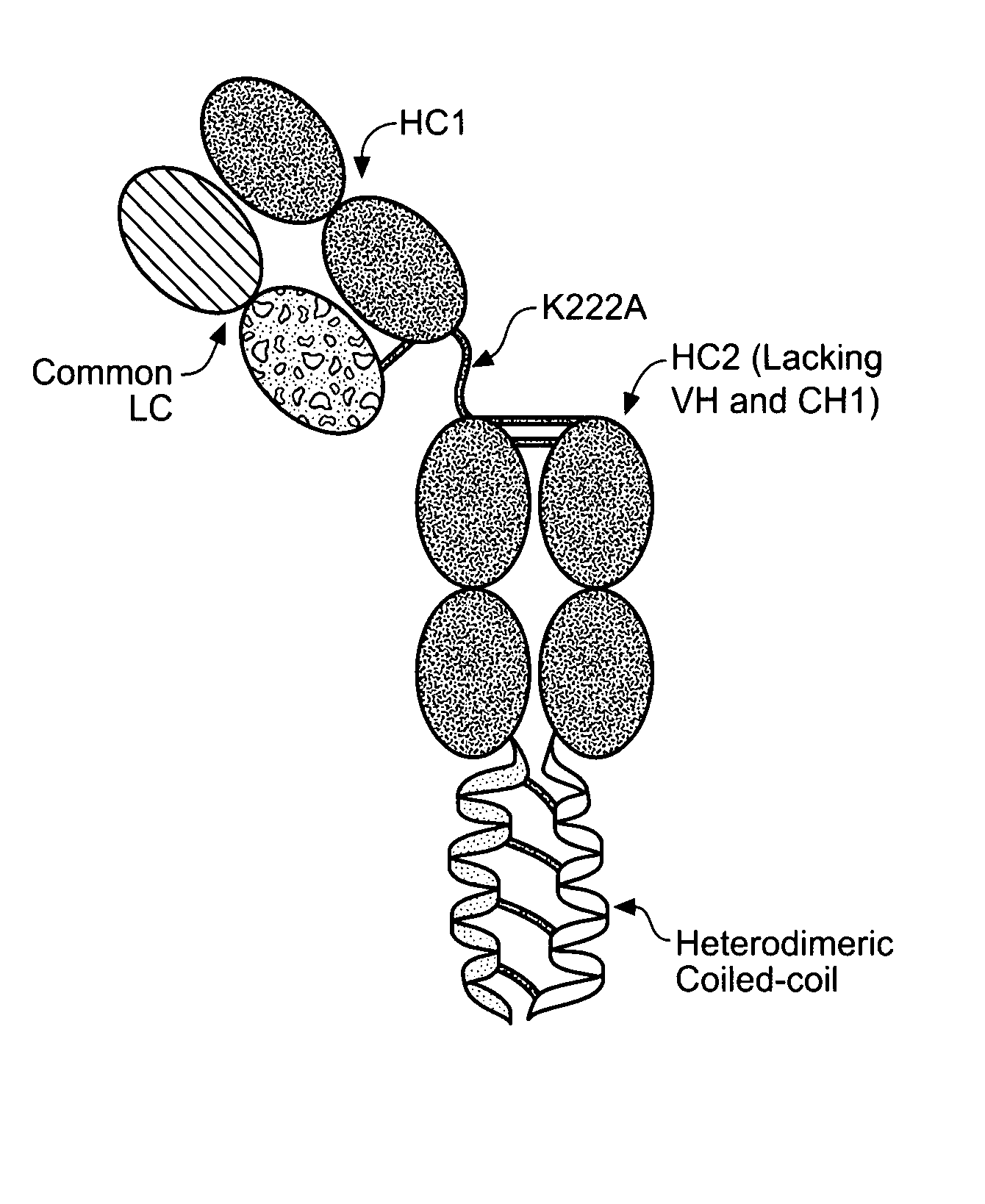
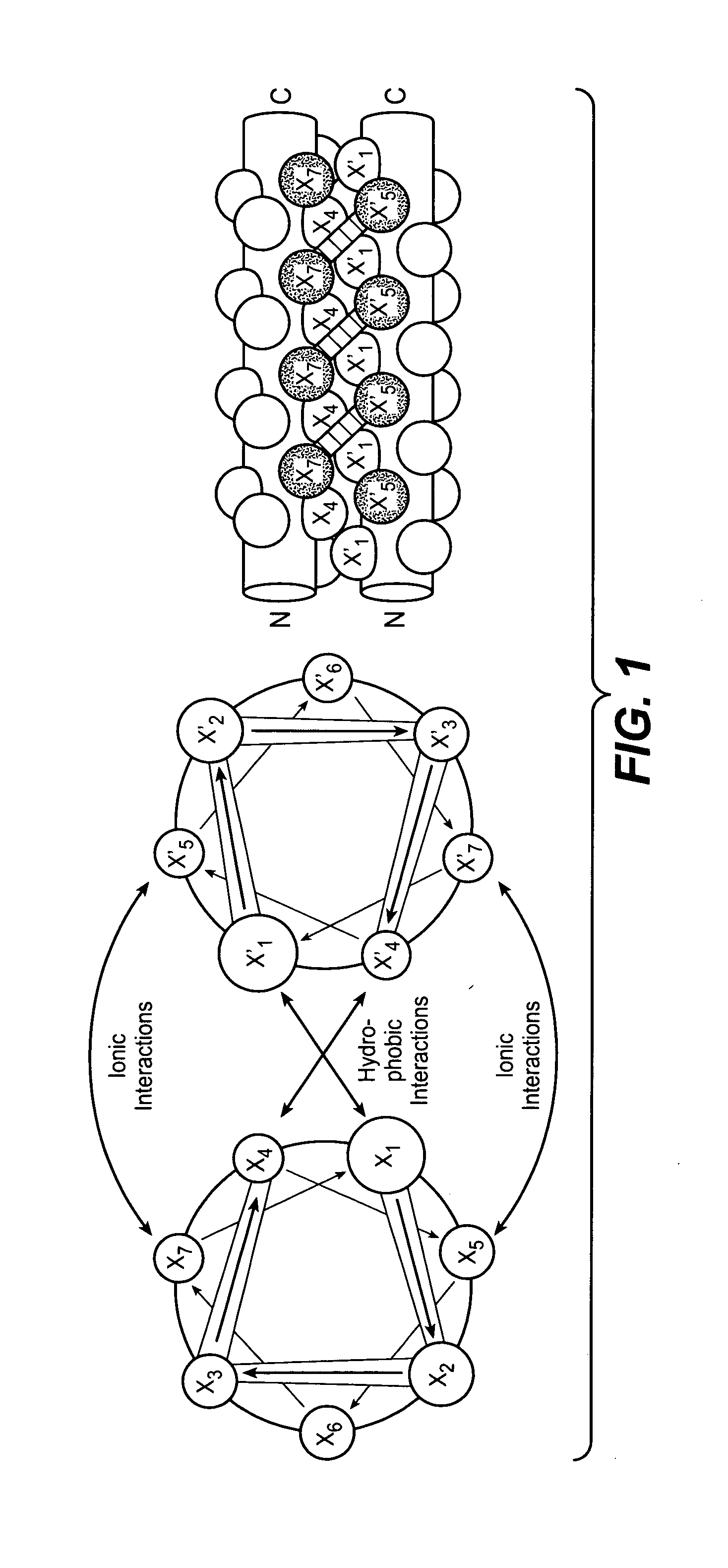
Coiled coil and/or tether containing protein complexes and uses thereof
InactiveUS20120302737A1Simple structureSimple and efficient and high yieldHybrid immunoglobulinsAntibody mimetics/scaffoldsAntiendomysial antibodiesEngineering
The invention provides engineered protein complexes constructed using a coiled coil and / or a tether and methods for making, using, and purifying such complexes, such as multispecific antibodies or other multispecific Fc containing complexes.
Owner:F HOFFMANN LA ROCHE & CO AG
Recombinant fibrin chains, fibrin and fibrin-homologs
The invention is directed to fibrin materials for use in fibrin compositions and methods that avoid the need to use thrombin as an activating agent for fibrin monomer-based sealants. The invention provides for substantially pure fibrin chains, fibrin chain precursors, fibrin chains with other N-terminal extensions, fibrin monomer, fibrin-homolog and fibrin-analog. The invention further provides for variant fibrin .gamma.-chains. The variant gamma-chain contains one or more mutations and / or deletions in the C-terminal region following the coiled-coil forming region such that, when incorporated into fibrin-homolog, the homolog lacks the ability to self-polymerize but has the ability to form non-covalent bonds, and thereby form mixed polymers useful as sealants, with fibrinogen. The invention also provides nucleotide sequences encoding fibrin chains or fibrin chain variants and cells expressing fibrin chains, fibrin chain variants, fibrin monomer, fibrin precursor or fibrinogen-analog. The invention further provides a method of forming fibrin-related proteins in vitro from their component fibrin chains. The invention additionally provides a method for forming a fibrin sealant by a reacting a first fibrin-related protein that is incapable of self-polymerizing with a second fibrin-related protein that is incapable of self-polymerizing. Fibrin chains produced by methods of the present invention may be used as sources of substantially pure starting material for the production of important fibrin-derived factors that regulate angiogenesis, platelet aggregation, and other physiological processes.
Owner:BRISTOL MYERS SQUIBB CO
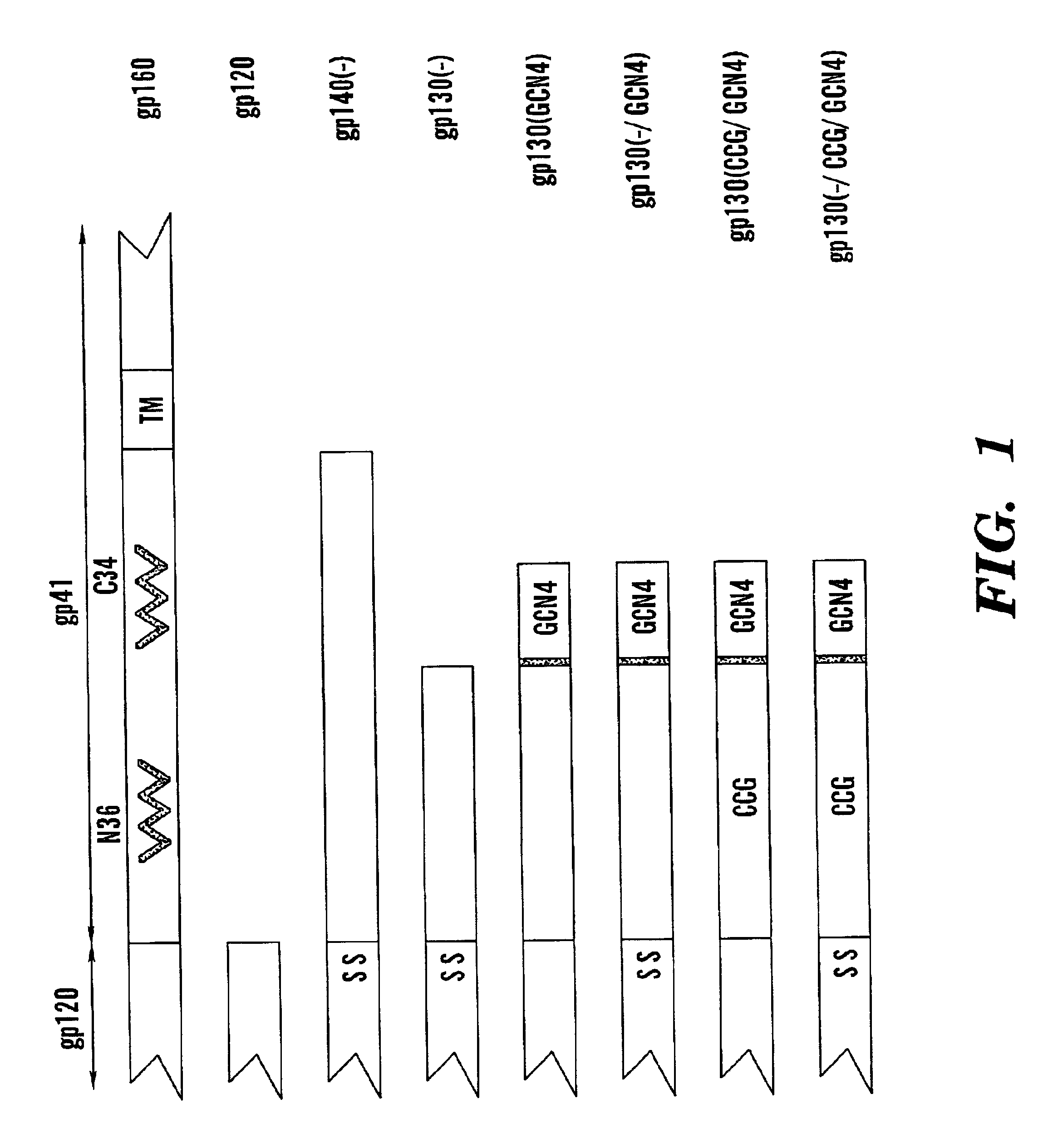
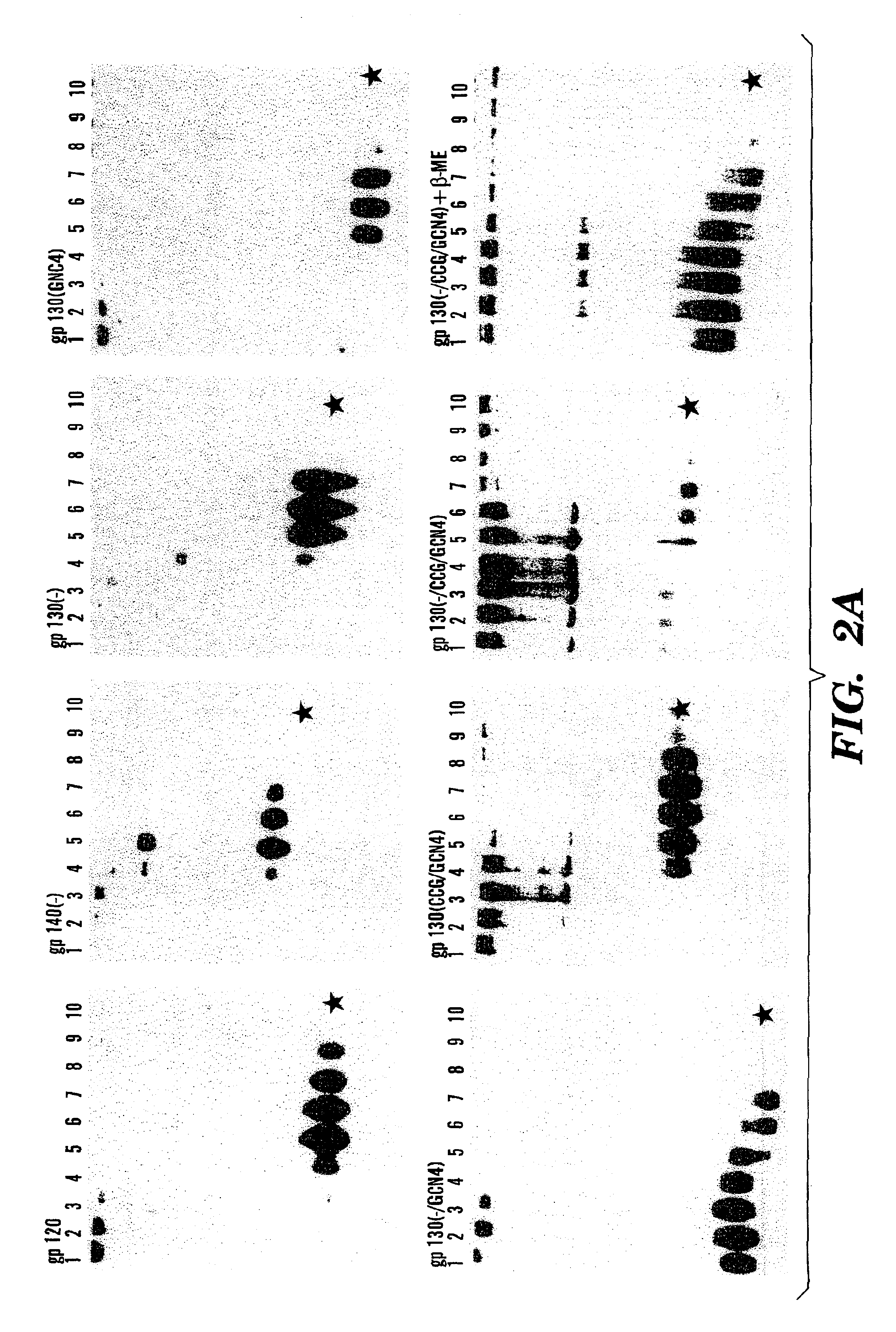
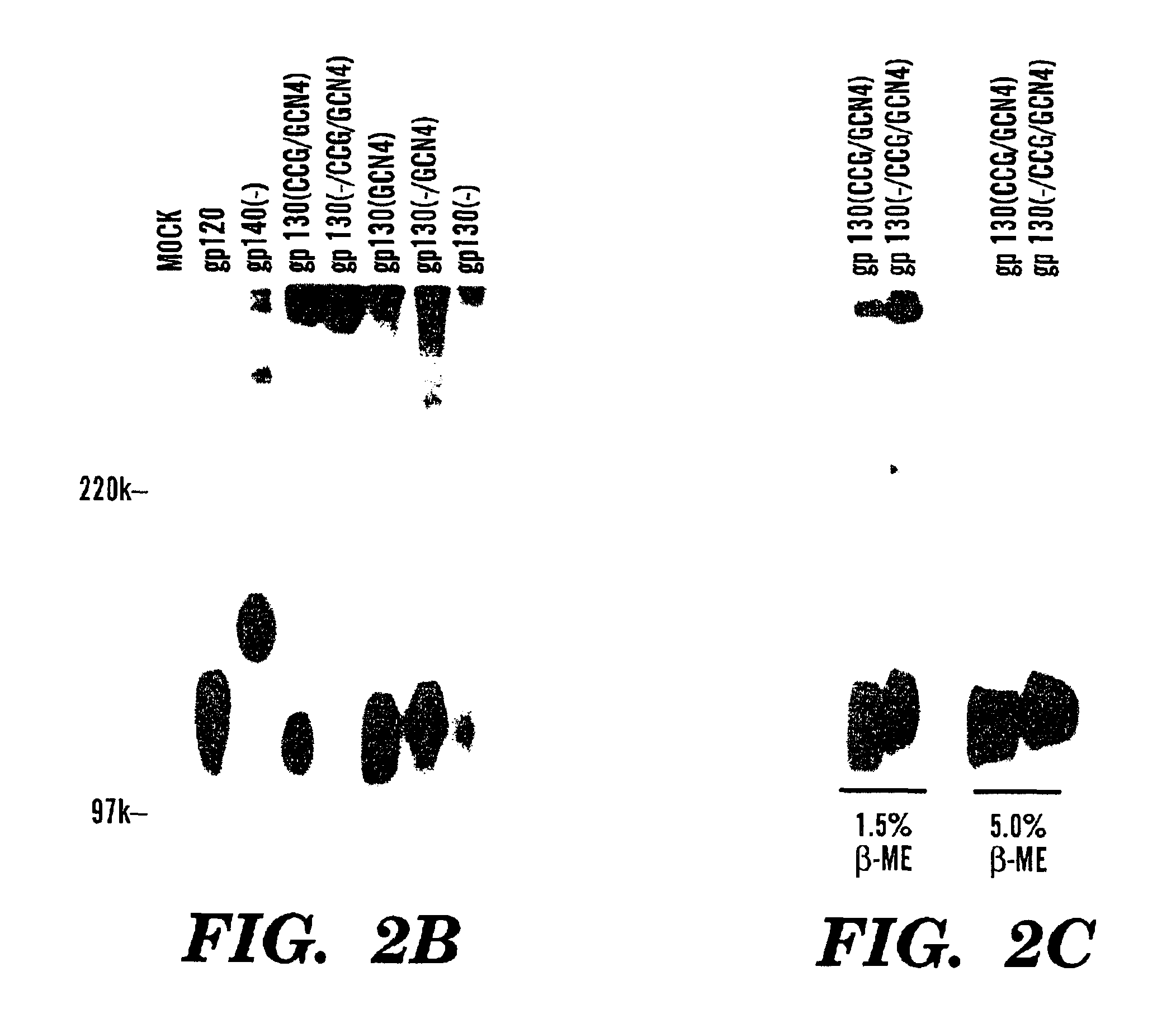
Stabilized soluble glycoprotein trimers
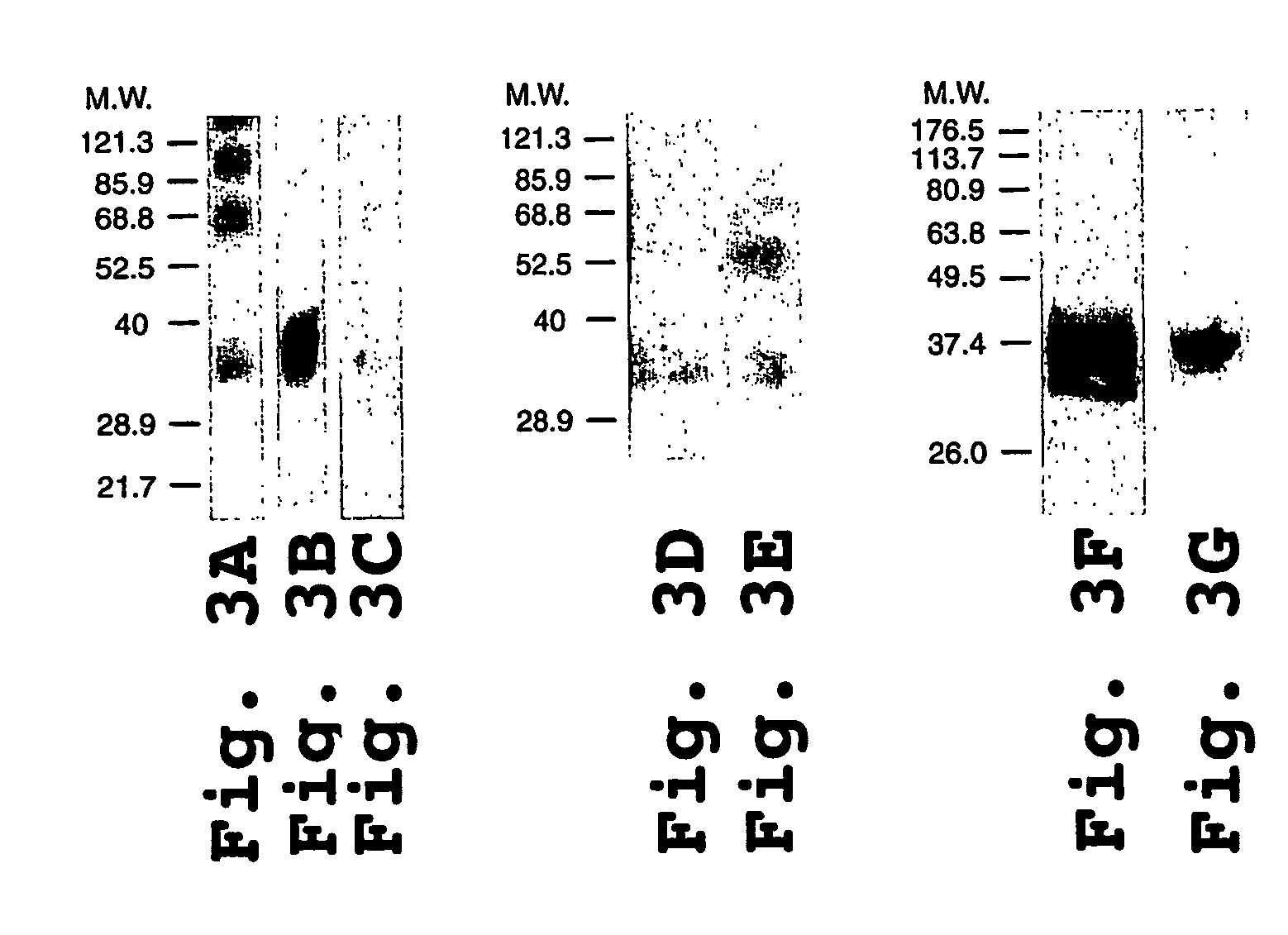
InactiveUS6911205B2Stabilize trimerSimplifies isolationPeptide/protein ingredientsAntibody mimetics/scaffoldsHiv envelopeGp41
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Methods for the inhibition of epstein-barr virus transmission employing anti-viral peptides capable of abrogating viral fusion and transmission
InactiveUS6518013B1SsRNA viruses negative-sensePeptide/protein ingredientsCell membraneViral life cycle
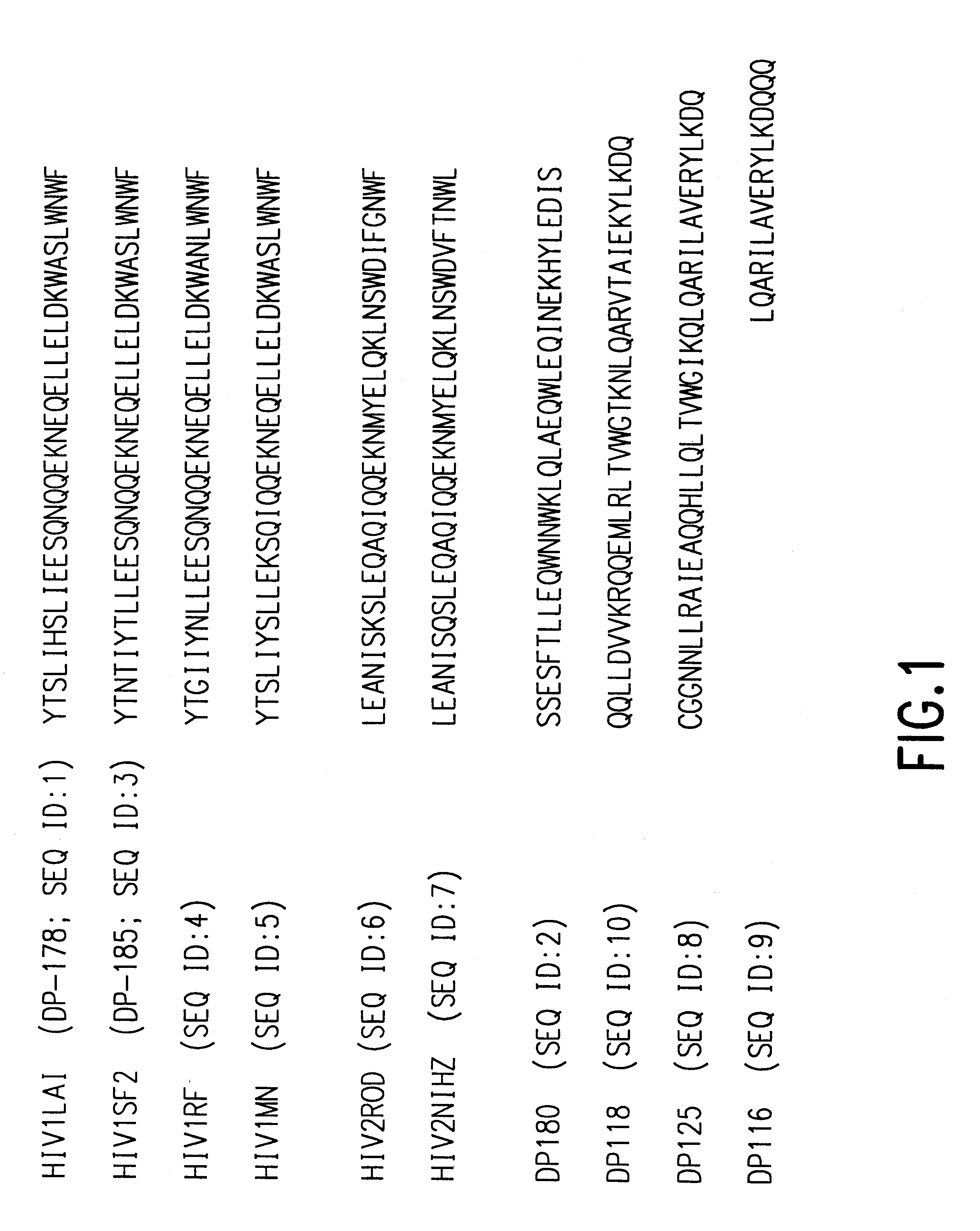
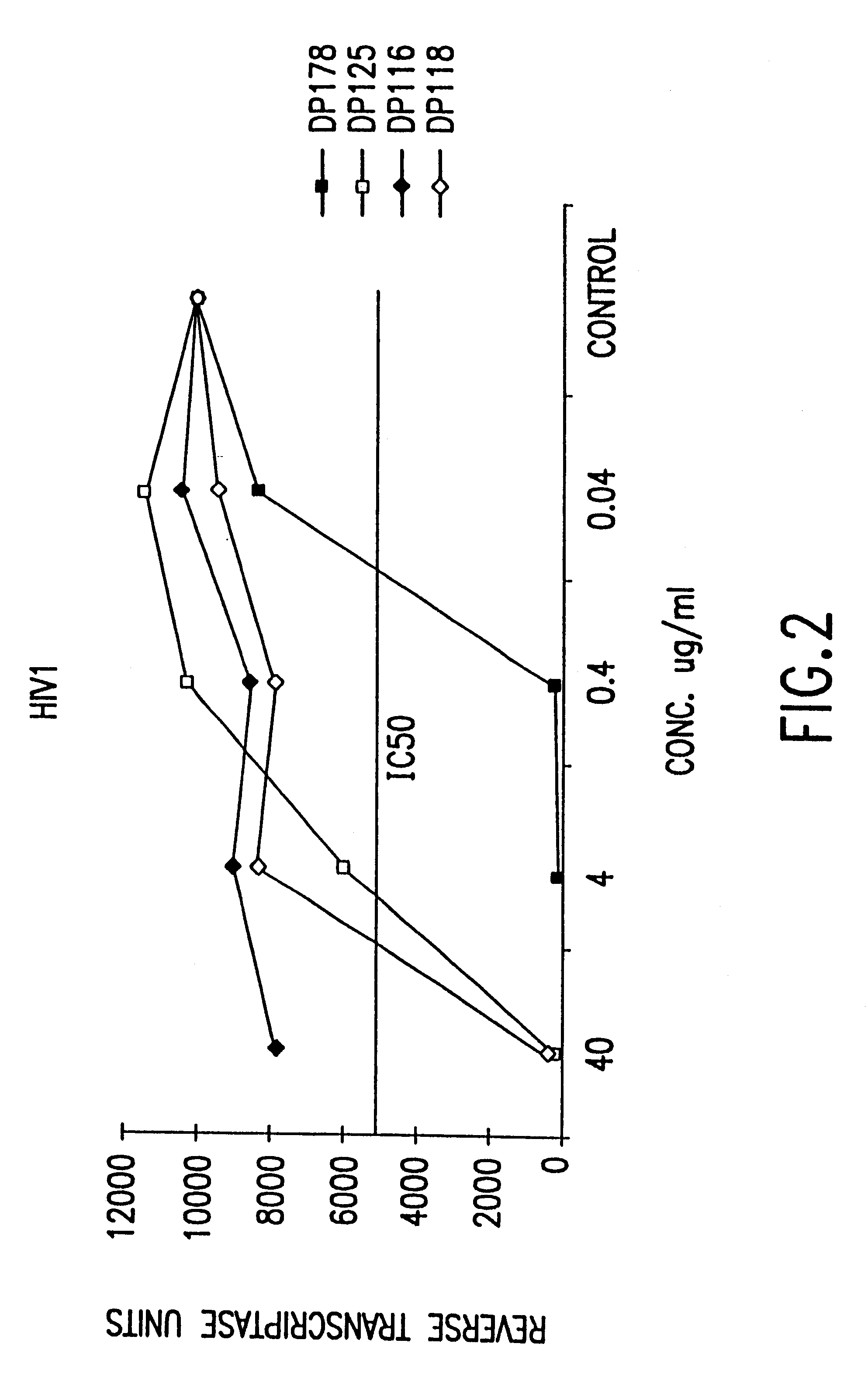
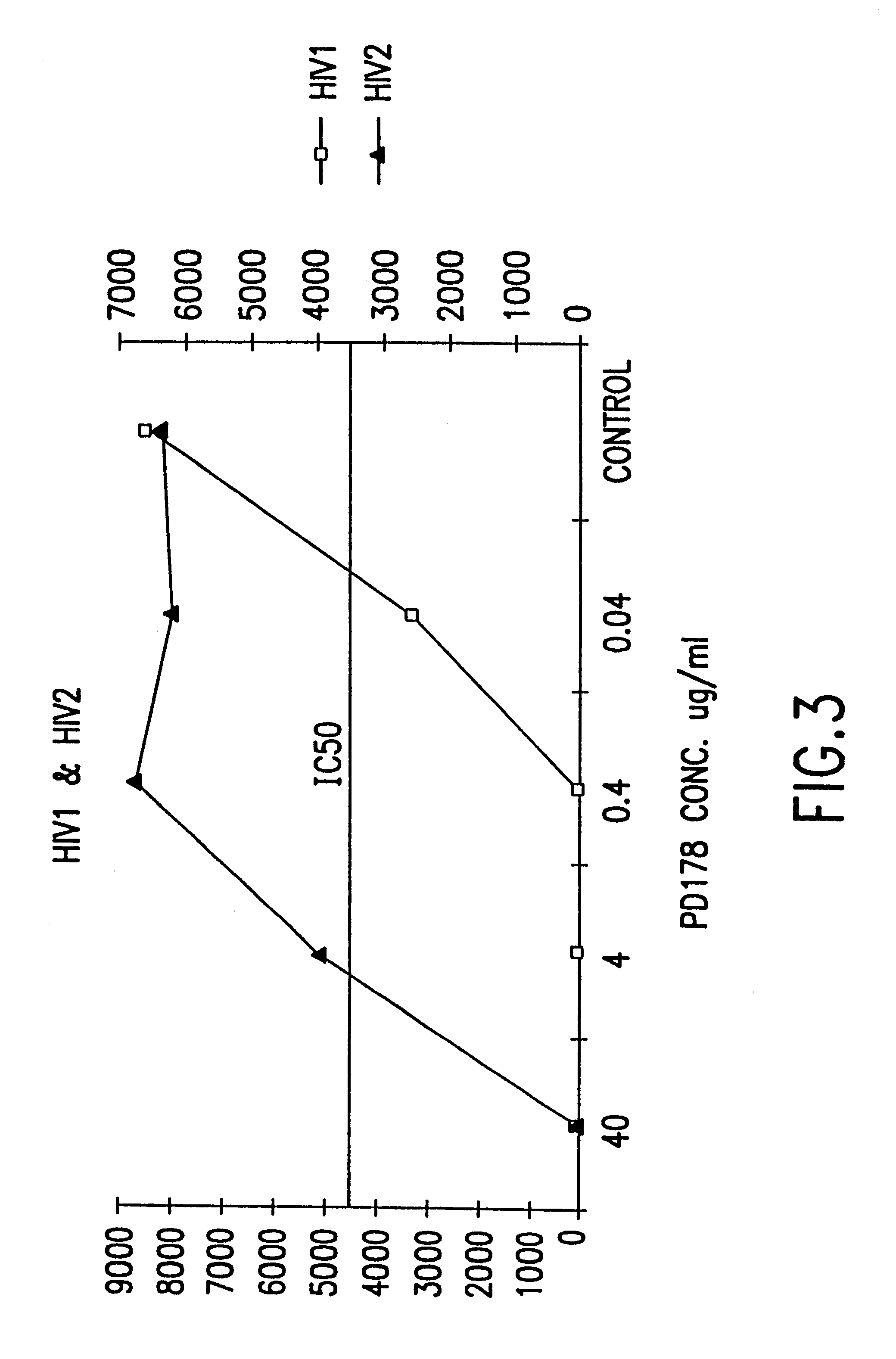
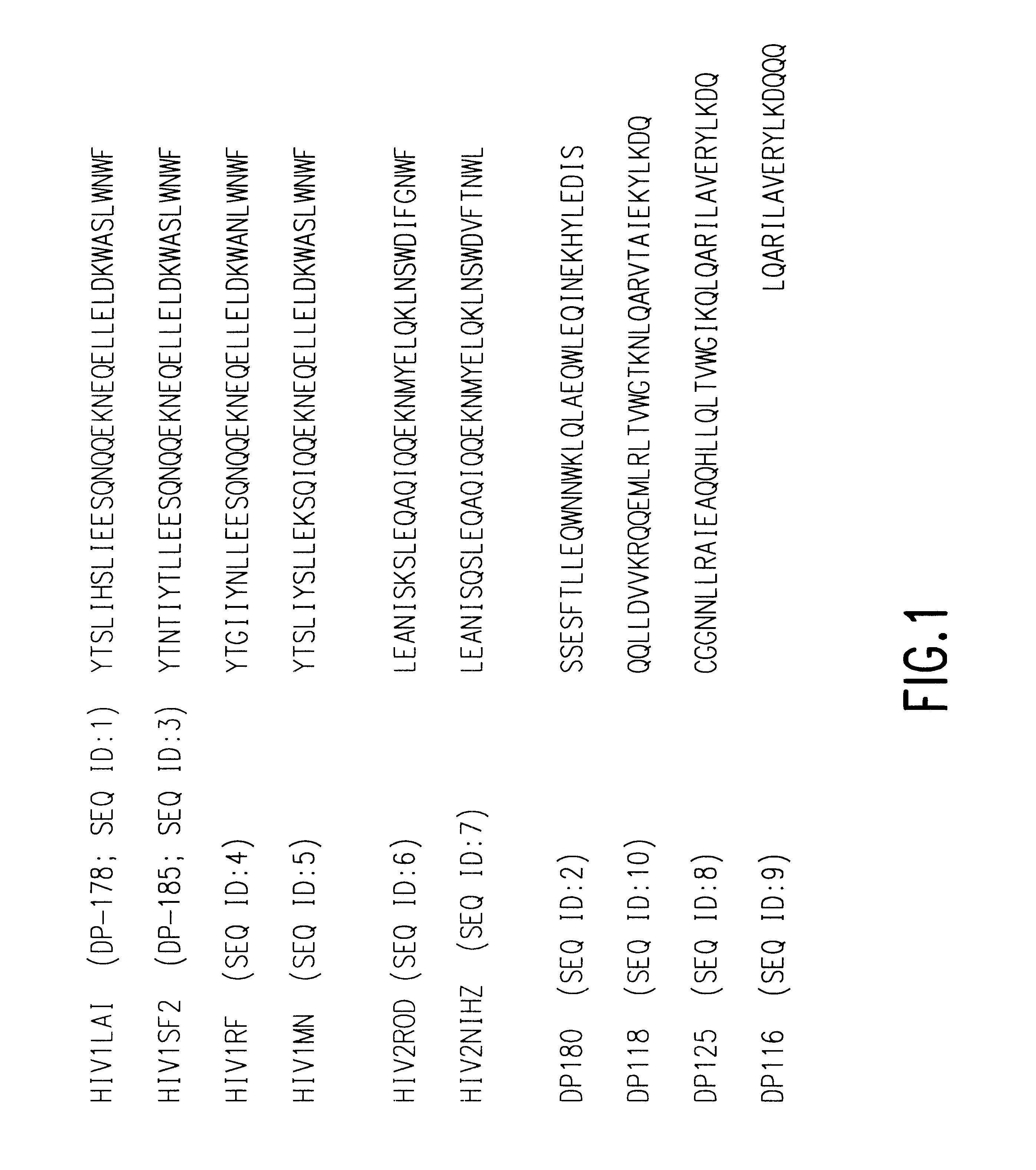
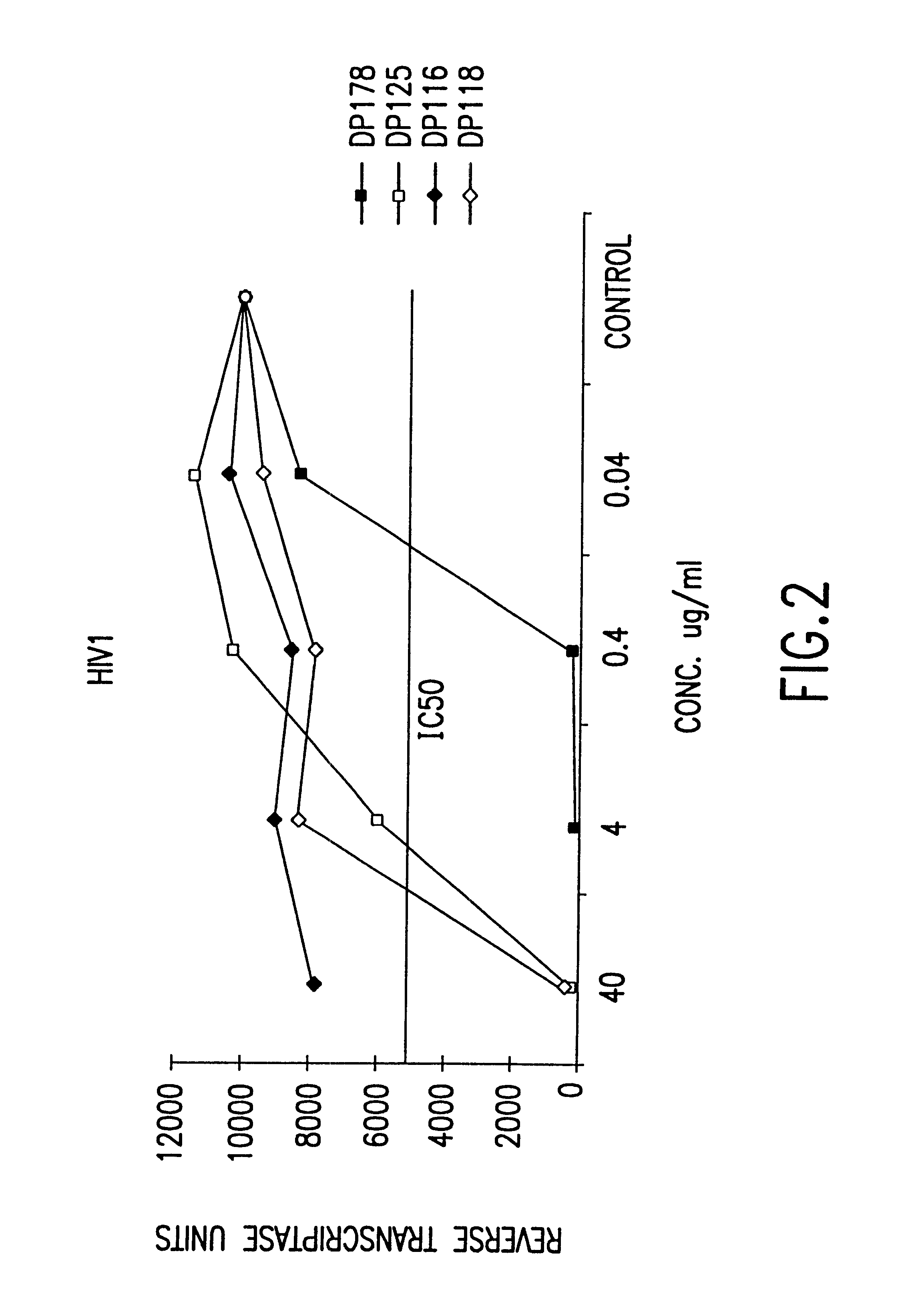
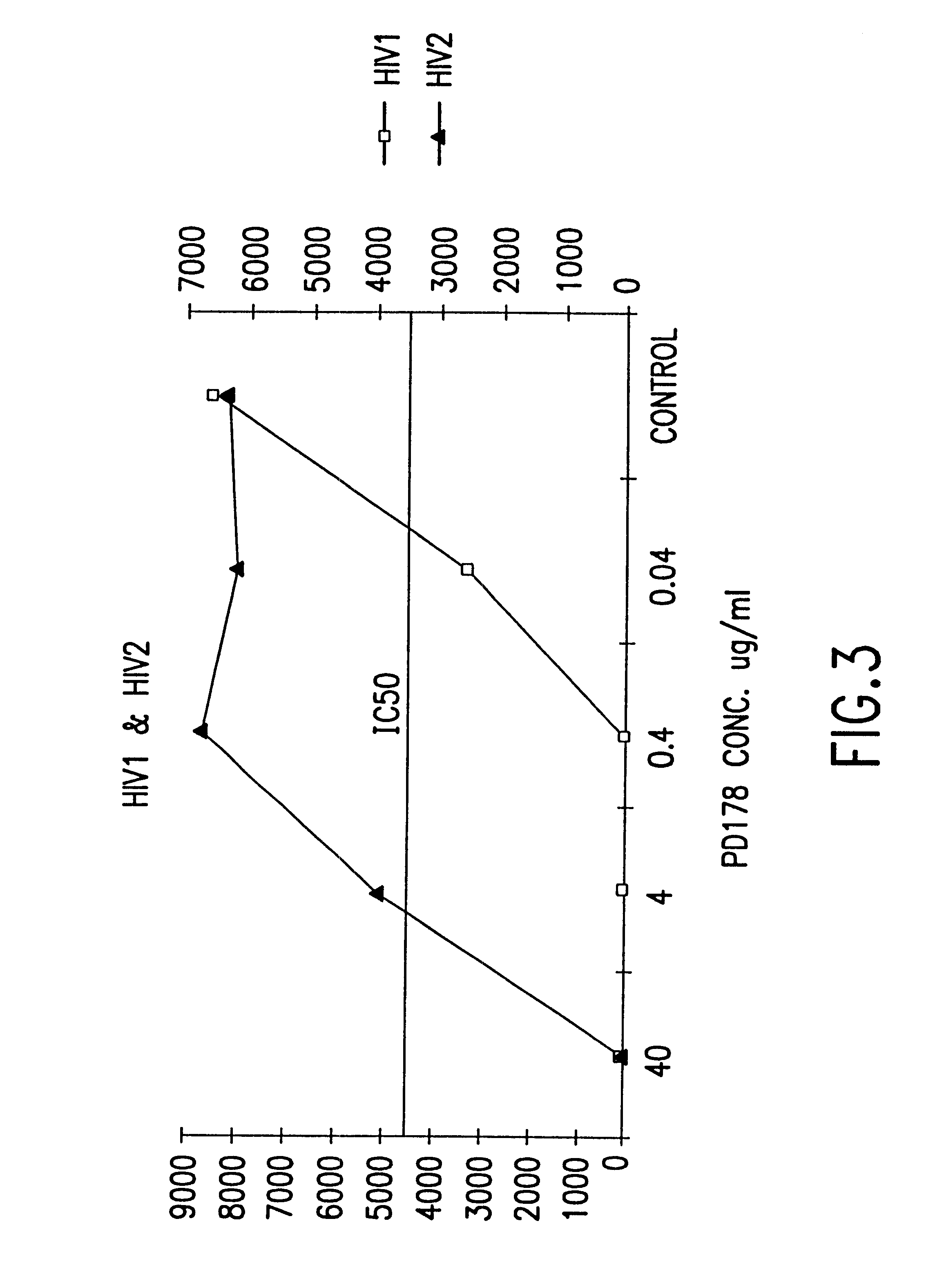
Fusion of the viral envelope, or infected cell membranes with uninfected cell membranes, is an essential step in the viral life cycle. Recent studies involving the human immunodeficiency virus type 1(HIV-1) demonstrated that synthetic peptides (designated DP-107 and DP-178) derived from potential helical regions of the transmembrane (TM) protein, gp41, were potent inhibitors of viral fusion and infection. A computerized antiviral searching technology (C.A.S.T.) that detects related structural motifs (e.g., ALLMOTI 5, 107x178x4, and PLZIP) in other viral proteins was employed to identify similar regions in the Epstein-Barr virus (EBV). Several conserved heptad repeat domains that are predicted to form coiled-coil structures with antiviral activity were identified in the EBV genome. Synthetic peptides of 16 to 39 amino acids derived from these regions were prepared and their antiviral activities assessed in a suitable in vitro screening assay. These peptides proved to be potent inhibitors of EBV fusion. Based upon their structural and functional equivalence to the known HIV-1 inhibitors DP-107 and DP-178, these peptides should provide a novel approach to the development of targeted therapies for the treatment of EBV infections.
Owner:TRIMERIS
Method and composition used for obtaining neuron-like cells from non-neuronal cells via reprogramming
ActiveCN105039258ALow costEasy to operateNervous disorderDrug screeningPTK InhibitorsEnzyme Inhibitor Agent
Owner:PEKING UNIV +1
Peptidic nanoparticles as drug delivery and antigen display systems
Described is a new type of nanoparticle using the concept of self-organization of a single continuous chain to form peptidic nanoparticles. In particular, nanoparticles of the invention consist of aggregates of a continuous chain comprising two peptidic oligomerization domains connected by a linker segment. Preferred are coiled-coil oligomerization domains with a contiguous pattern of hydrophobic residues spaced 3 and 4 residues apart. The invention provides a drug targeting and delivery system comprising a functionalized peptidic nanoparticle comprising ligands capable of binding a receptor and drugs, and a method of treating or diagnosing humans using such functionalized peptidic nanoparticles. The invention further provides an antigen display system to be used as efficient vaccines comprising a functionalized peptidic nanoparticle comprising an antigen, and a method of vaccinating humans or non-human animals using such functionalized peptidic nanoparticles. The invention also provides processes for making peptidic nanoparticles and functionalized peptidic nanoparticles, and monomeric building blocks suitable for forming such nanoparticles.
Owner:ALPHA O PEPTIDES
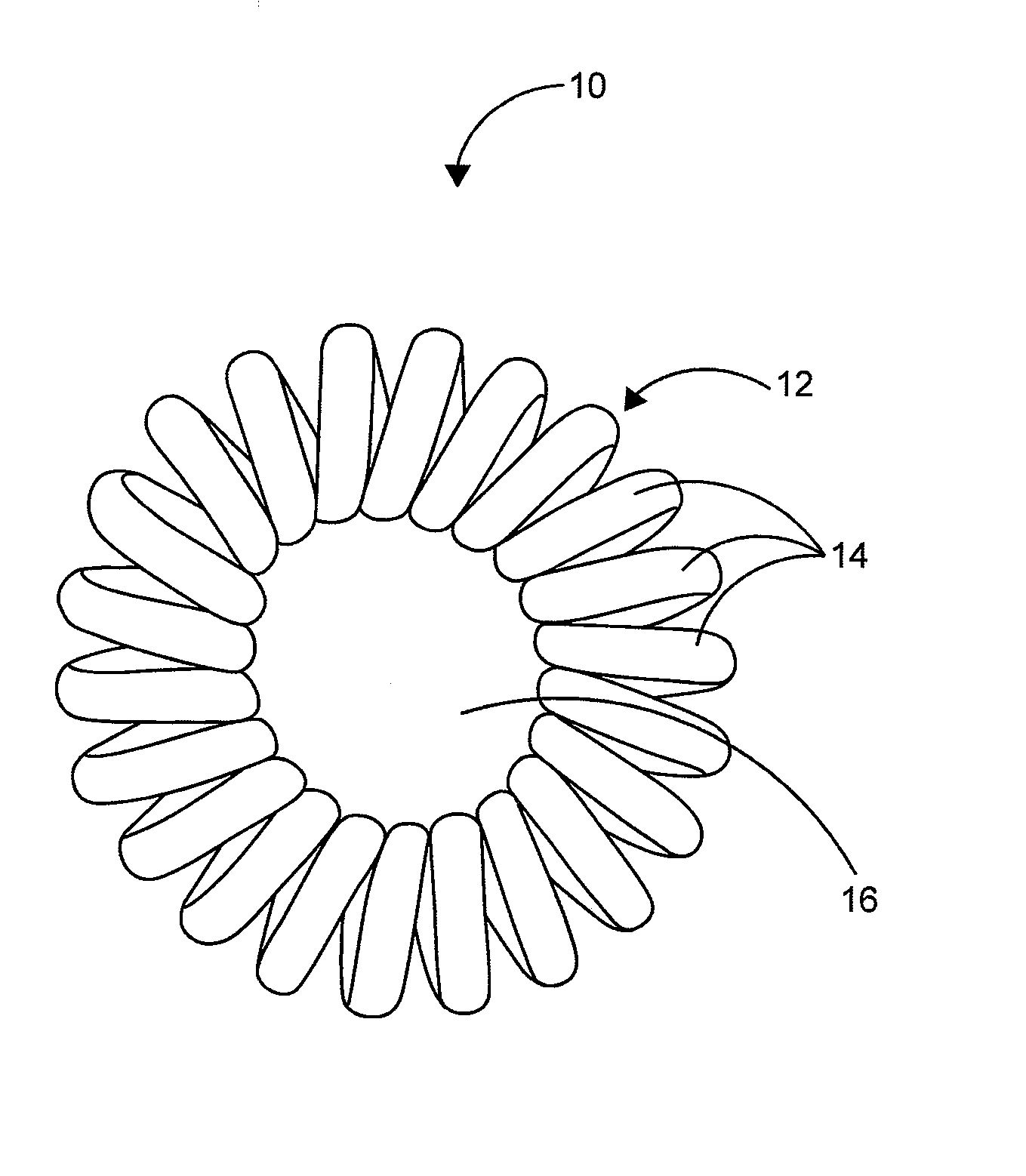
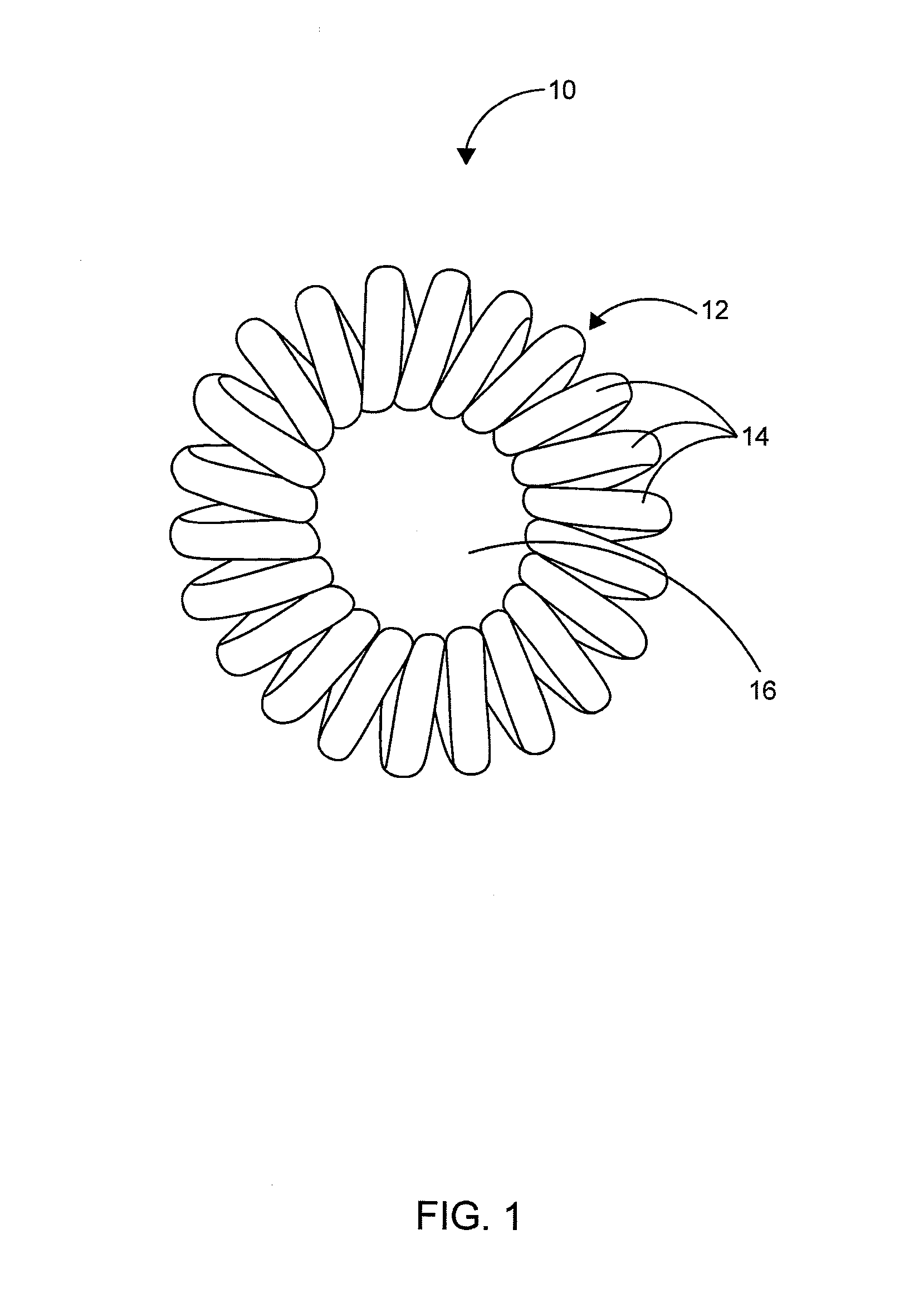
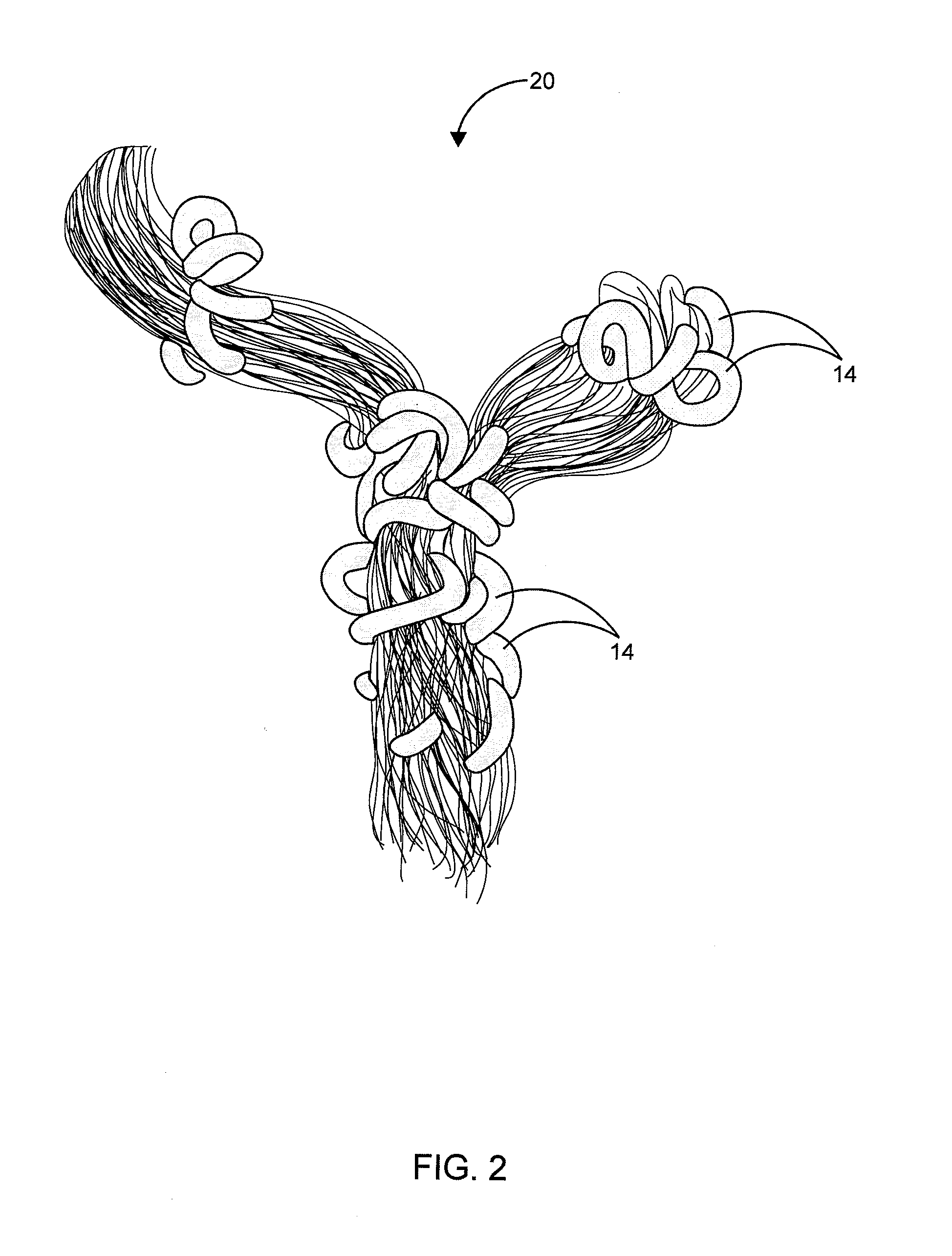
Hair Ties
InactiveUS20140096788A1Reduce frictionRegain shapeCurling devicesTravelling articlesEngineeringCoiled coil
The present invention is a hair accessory having a special coil design. The hair accessory comprising a substantially circular shaped member having a plurality of coiled coil structures, each of the plurality of coiled coil structures is arranged in a way to form a closed helical loop. When the coiled coil structure is twisted and folded for holding the hair, its special structure permits it to lock with itself so that the hair accessory remains in hair even if strenuous movement is performed by the wearer. The hardness of the material results in less friction with the hair thereby allowing easy gliding during pull out of hair. The twists around the hair act as anchors thereby not allowing the hair accessory to dislodge. The constant use of the hair accessory may tend to loose its shape and it can regain its shape by placing the hair accessory in boiling water.
Owner:KRASNIANSKY NOAM
Anti-viral compositions comprising heterocyclic substituted phenyl furans and related compounds
InactiveUS20060287319A1Potent anti-HIV activityInhibit HIV replicationBiocideAntiviralsFuranFluorescence
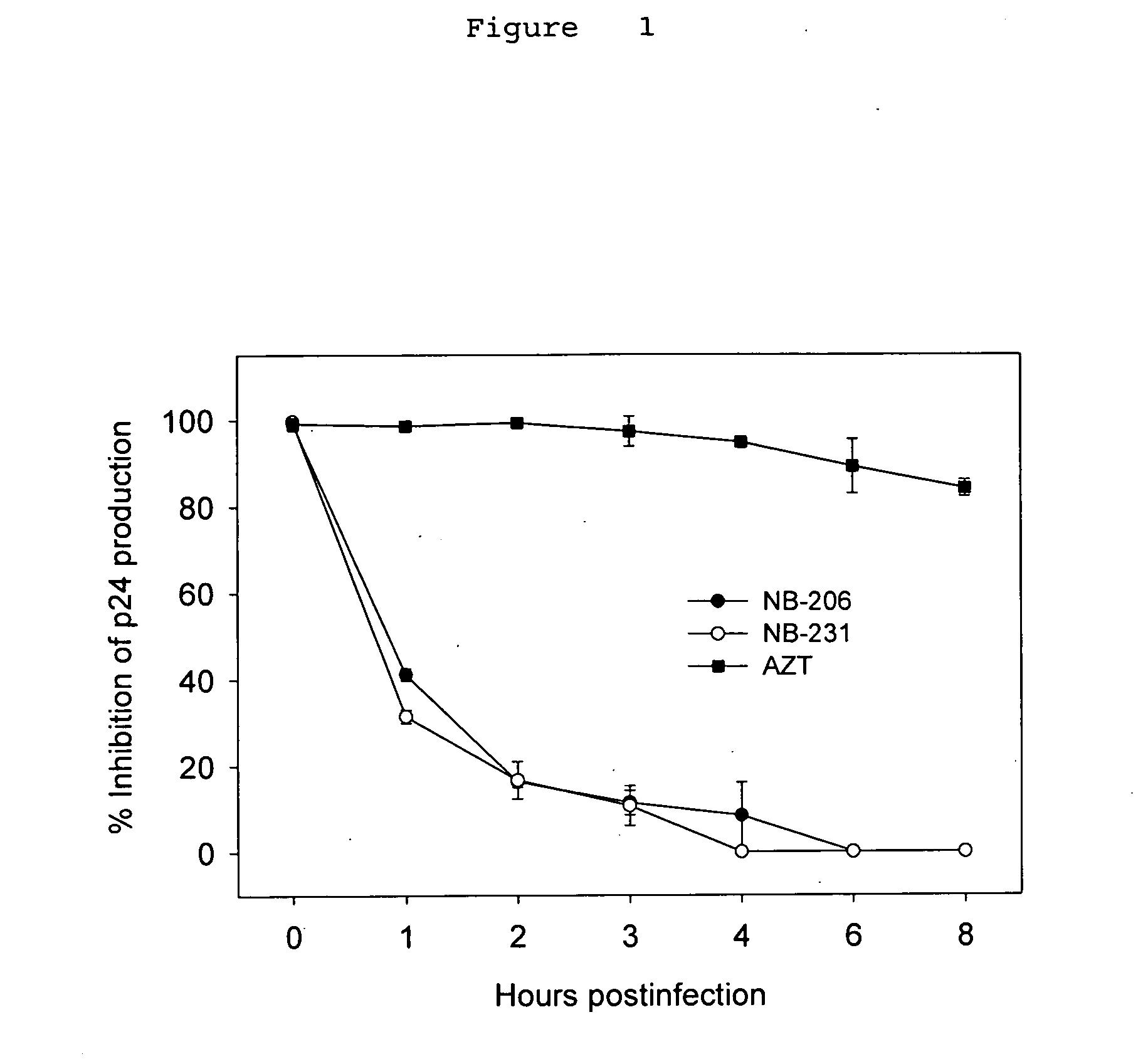
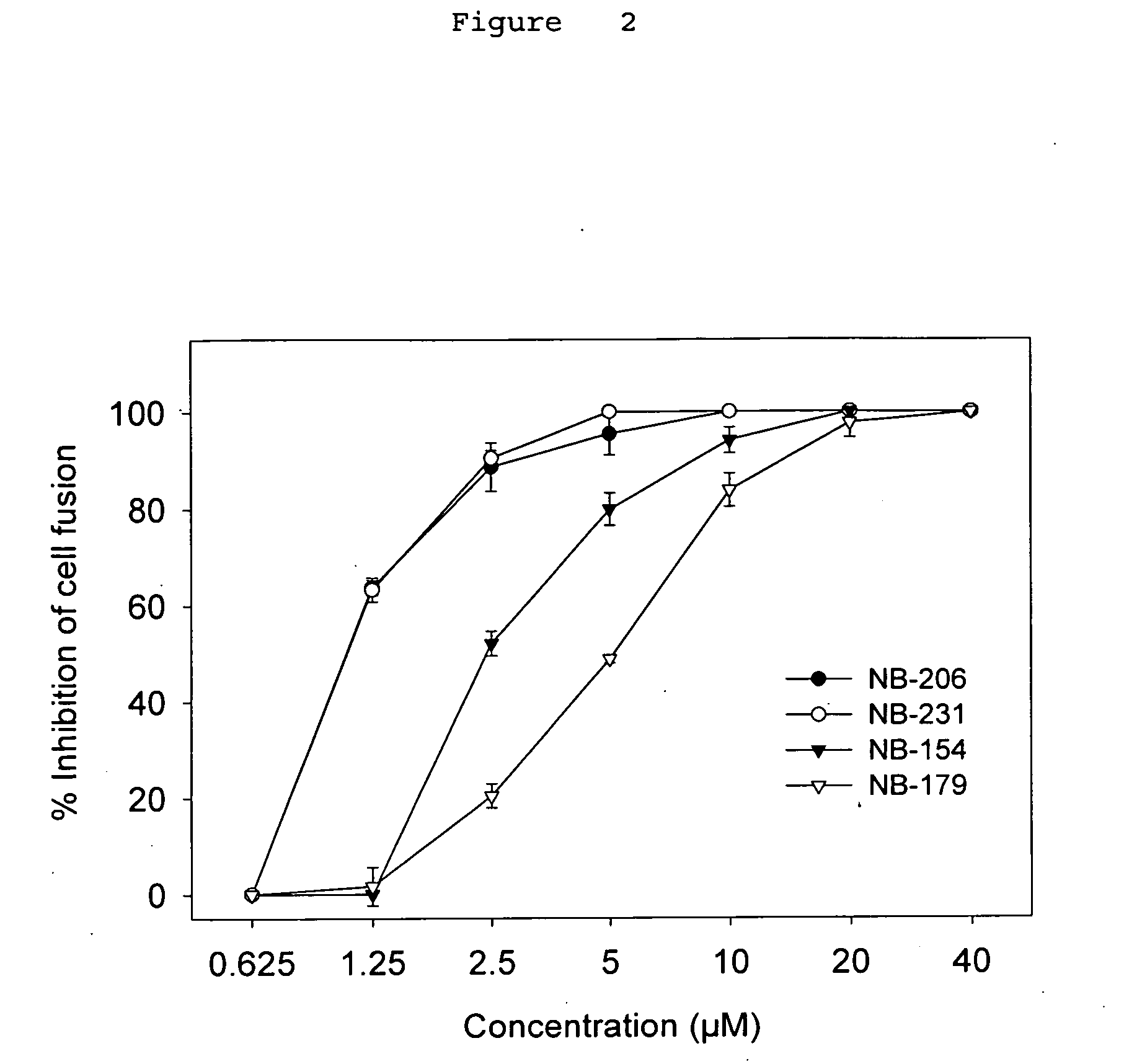
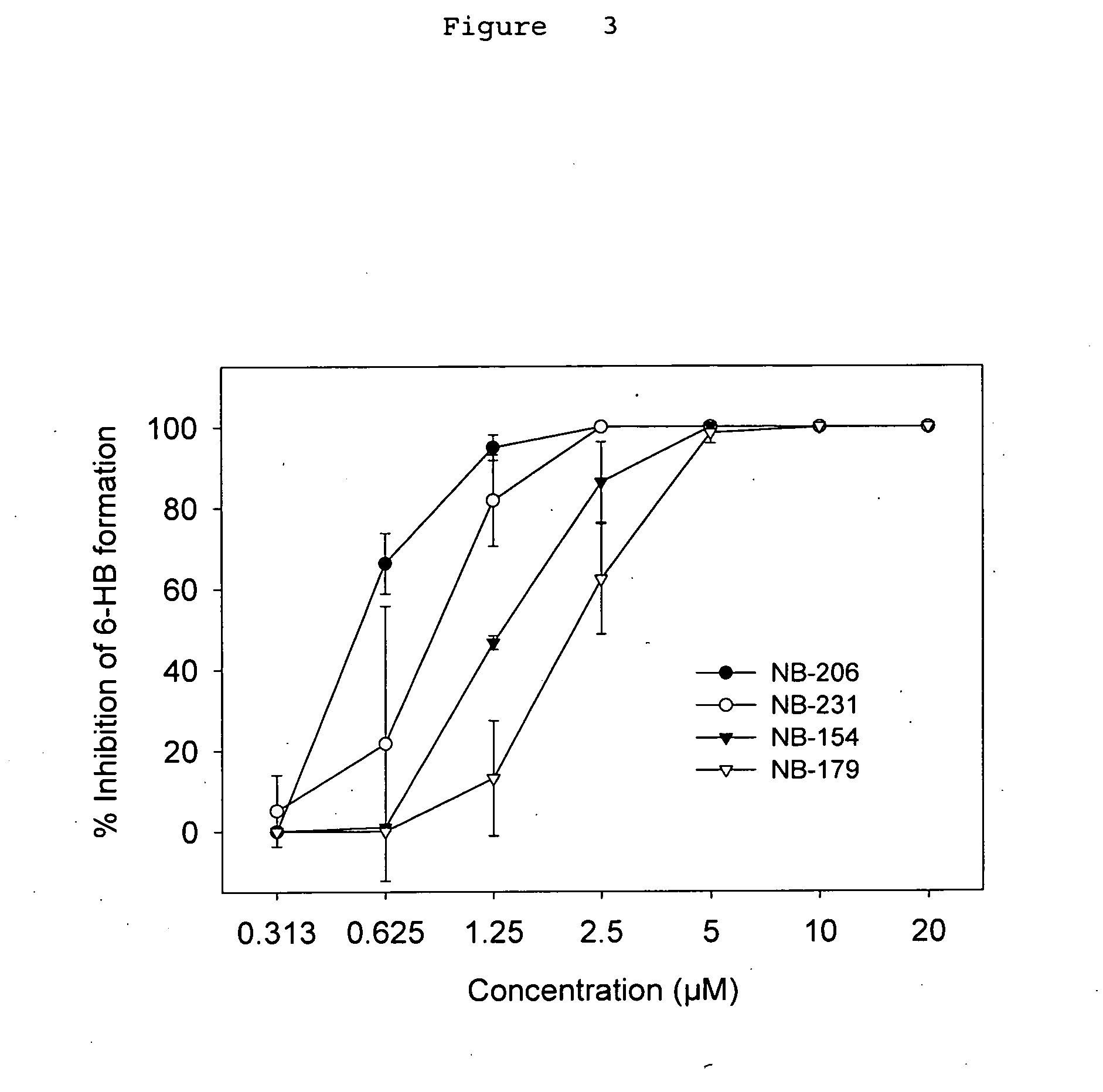
A group of compounds that inhibit HIV replication by blocking HIV entry was identified. One representative compound, designated NB-206, and its analogs inhibited HIV replication (p24 production) with IC50 values at nanomolar levels. It was proved that NB-206 and its analogs are HIV entry inhibitors by targeting the HIV gp41 since: 1) they inhibited HIV-mediated cell fusion; 2) they inhibited HIV replication only when they were added to the cells less than one hour after virus addition; 3) they blocked the formation of the gp41 core that is detected by sandwich enzyme linked immunosorbent assay (ELISA) using a conformation-specific MAb NC-1; and 4) they inhibited the formation of the gp41 six-helix bundle revealed by fluorescence native-polyacrylamide gel electrophoresis (FN-PAGE). These results suggested that NB-206 and its analogs may interact with the hydrophobic cavity and block the formation of the fusion-active gp41 coiled coil domain, resulting in inhibition of HIV-1 mediated membrane fusion and virus entry.
Owner:NEW YORK BLOOD CENT
Soluble T cell receptor
InactiveUS20020142389A1High affinityStrong specificityBacteriaPeptide/protein ingredientsHeterologousExtracellular Structure
The present invention relates to a recombinant soluble T cell receptor. The T cell receptor (TCR) is refolded and comprises a recombinant TCR alpha or gamma chain extracellular domain having a first heterologous C-terminal dimerisation peptide; and a recombinant TCR beta or delta chain extracellular domain having a second C-terminal dimerisation peptide which is specifically heterodimerised with the first dimerisation peptide to form a heterodimerisation domain, which may be a coiled coil domain. The invention also provides nucleic acid sequences encoding the recombinant TCR and a method for producing the recombinant TCR. The TCR may be labelled with a detectable label so as to enable the detection of specific MHC-peptide complexes. Alternatively, it can be linked to a therapeutic agent such as a cytotoxic agent or an immunostimulating agent so as to deliver such an agent to the site of a specific MHC-peptide complex.
Owner:JAKOBSEN BENT KARSTEN +4
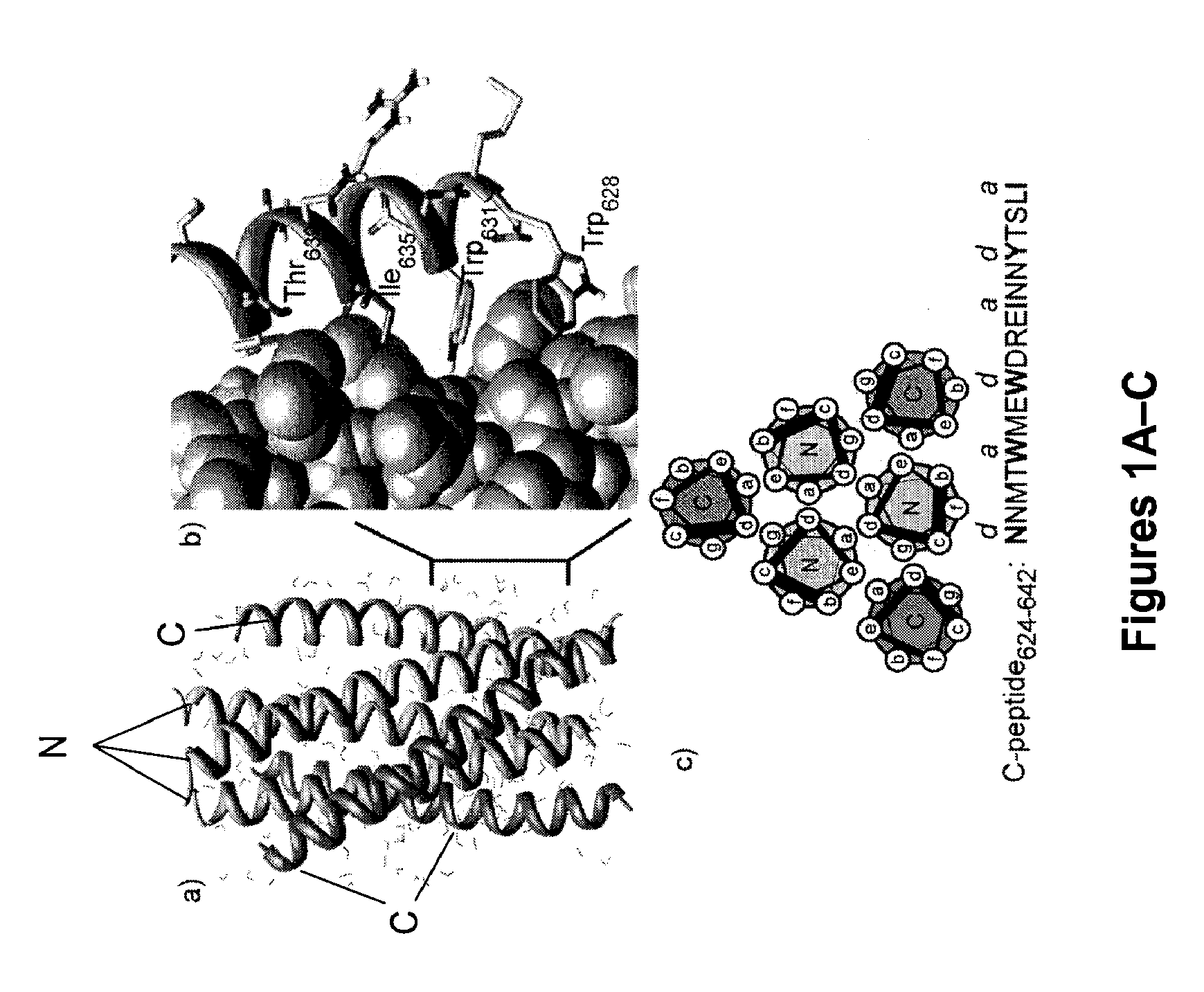
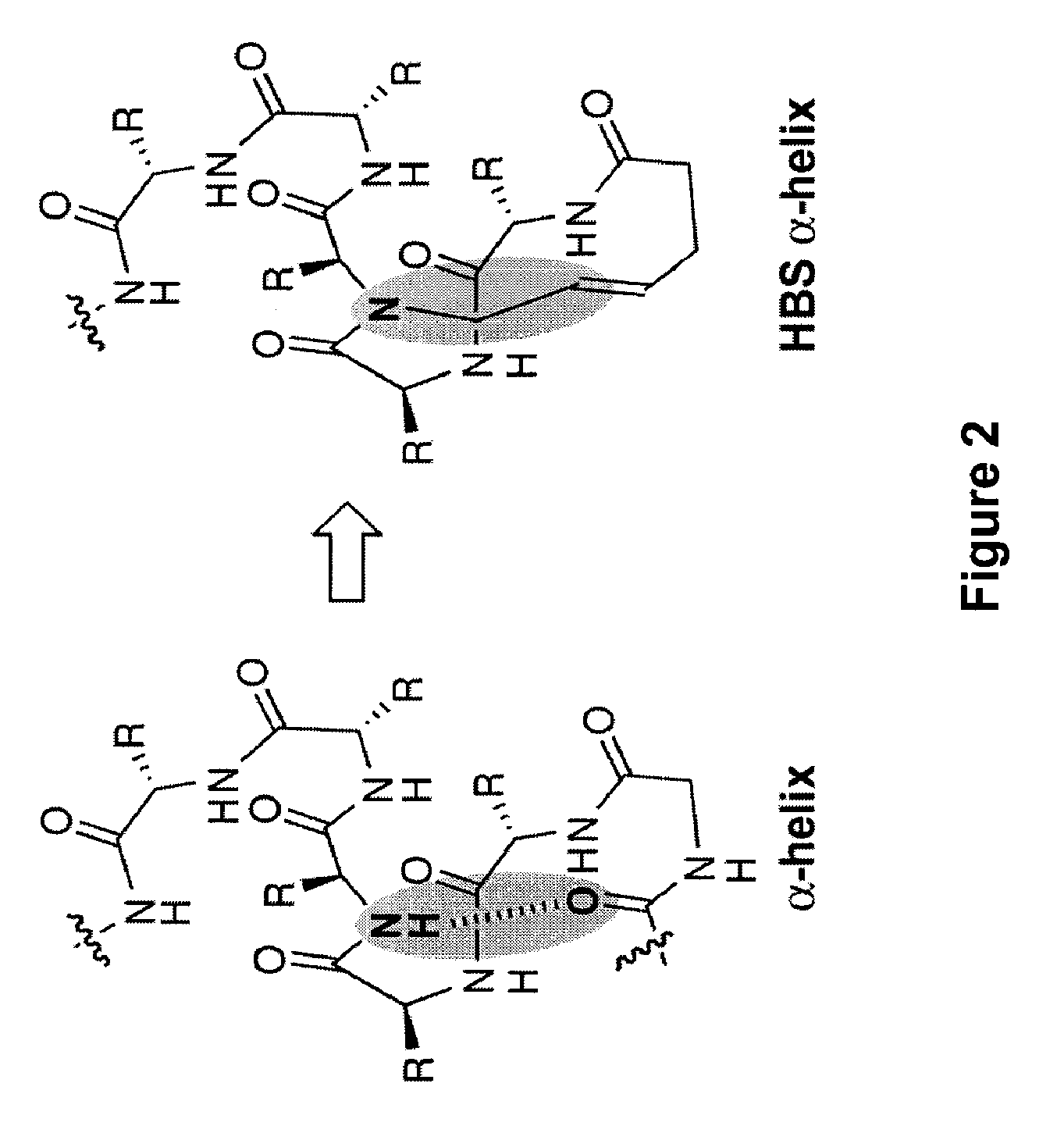
Control of viral-host membrane fusion with hydrogen bond surrogate-based artificial helices
The present invention relates to a peptide having one or more stable, internally-constrained HBS α-helices, where the peptide mimics at least a portion of a class I C-peptide helix or at least a portion of a class I N-peptide helix of a viral (e.g., HIV-I) coiled-coil assembly. Methods of inhibiting viral infectivity of a subject by administering these peptides are also disclosed.
Owner:NEW YORK UNIV
Stabilized soluble glycoprotein trimers
InactiveUS20050106177A1Stabilize trimerSimplifies isolationPeptide/protein ingredientsAntibody mimetics/scaffoldsHiv envelopeGp41
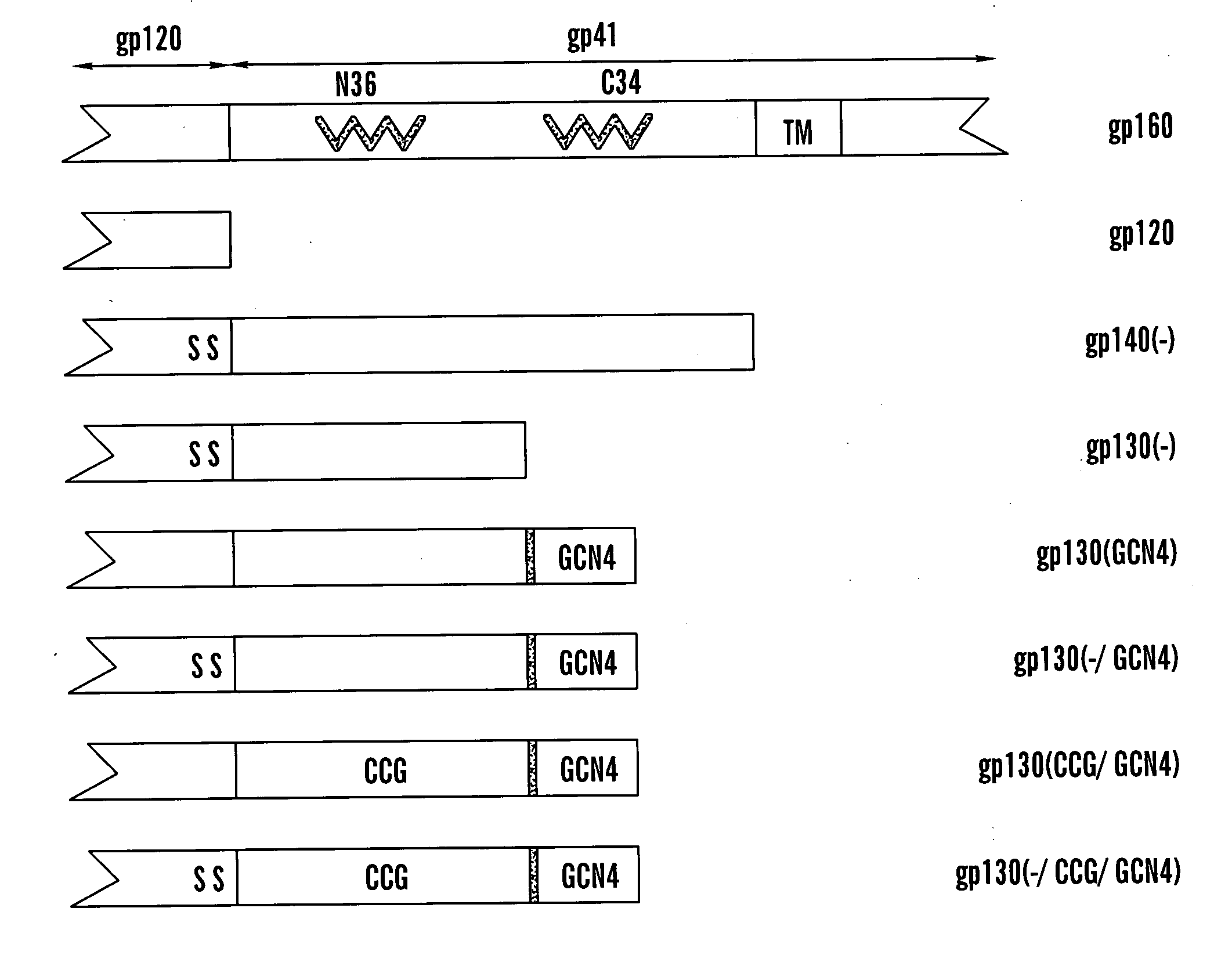
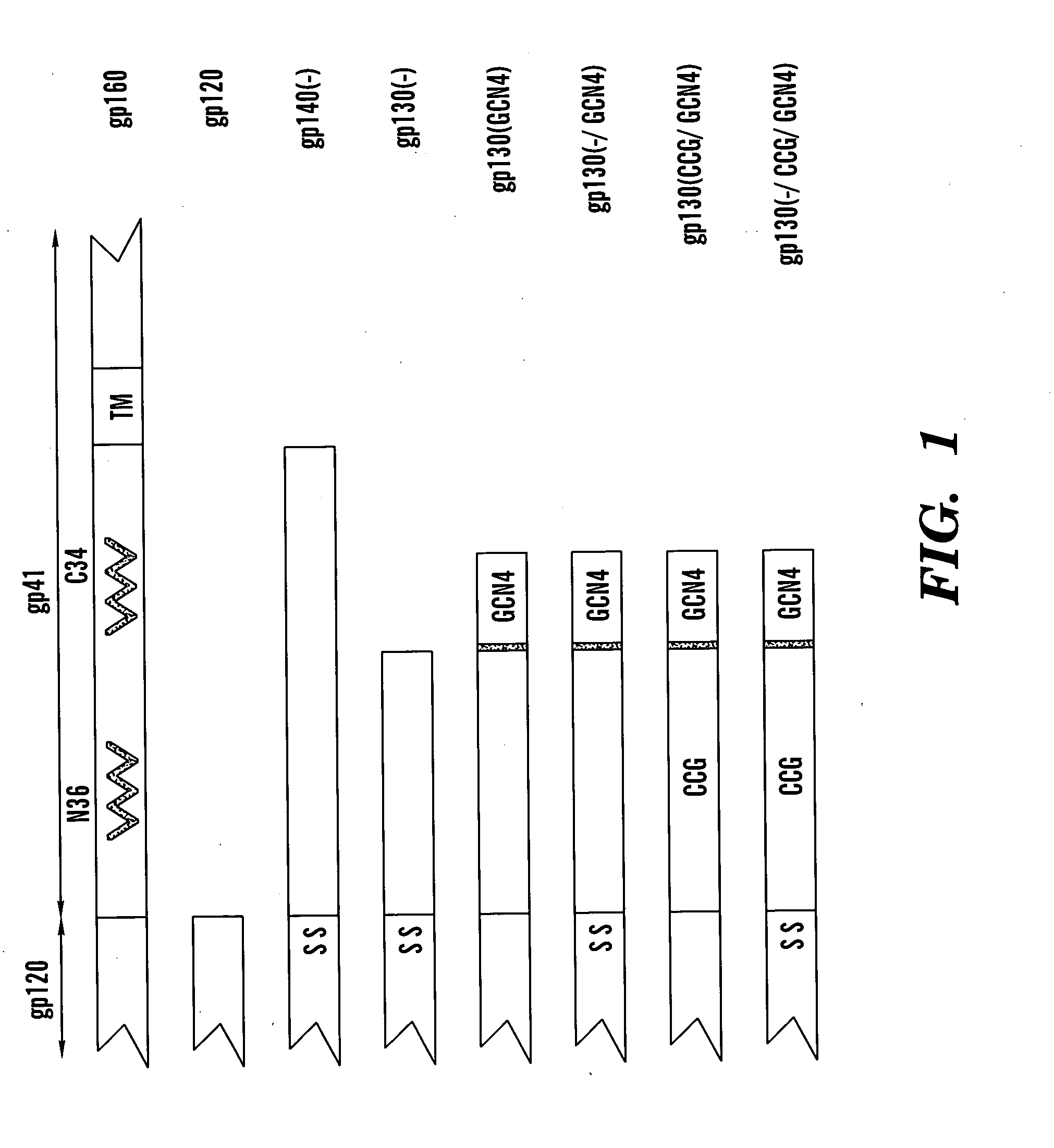
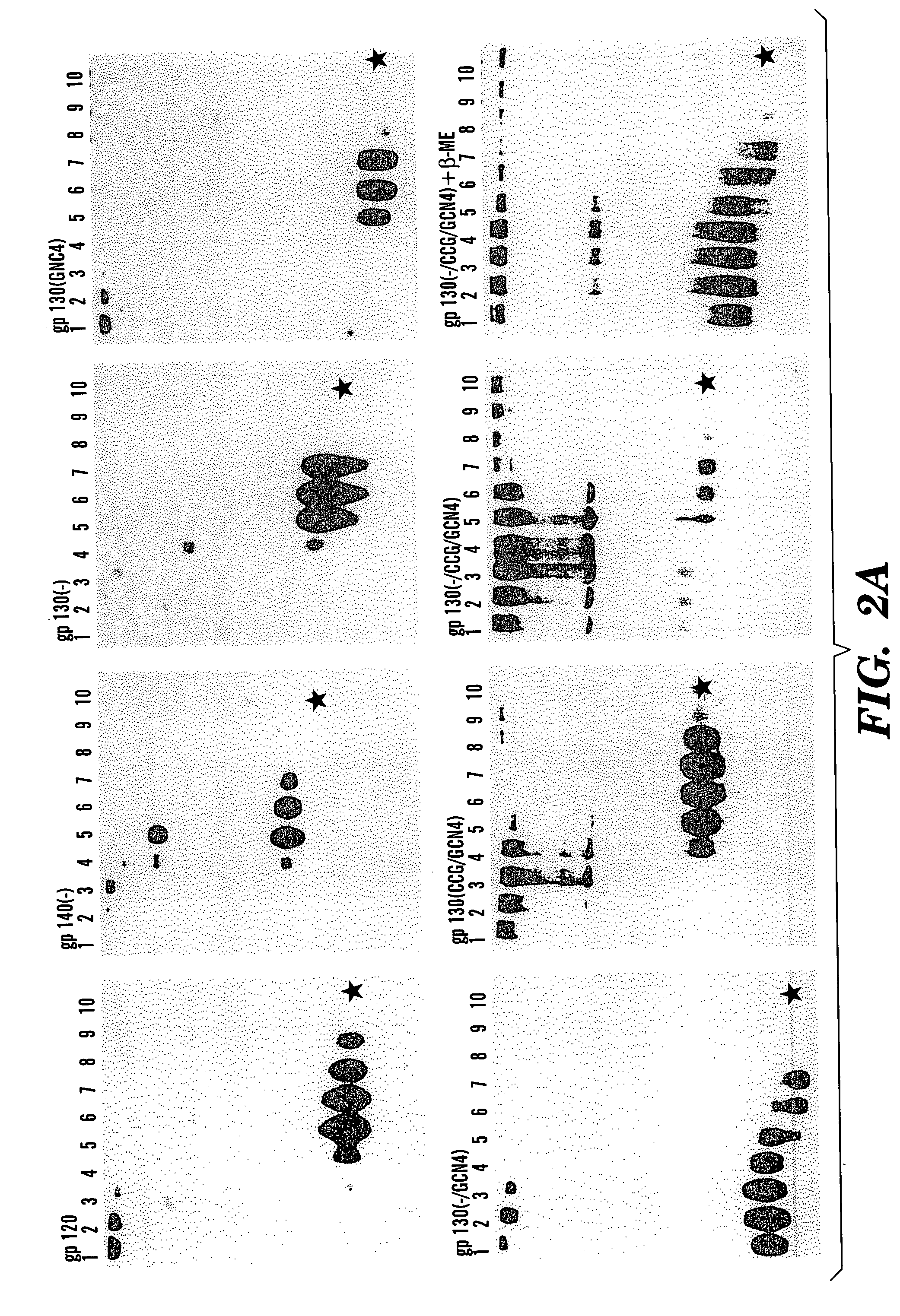
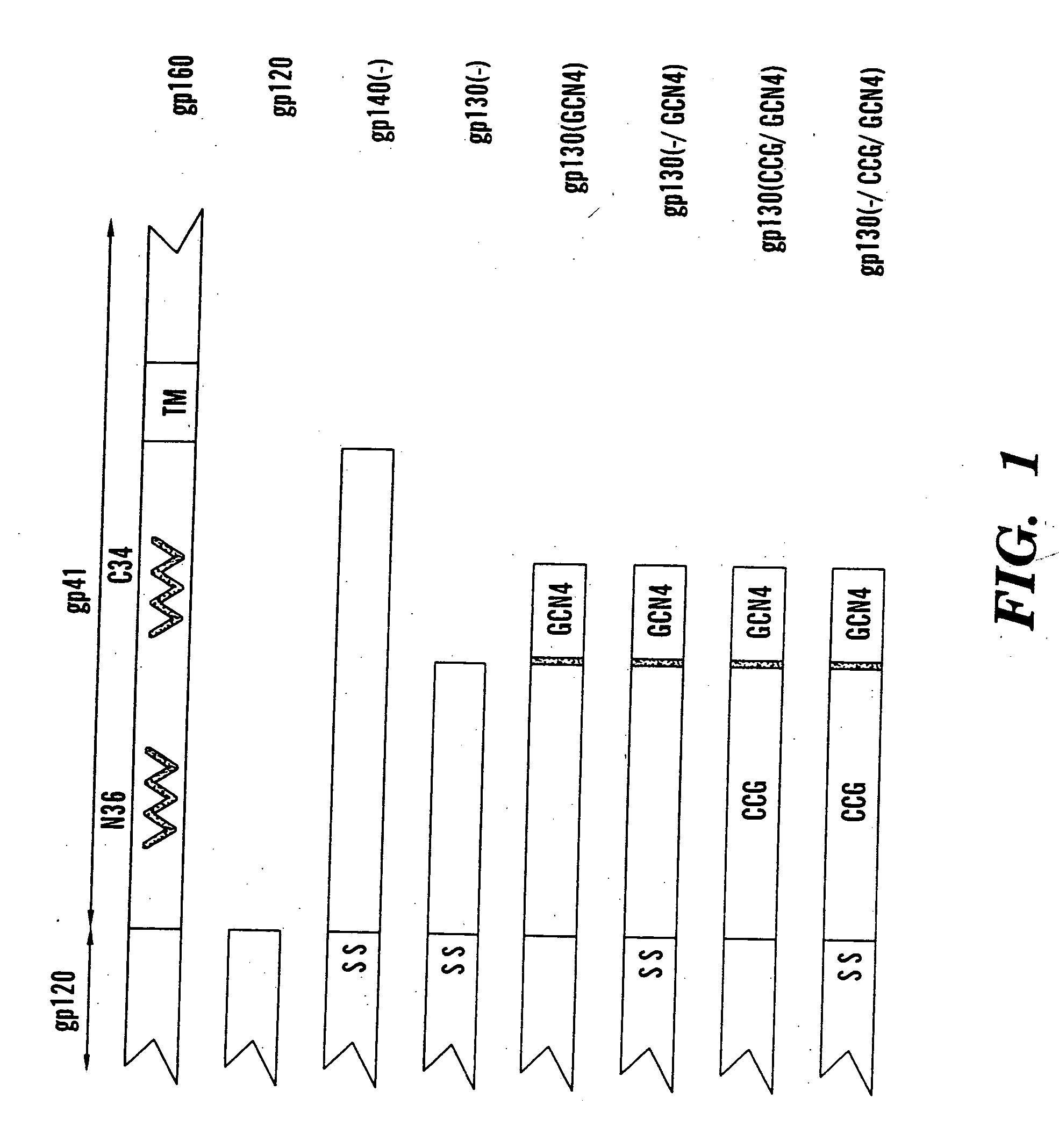
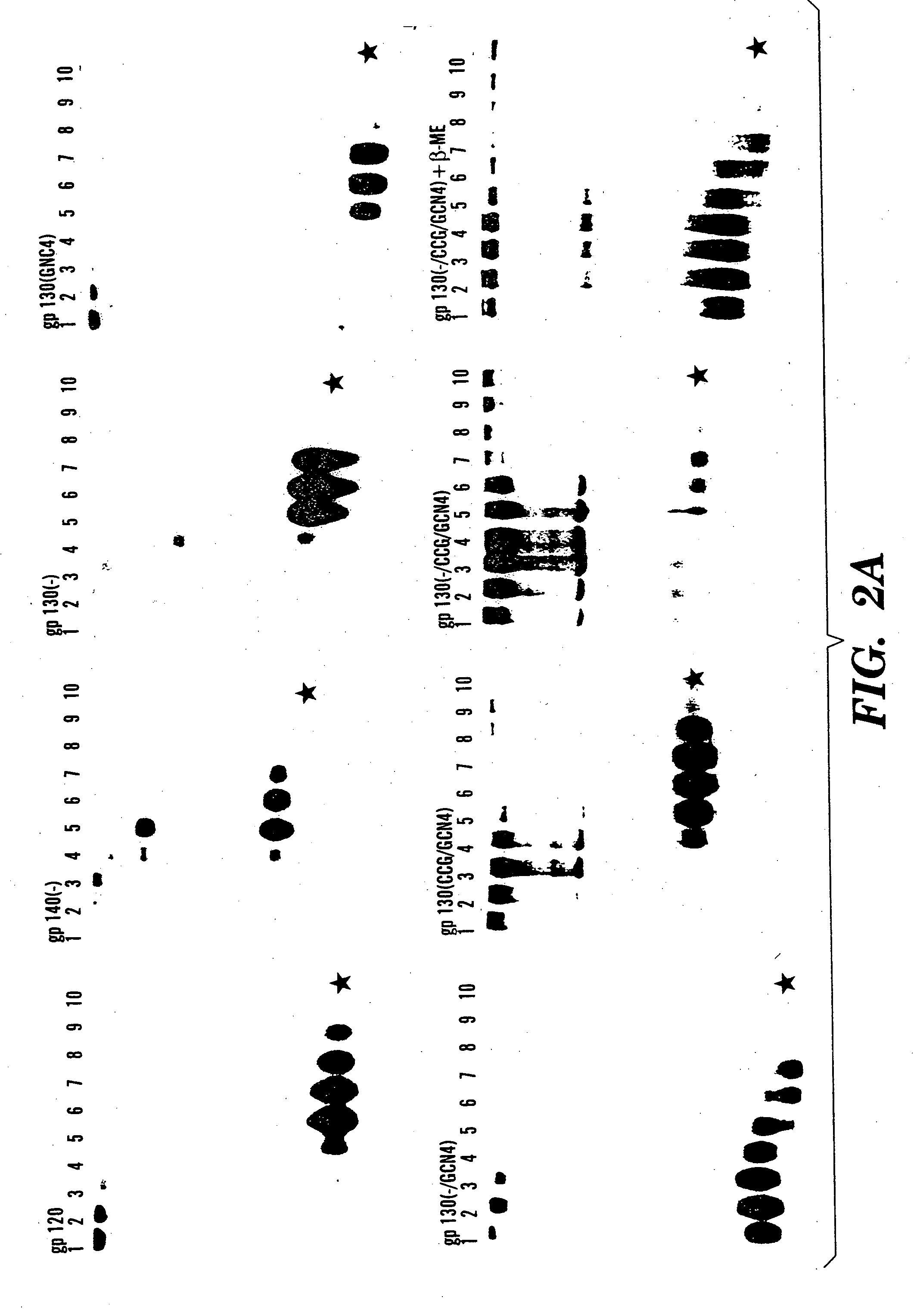
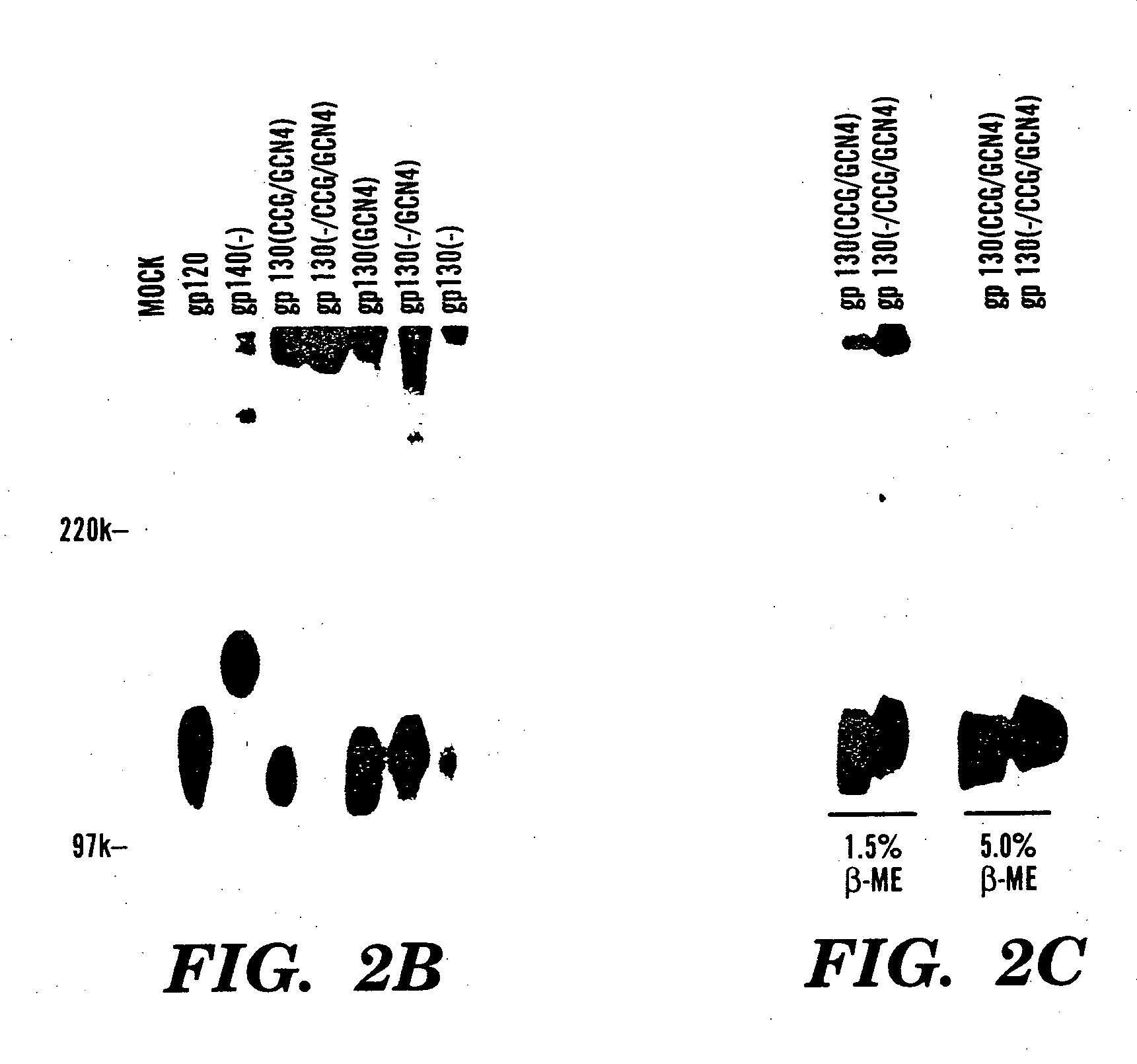
The present application is directed to stabilized HIV envelope glycoprotein trimers. The trimers are stabilized by introducing trimeric motifs, preferably the GCN4 coiled coil or the fibritin trimeric domain, at certain sites, for example in the gp41 ectodomain. These stabilized trimers or DNA molecules encoding such trimers can be used to generate an immunogenic reaction. The trimers can also be used in assays to screen for molecules that interact with them—and to identify molecules that interact with specific sites.
Owner:THE TRUSTEES OF COLUMBIA UNIV IN THE CITY OF NEW YORK
Methods for the inhibition of respiratory syncytial virus transmission
Fusion of the viral envelope, or infected cell membranes with uninfected cell membranes, is an essential step in the viral life cycle. Recent studies involving the human immunodeficiency virus type 1 (HIV-1) demonstrated that synthetic peptides (designated DP-107 and DP-178) derived from potential helical regions of the transmembrane (TM) protein, gp41, were potent inhibitors of viral fusion and infection. A computerized antiviral searching technology (C.A.S.T.) that detects related structural motifs (e.g., ALLMOTI5, 107x178x4, and PLZIP) in other viral proteins was employed to identify similar regions in the respiratory syncytial virus (RSV). Several conserved heptad repeat domains that are predicted to form coiled-coil structures with antiviral activity were identified in the RSV genome. Synthetic peptides of 16 to 39 amino acids derived from these regions were prepared and their antiviral activities assessed in a suitable in vitro screening assay. These peptides proved to be potent inhibitors of RSV fusion. Based upon their structural and functional equivalence to the known HIV-1 inhibitors DP-107 and DP-178, these peptides should provide a novel approach to the development of targeted therapies for the treatment of RSV infections.
Owner:TRIMERIS
Coiled-coil fusion proteins comprising cell receptor domains
Fusion proteins and coiled-coil induced dimers prepared from both the ectodomains and the kinase domains are disclosed. The receptor domains when presented in the form of a homodimer or heterodimer by virtue of the coiled-coil tag have enhanced ligand binding activity or enhanced kinase activity. The kinetics of binding and the antagonistic potencies of the ectodomain dimers, and their use to alter or inhibit signaling is described. Application of the ectodomain and kinase domain dimers in assays for selecting compounds capable of inhibiting ligand binding and kinase activity, respectively, is described.
Owner:NAT RES COUNCIL OF CANADA
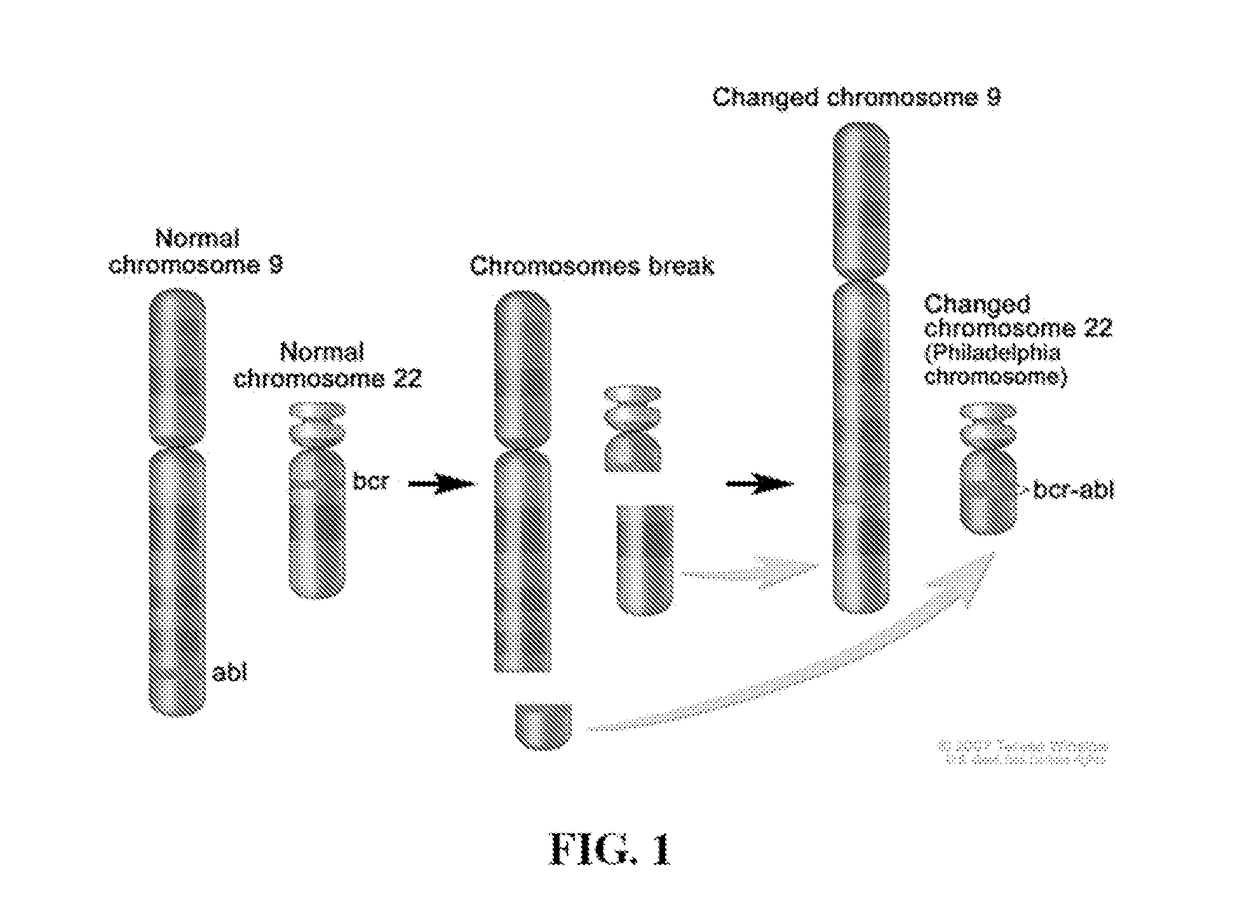
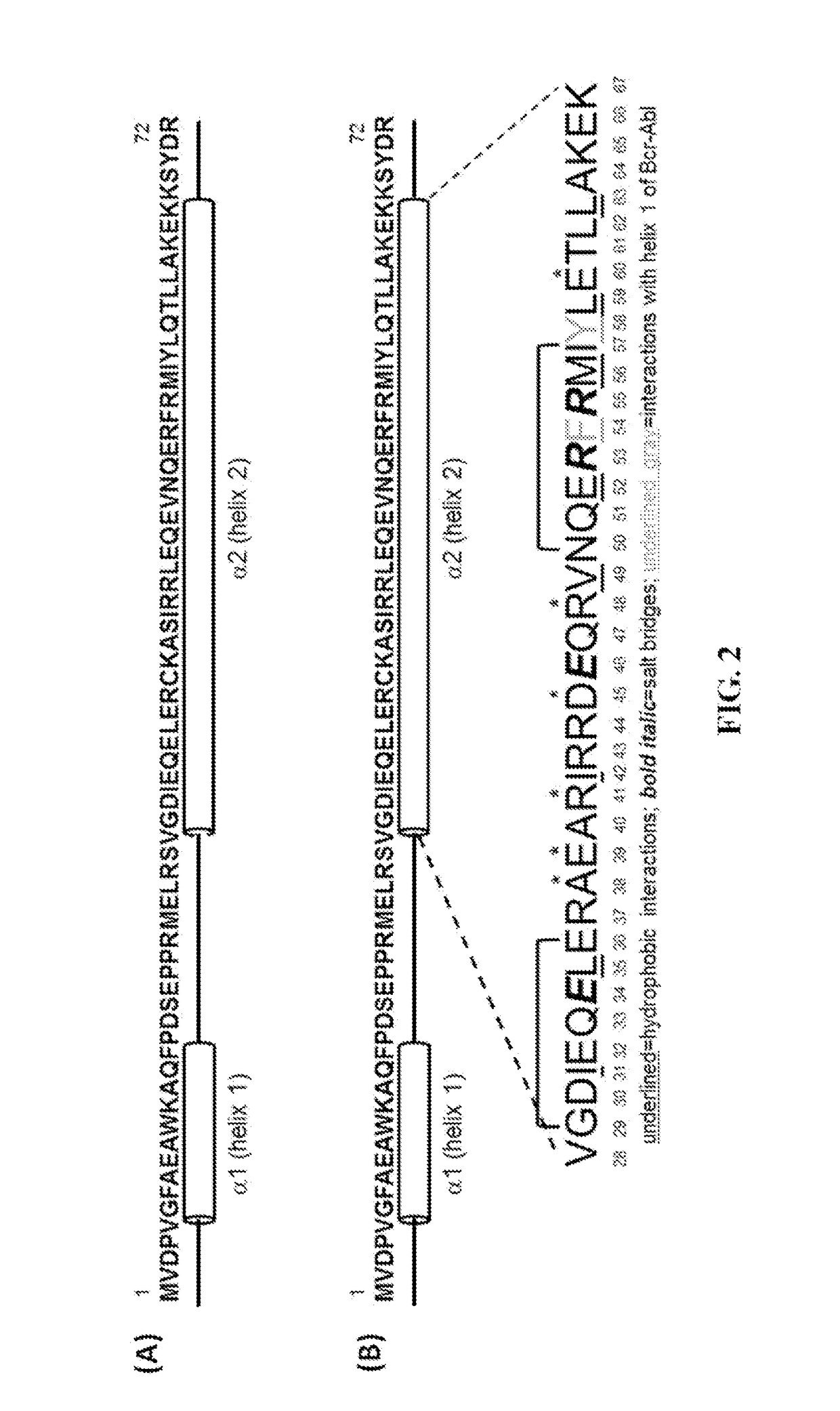
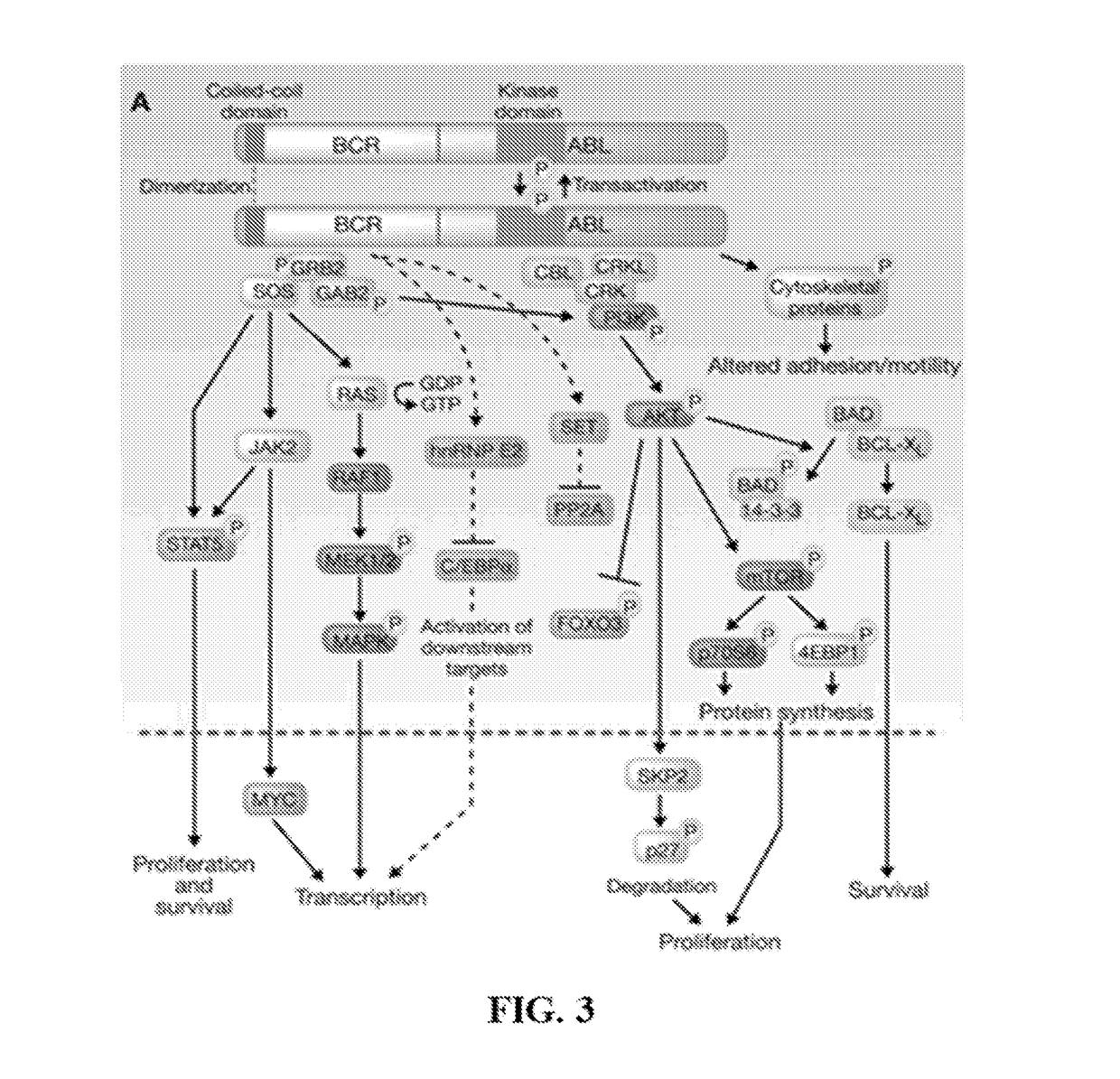
Peptide Inhibitors of BCR-ABL Oligomerization
In one aspect, the invention relates to peptides comprising the Bcr-Abl coiled-coil oligomerization domain and an alpha helix stabilizing moiety, mutant forms thereof, truncated forms thereof, derivatives thereof, and related peptides, which are useful as inhibitors of the Bcr-Abl chimeric protein; pharmaceutical compositions comprising the compounds; and methods of treating hyperproliferative disorders associated with Bcr-Abl using the compounds and compositions. This abstract is intended as a scanning tool for purposes of searching in the particular art and is not intended to be limiting of the present invention.
Owner:UNIV OF UTAH RES FOUND
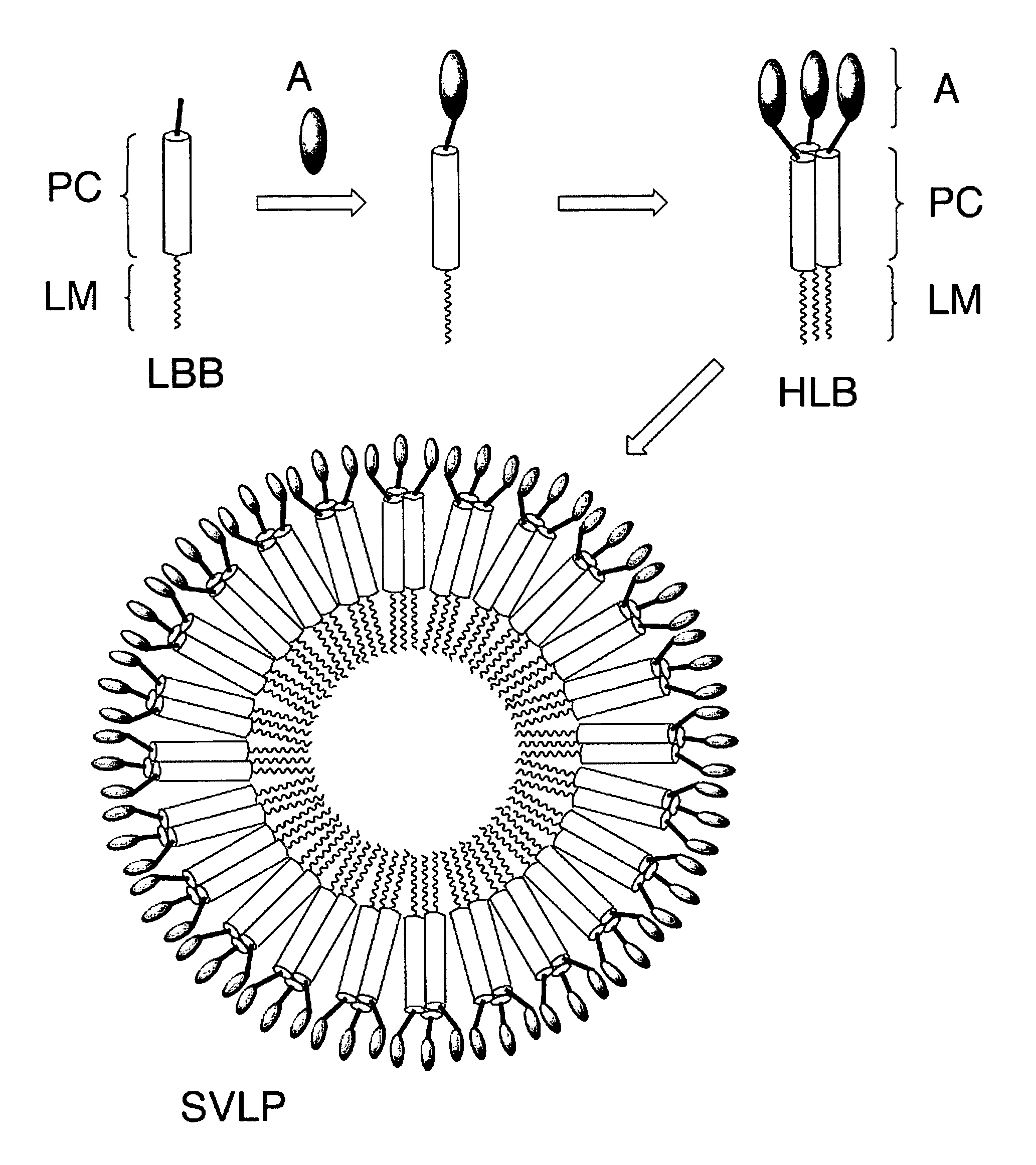
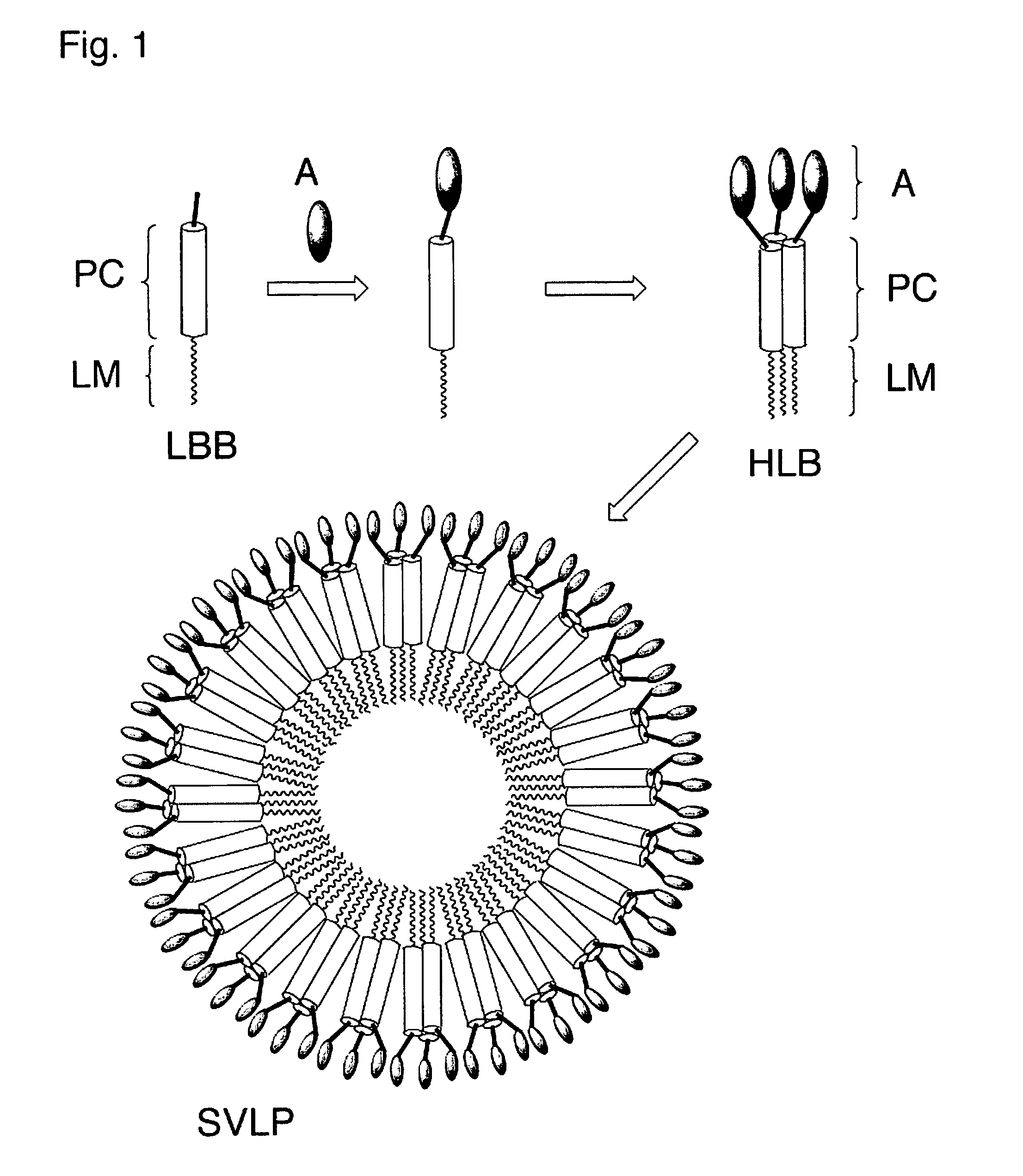
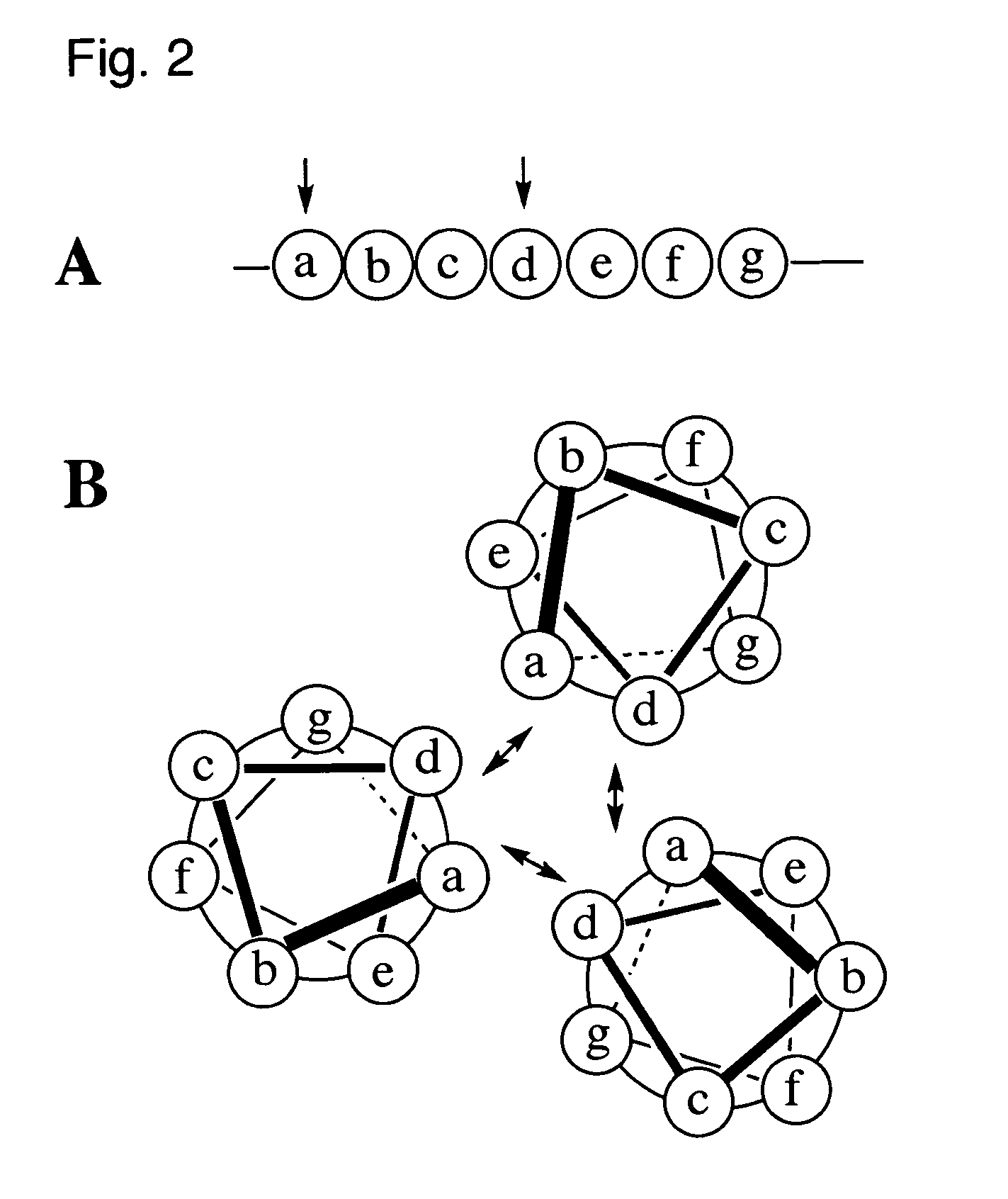
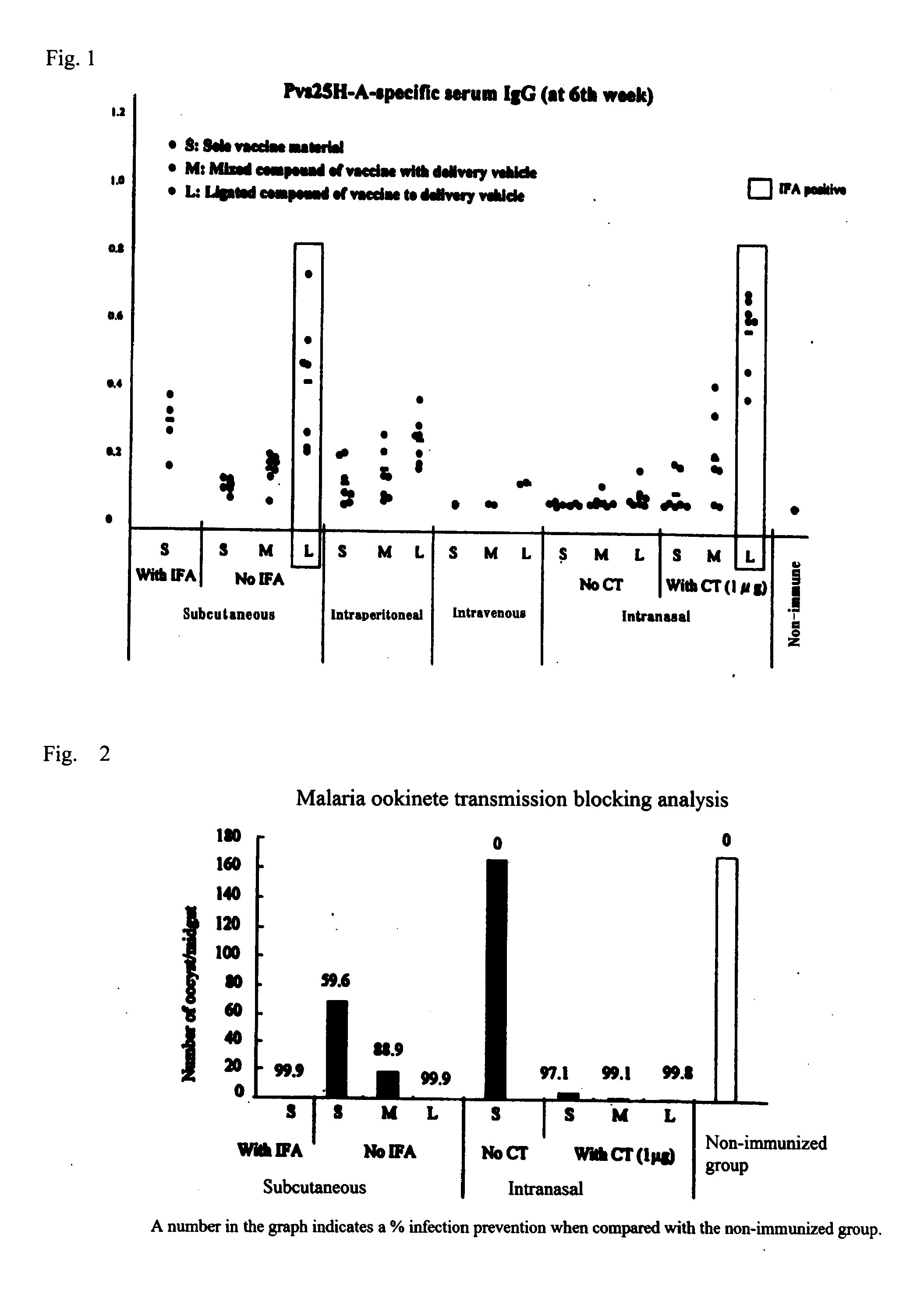
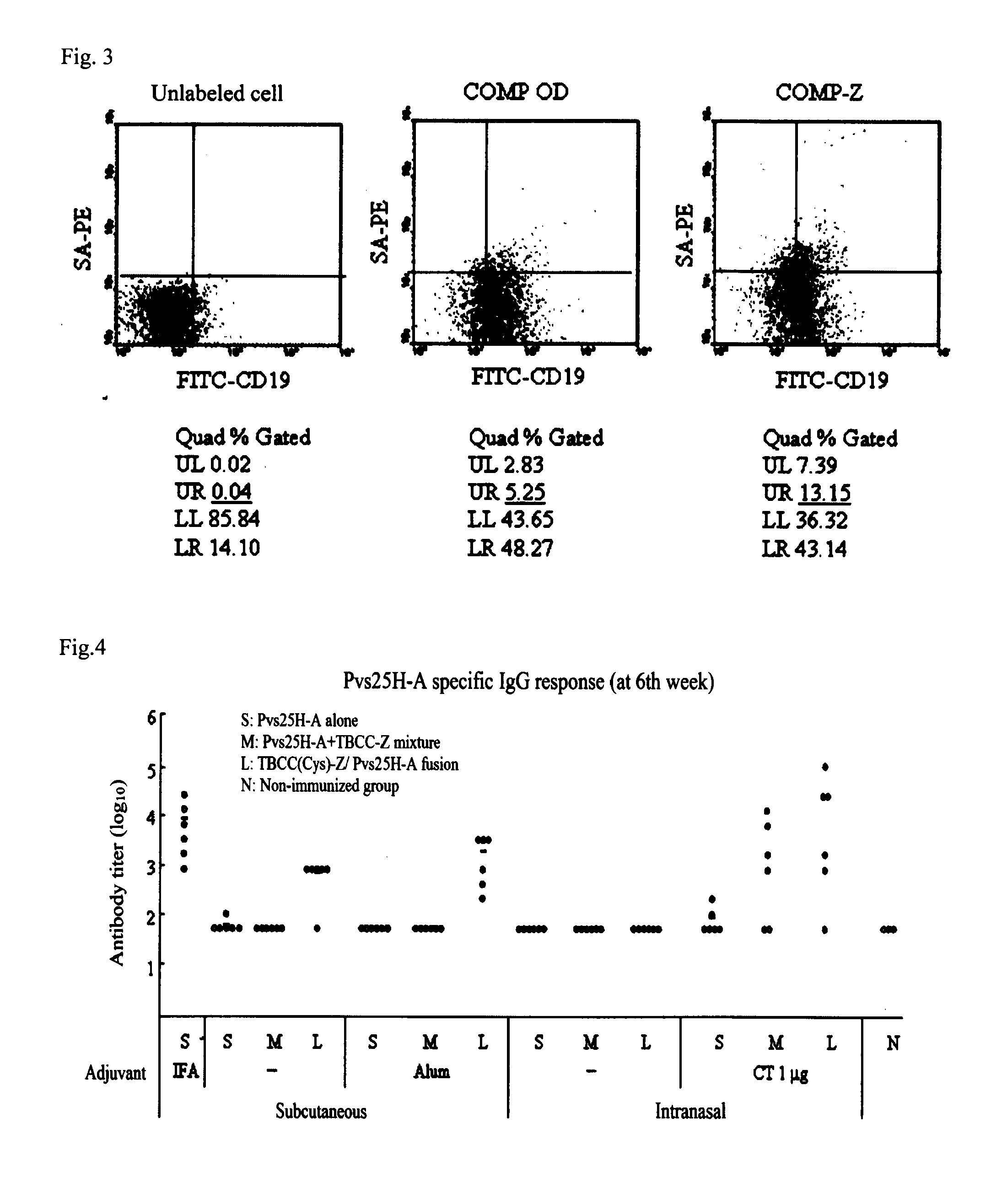
Coiled-coil lipopeptide helical bundles and synthetic virus-like particles
ActiveUS20100015173A1Efficient inductionVirus peptidesSaccharide peptide ingredientsChemical synthesisCoiled coil
The invention relates to lipopeptide building blocks consisting of a peptide chain comprising a coiled-coil domain, linked covalently to a lipid moiety comprising long alkyl or alkenyl chains, and optionally linked to an antigen; and to helical lipopeptide bundles and synthetic virus-like particles formed by aggregation. The nanometer size and shape of these bundles and particles, their stability under aqueous physiological conditions, their chemical composition, the possibility to incorporate B- and T-cell epitopes, and their production by chemical synthesis, make them highly suitable as vaccine delivery vehicles.
Owner:UNIV ZURICH
Drug transporter, and adjuvant and vaccine each utilizing same
InactiveUS20120100165A1Good effectImproving immunogenicityAntibody mimetics/scaffoldsAntiviralsAdjuvantImmunogenicity
An objective of the invention is to provide a drug delivery vehicle capable of allowing a vaccine or adjuvant to reach a target cell or tissue efficiently while being capable of improving the immunogenicity of the vaccine or capable of enhancing the immunostimulating effect of the adjuvant as well as a vaccine or adjuvant utilizing the same. Said drug delivery vehicle contains a multimeric protein having a coiled coil structure and a ligand molecule to a receptor of an immune cell.
Owner:UNIVERSITY OF THE RYUKYUS
Soluble T cell receptor
The present invention relates to a recombinant soluble T cell receptor. The T cell receptor (TCR) is refolded and comprises a recombinant TCR α or γ chain extracellular domain having a first heterologous C-terminal dimerization peptide; and a recombinant TCR β or δ chain extracellular domain having a second C-terminal dimerization peptide which is specifically heterodimerized with the first dimerization peptide to form a heterodimerization domain, which may be a coiled coil domain. The invention also provides nucleic acid sequences encoding the recombinant TCR and a method for producing the recombinant TCR. The TCR may be labelled with a detectable label so as to enable the detection of specific MHC-peptide complexes. Alternatively, it can be linked to a therapeutic agent such as a cytotoxic agent or an immunostimulating agent so as to deliver such an agent to the site of a specific MHC-peptide complex.
Owner:AVIDEX LTD
MG53 (mitsugumin53) mutant as well as preparation method and application thereof
The invention provides an MG53 (mitsugumin53) mutant and a preparation method thereof. The mutant is a serine-site mutant of MG53 protein in a Coiled-coil structural domain, and the mutant results in inactivation of MG53 phosphorylation. Meanwhile, the MG53 protein mutant can be used for preventing or treating diseases related to cellular membrane damage, such as diseases related to myocardial cell injury, diseases related to ulcer, trauma with a wound, particularly a difficult-to-heal wound, intestinal leakage, kidney injury and the like. The diseases related to myocardial cell injury can include one or more diseases related to following symptoms: myocardial ischemia, heart ischemia, heart ischemia / reperfusion injury, myocardial infarction, cardiac failure, arrhythmia and cardiac rupture. The diseases related to ulcer include one or more of following diseases: chronic ulcer, peptic ulcer, diabetic foot ulcer, diabetic foot gangrene and chronic gastric ulcer. Besides, the invention further provides a dry powder preparation, a spray preparation, a gel preparation and an emulsion containing MG53 or MG53 mutant as well as preparation methods of the preparations.
Owner:MUDANJIANG YOUBO PHARMA CO LTD
Coiled-coil fusion proteins comprising cell receptor domains
Fusion proteins and coiled-coil induced dimers prepared from both the ectodomains and the kinase domains are disclosed. The receptor domains when presented in the form of a homodimer or heterodimer by virtue of the coiled-coil tag have enhanced ligand binding activity or enhanced kinase activity. The kinetics of binding and the antagonistic potencies of the ectodomain dimers, and their use to alter or inhibit signaling is described. Application of the ectodomain and kinase domain dimers in assays for selecting compounds capable of inhibiting ligand binding and kinase activity, respectively, is described.
Owner:NAT RES COUNCIL OF CANADA
Stabilized soluble glycoprotein trimers
InactiveUS20050220817A1Stabilize trimerSimplifies isolationAntibody mimetics/scaffoldsVirus peptidesHiv envelopeGp41
The present application is directed to stabilized HIV envelope glycoprotein trimers. The trimers are stabilized by introducing trimeric motifs, preferably the GCN4 coiled coil or the fibritin trimeric domain, at certain sites, for example in the gp41 ectodomain. These stabilized trimers or DNA molecules encoding such trimers can be used to generate an immunogenic reaction. The trimers can also be used in assays to screen for molecules that interact with them—and to identify molecules that interact with specific sites.
Owner:DANA FARBER CANCER INST INC
Inhibitors of HIV membrane fusion
InactiveUS20050221294A1Improve stabilityImprove solubilityCompound screeningApoptosis detectionCoiled coilMembrane configuration
Owner:WHITEHEAD INST FOR BIOMEDICAL RES
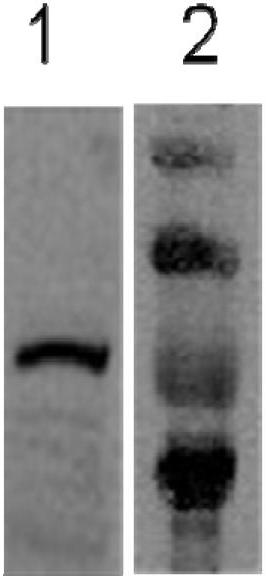
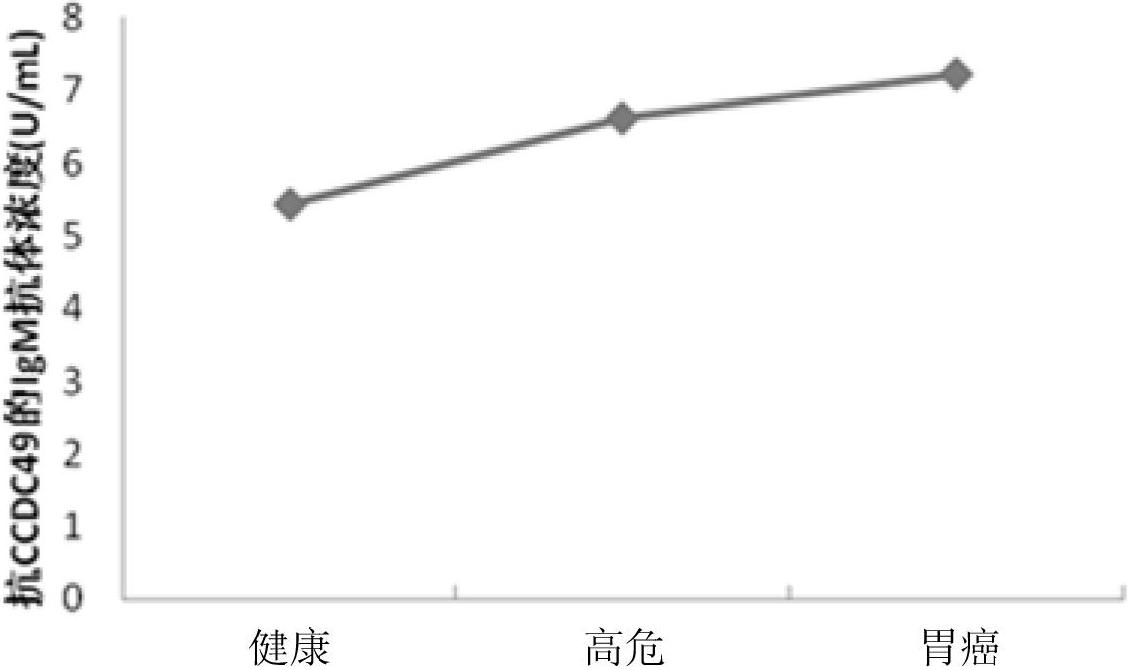
Diagnostic kit and application of coiled-coil domain containing protein 49 (CCDC49) in preparing gastric cancer early-stage diagnostic reagent
The invention relates to the field of the biological science, in particular to a diagnostic kit and an application of coiled-coil domain containing protein 49 (CCDC49) in preparing a gastric cancer early-stage diagnostic reagent. The diagnostic kit is used for diagnosing the early-stage gastric cancer. The diagnostic kit comprises an elisa plate, human protein CCDC49, standard serum, an enzyme labeled reagent, enzyme substrate solution, confining liquid, sample diluent, detergent liquid and termination liquid, wherein the human protein CCDC49 is wrapped on the elisa plate. Compared with the prior art, the diagnostic kit has advantages that 1, a commodity diagnostic kit which is sensitive, safe, reliable and easy to operate is provided, the immunoglobulin m (IgM) antibody for resisting the protein CCDC49 in the human serum can be qualitatively determined, and the early diagnosis of the gastric cancer can be assisted; and 2, the specificity of the provided serum biological labeled protein CCDC49 is 81 percent, the sensitivity is 82 percent, and the characteristics of high specificity and high sensitivity can be realized.
Owner:SHANGHAI JIAO TONG UNIV
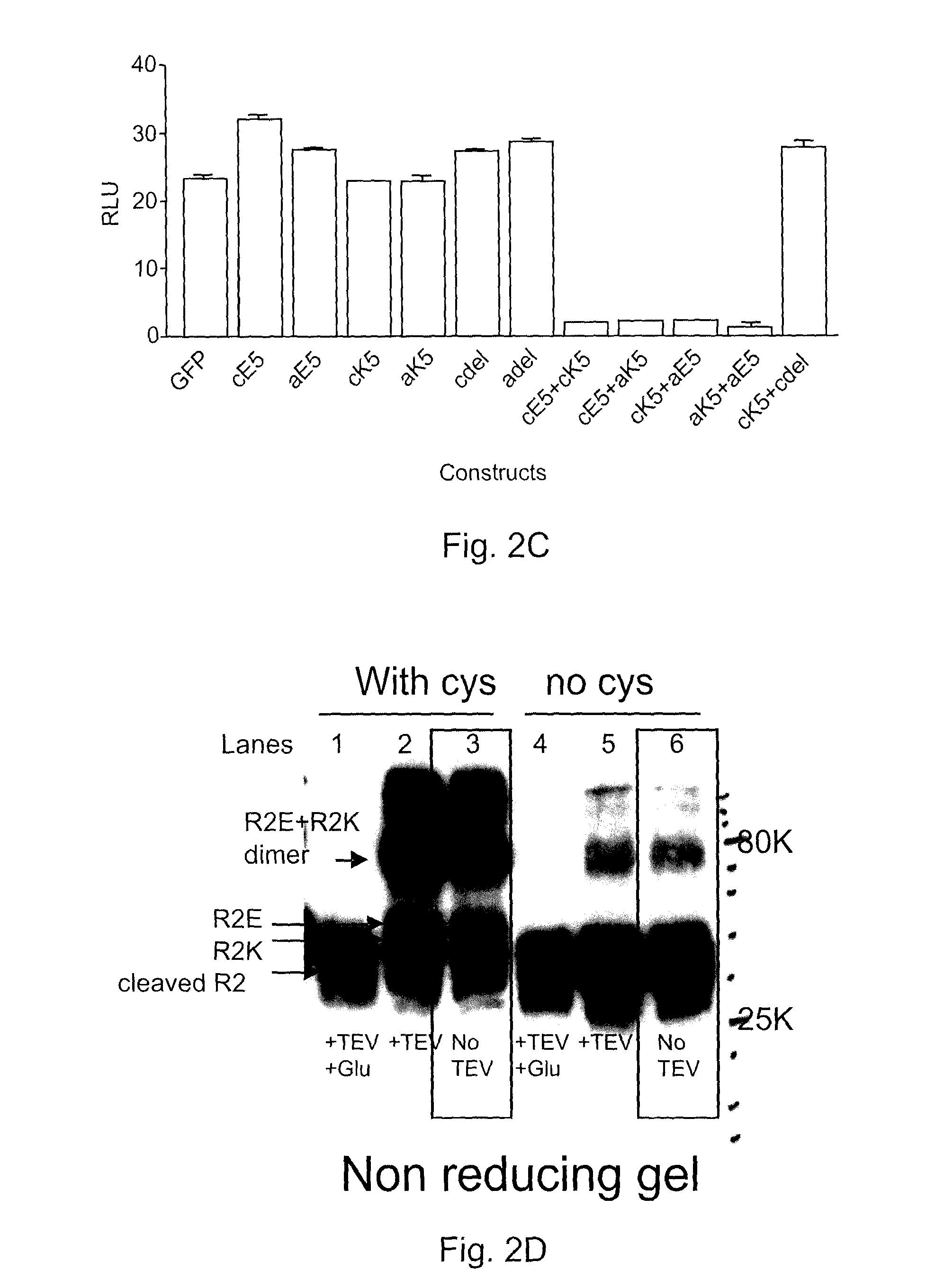
Covalently dimerized bivalent binding agents
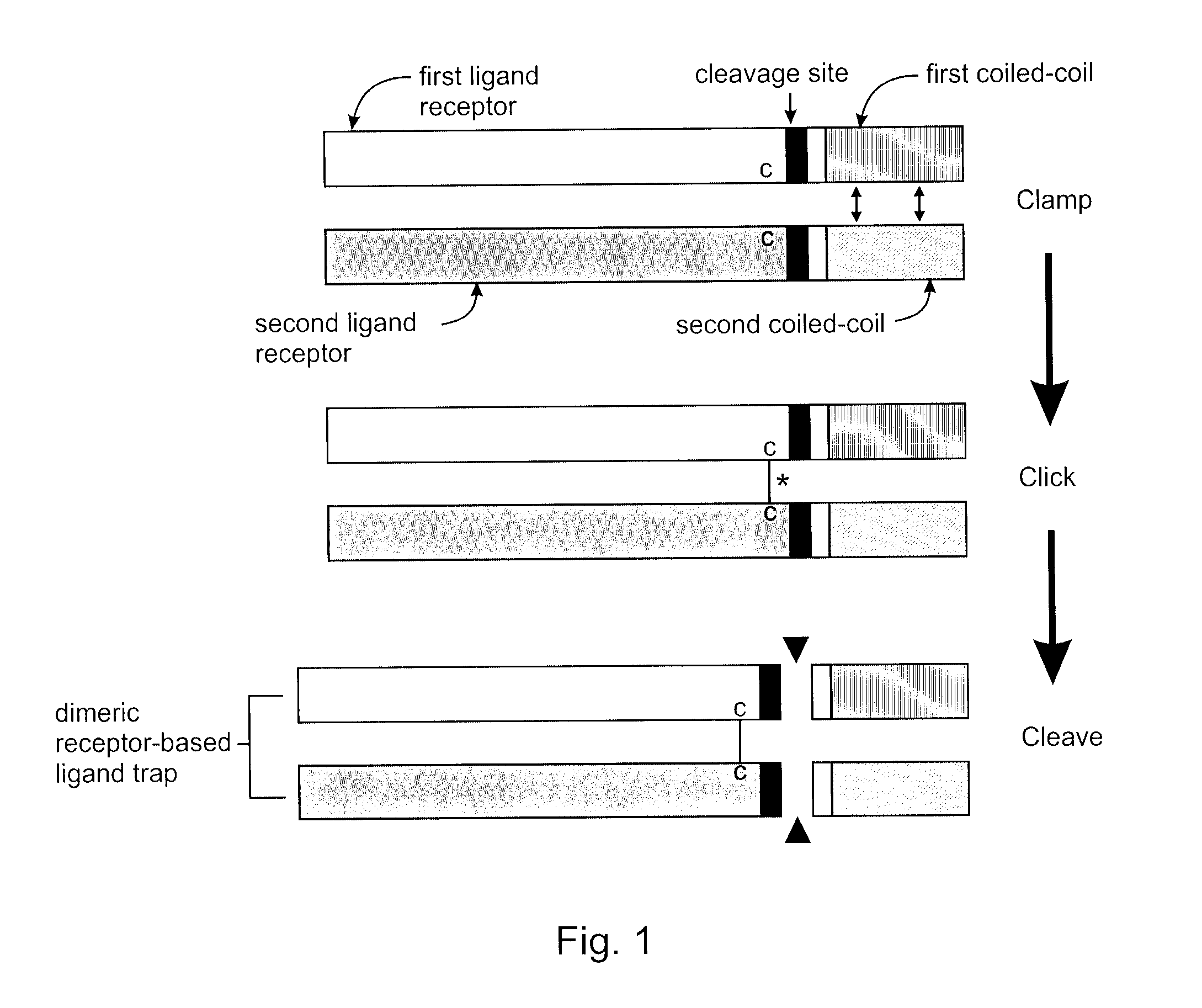
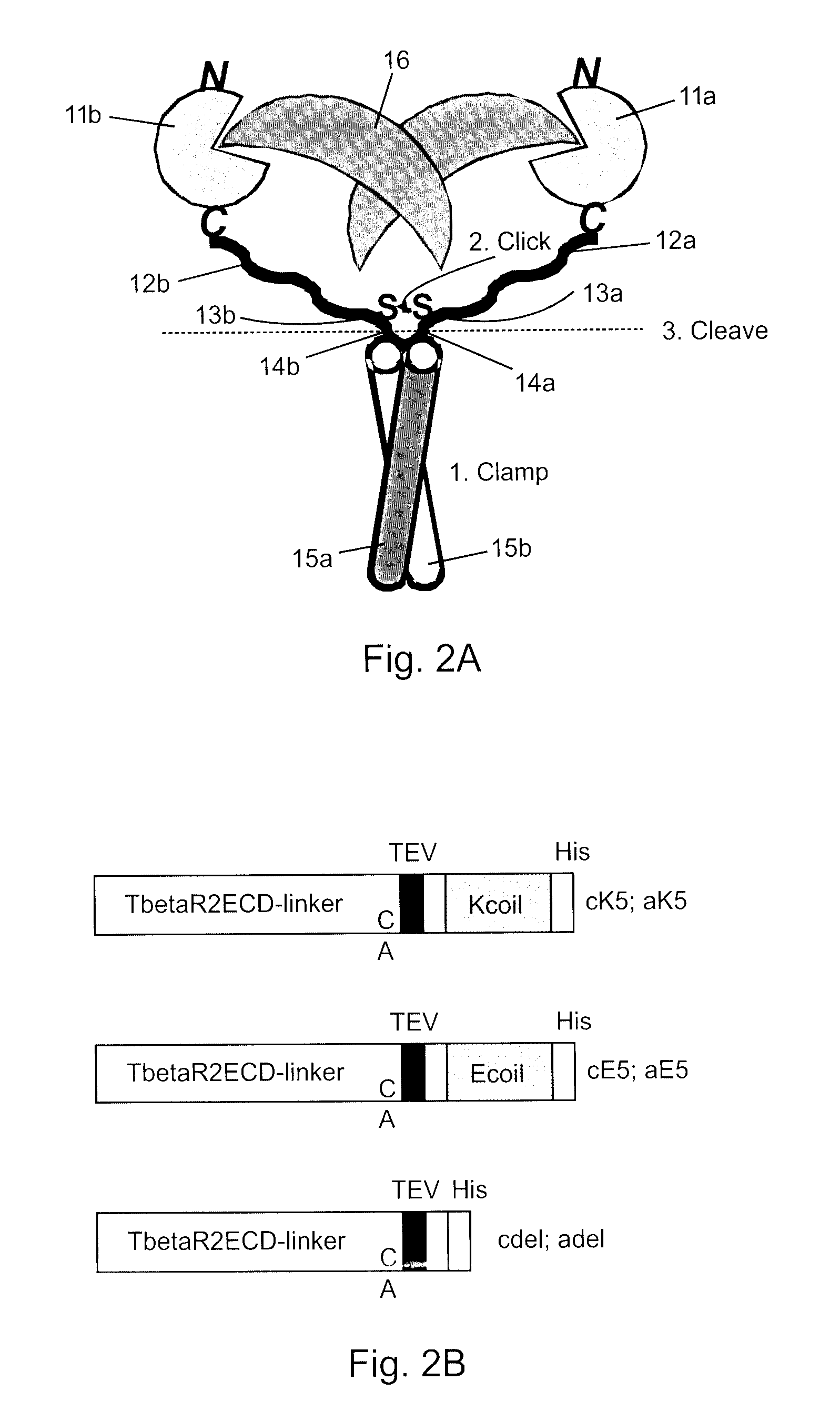
The present invention addresses limitations of prior art receptor-based traps through a methodology called the clamp / click / cleave (CCC) approach. Two fusion proteins each comprising a binding domain fused to a coiled-coil are non-covalently dimerized through the coiled-coil (clamp), and the dimer so formed is stabilized by a covalent disulphide bond (click) between cysteine residues located on the fusion proteins between the binding domains and coiled-coils. Once the disulphide bond has formed, the coiled-coils are subsequently removed (cleave) by cleaving the fusions proteins at cleavage sites located between the cysteine residues and the coiled-coils to provide the covalently dimerized bivalent binding agent of the present invention. Such binding agents are useful in the treatment and diagnosis of disease states characterized by production and / or overexpression of a ligand to which the binding domains bind. The invention is particularly useful for covalently dimerized receptor-based ligand traps where the binding domains are receptor ligand-binding domains, such as those of TGF-β receptors.
Owner:NAT RES COUNCIL OF CANADA
Mitofusins, Fzo Homologs and functional derivatives thereof
Mitofusin genes and encoded polypeptides are provided, including the Drosophila Fzo protein and its homologs from insects, other invertebrates, yeast, and vertebrates including mouse and humans. Mitofusins are large predicted GTPases with a predicted trans-membrane domain, coiled-coil regions, and a C-terminal region showing a high pI. The mitofusins are the first known protein mediator of mitochondrial fusion, and mediate developmentally regulated post-meiotic fusion of mitochondria. Missense mutations that alter conserved residues required for GTP binding in other GTPases inhibit the in vivo fusogenic activity of Fzo but do not affect its localization. Fusion proteins having amino acid sequences from mitofusin transmembrane regions localize to mitochondria. Mitofusins may be used in methods of identifying anti-insect and antifungal agents. Functional derivatives of mitofusins useful for such methods are described.
Owner:THE BOARD OF TRUSTEES OF THE LELAND STANFORD JUNIOR UNIV
Compounds for inhibition of HIV infection by blocking HIV entry
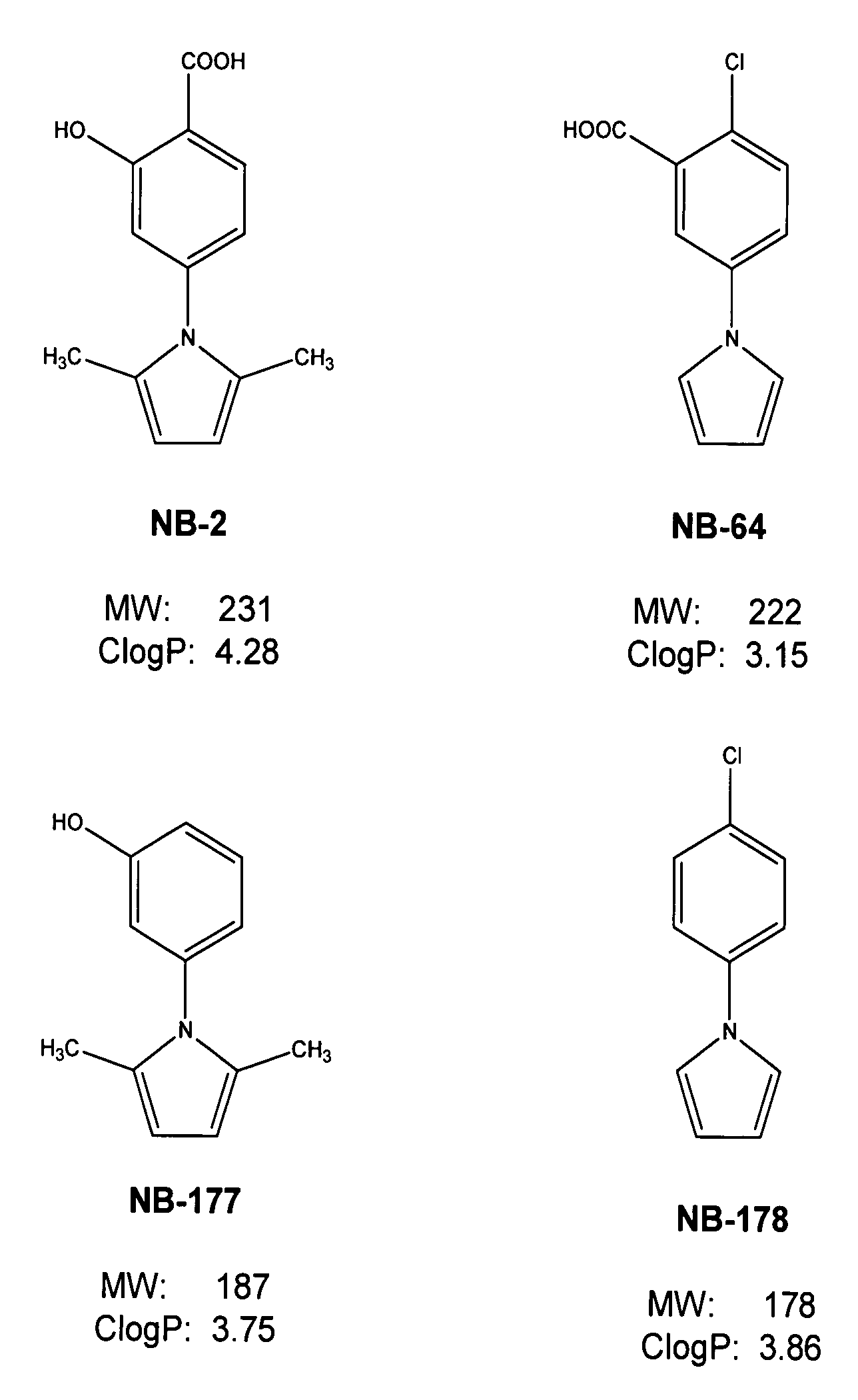
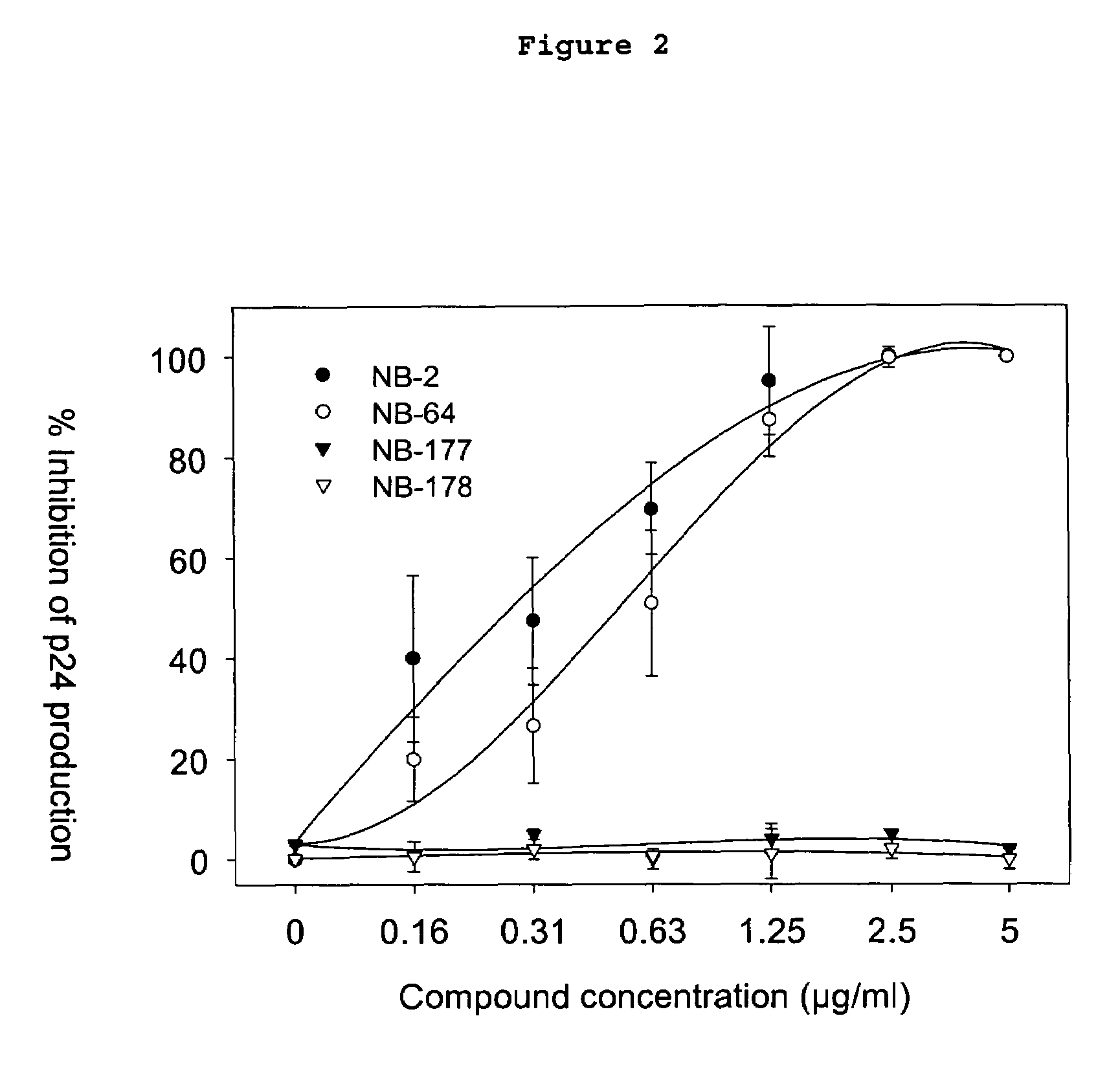
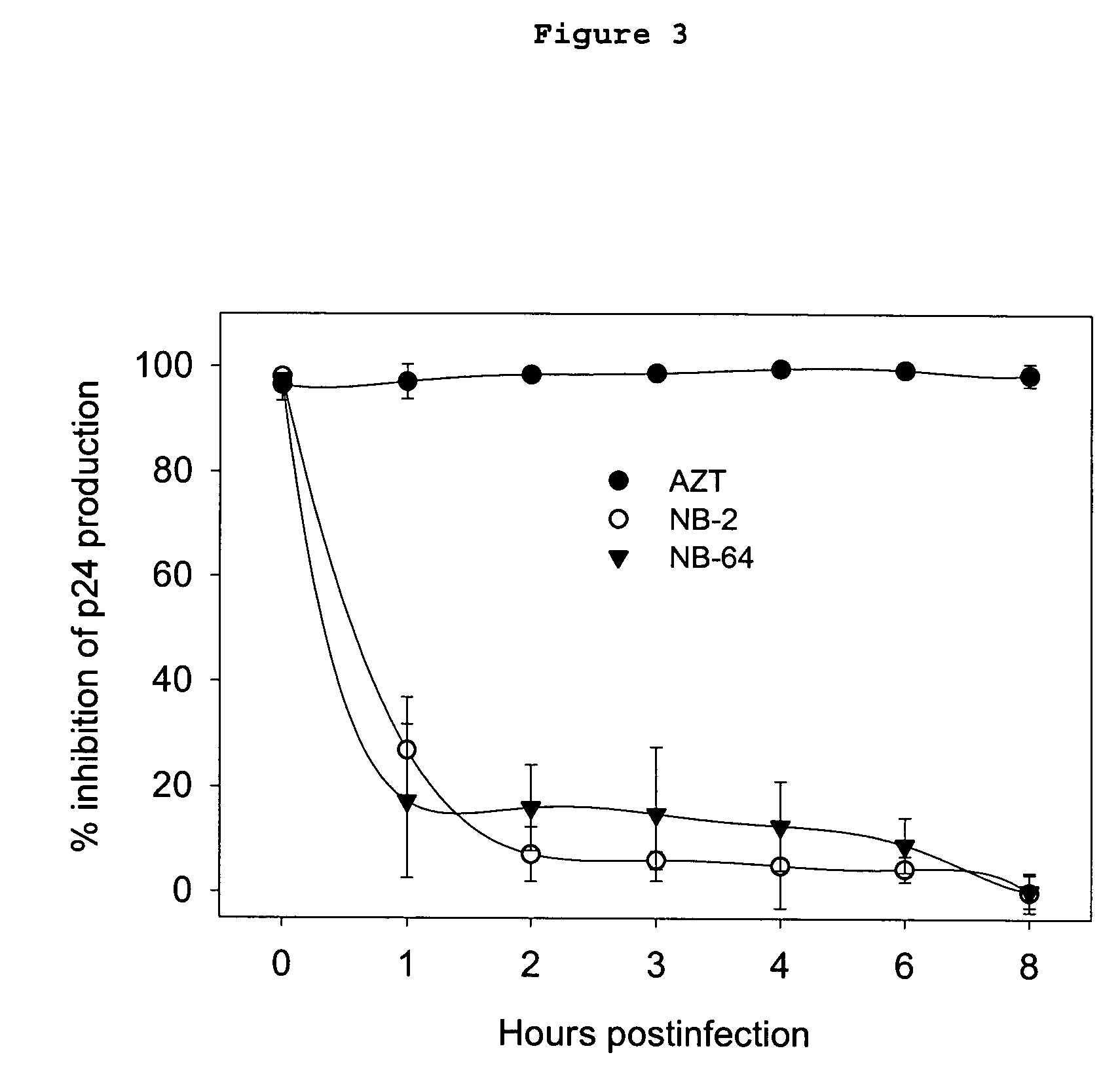
ActiveUS7241803B2Potent anti-HIV activityInhibit HIV replicationBiocideOrganic chemistryFluorescenceCoiled coil
A group of compounds that inhibit HIV replication by blocking HIV entry was identified. Two representative compounds, designated NB-2 and NB-64, inhibited HIV replication (p24 production) with IC50 values <0.5 μg / ml. It was proved that NB-2 and NB-64 are HIV entry inhibitors by targeting the HIV gp41 since: 1) they inhibited HIV-mediated cell fusion; 2) they inhibited HIV replication only when they were added to the cells less than one hour after virus addition; 3) they did not block the gp120-CD4 binding; 4) they did not interact with the coreceptor CXCR4 since they failed to block anti-CXCR4 antibody binding to CXCR4-expressing cells; 5) they blocked the formation of the gp41 core that is detected by sandwich enzyme linked immunosorbent assay (ELISA) using a conformation-specific MAb NC-1; 6) they inhibited the formation of the gp41 six-helix bundle revealed by fluorescence native-polyacrylamide gel electrophoresis (FN-PAGE); and 7) they blocked binding of D-peptide to the hydrophobic cavity within gp41 coiled coil domain, modeled by peptide IQN17. These results suggested that NB-2 and NB-64 may interact with the hydrophobic cavity and block the formation of the fusion-active gp41 coiled coil domain, resulting in inhibition of HIV-1 mediated membrane fusion and virus entry.
Owner:NEW YORK BLOOD CENT
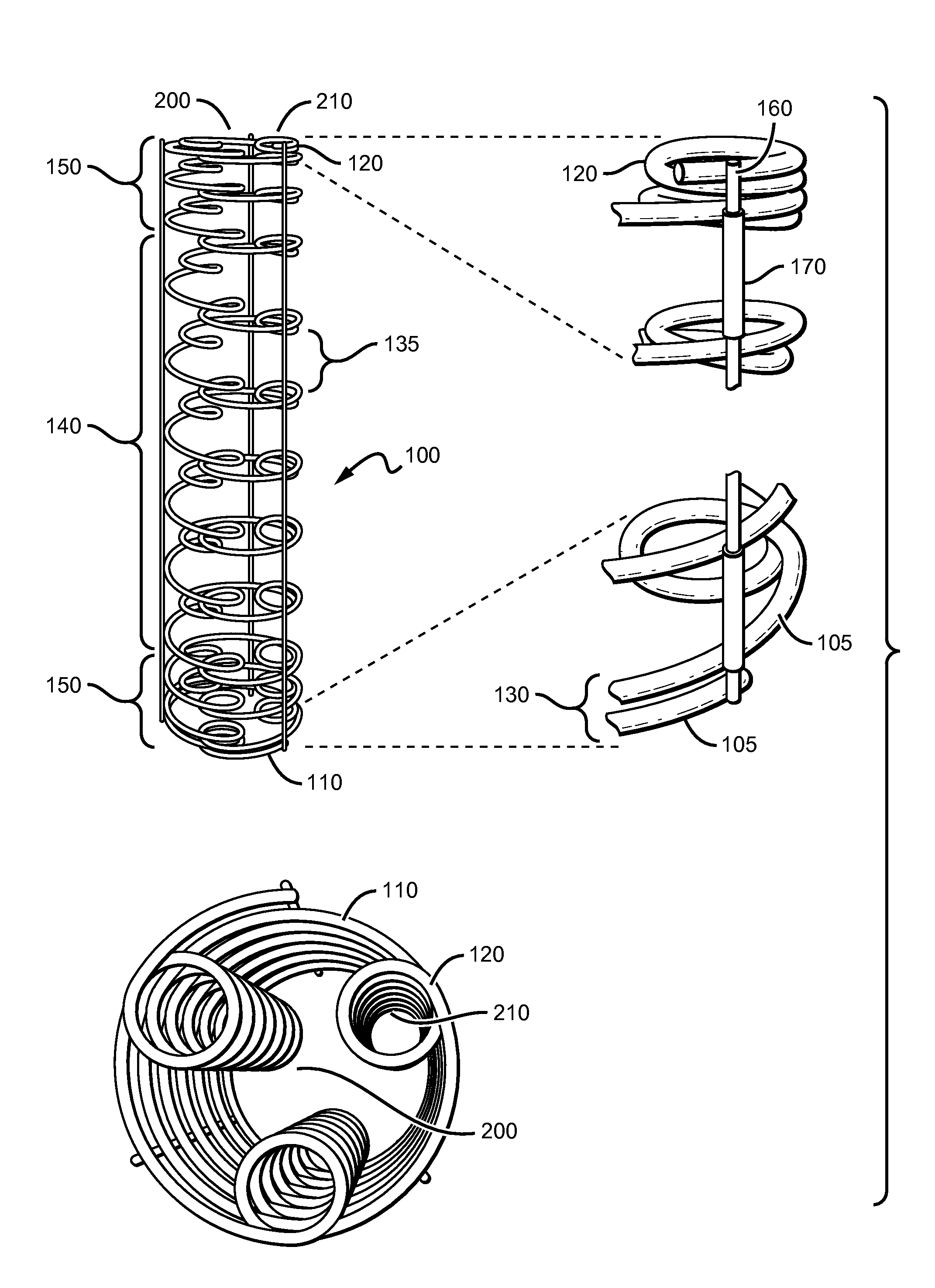
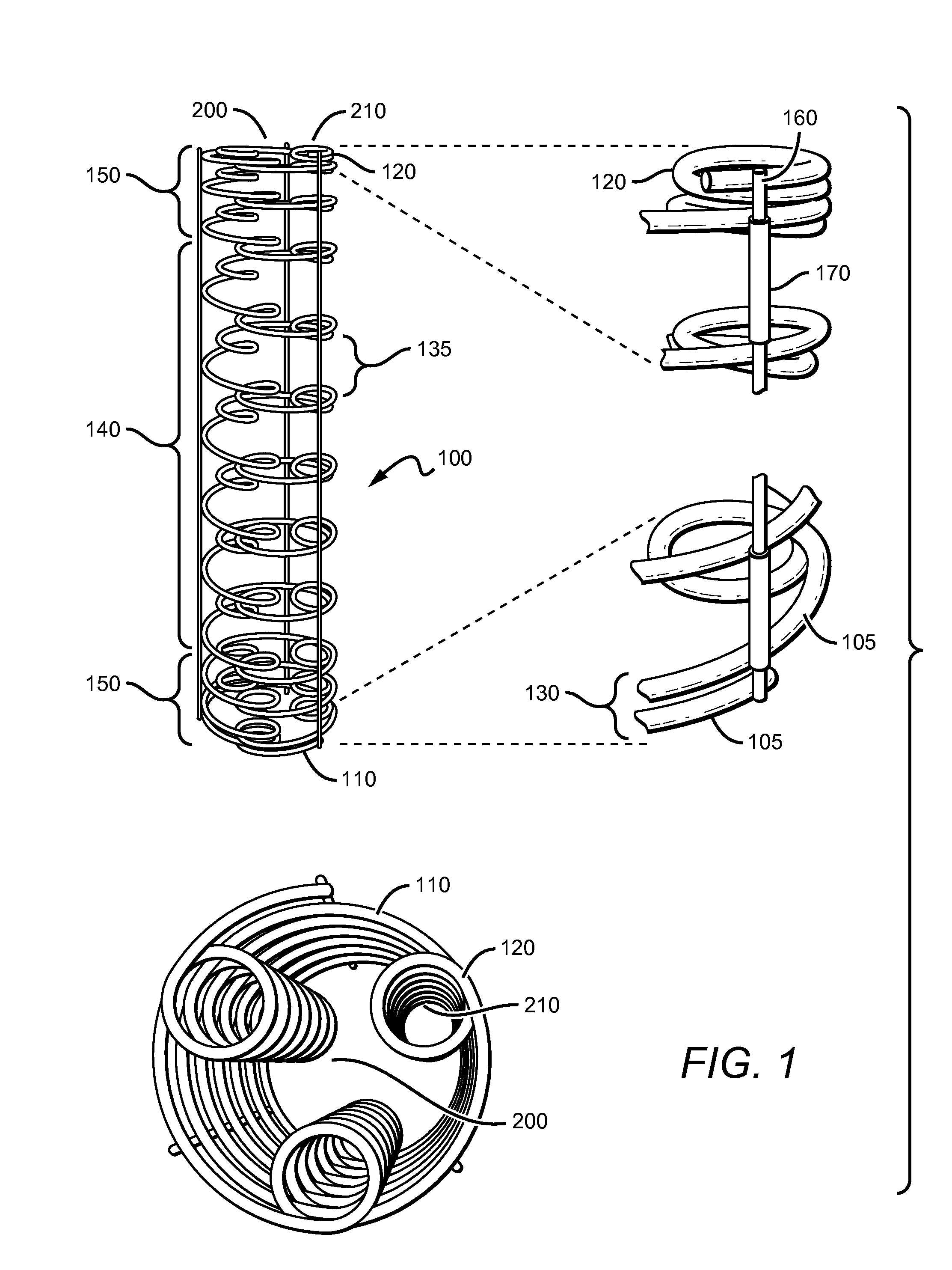
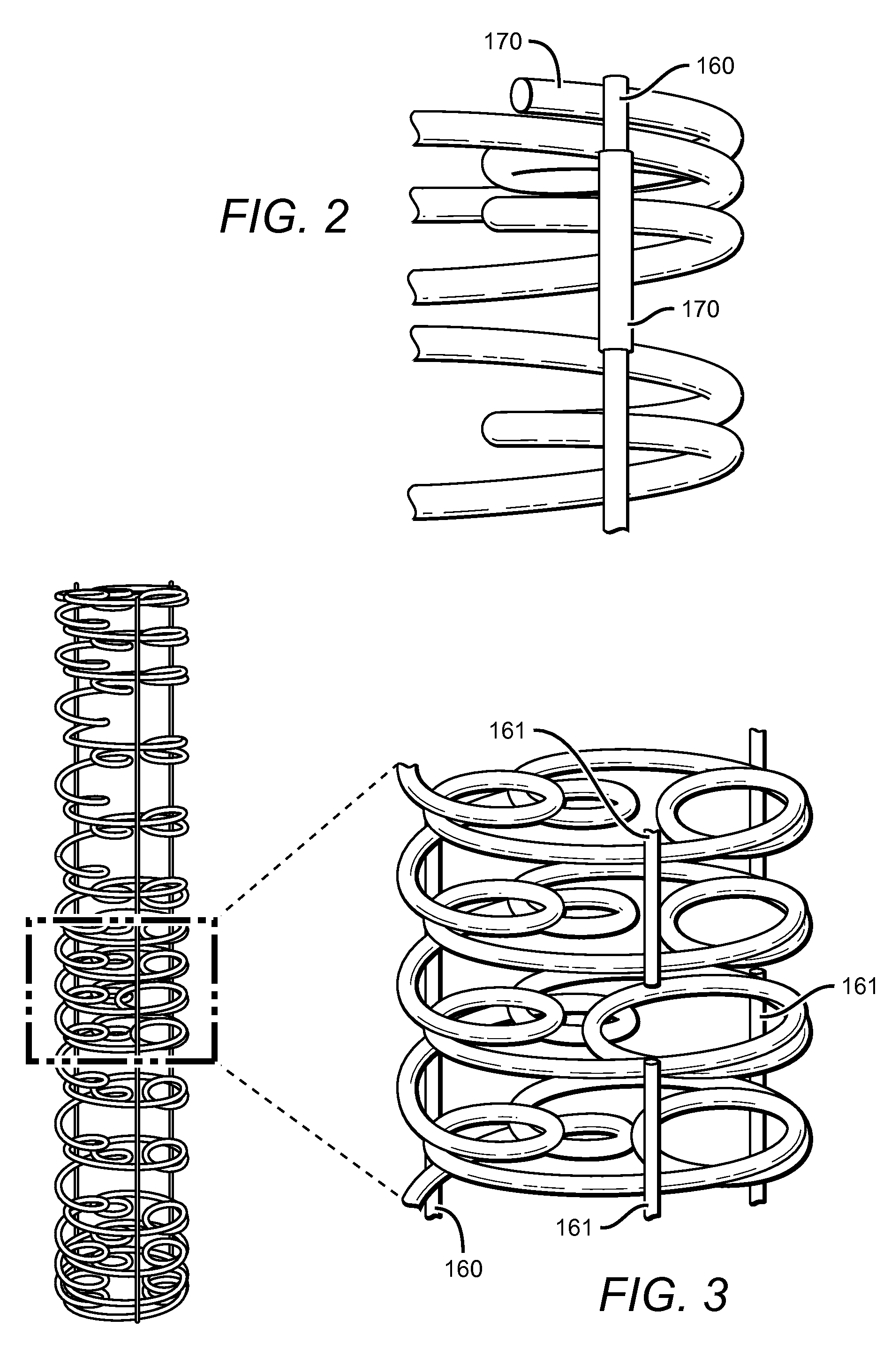
Coil Bioabsorbable Stents
ActiveUS20130261733A1Variation in rigidityVariation in stent flexibilityStentsBlood vesselsFiberIliac Aneurysm
An expandable, bioabsorbable stent having a coiled-coil configuration is described. The stent further comprises regions of variable pitch that allow for variation in either rigidity, or variability diameter within sub-regions along the length of the deployed stent. By varying the diameter allows the stent to extend into regions such as branched vessels, or into the neck of aneurysms. In some embodiments, the stent comprises longitudinal support fibers that run substantially the length of the deployed stent to provide additional strength. In addition, the stent may also comprise regional support fibers that run less than the length of the stent, and which provide increased regional strength while permitting flexibility of the stent. The stent further comprises micro-tubes that are configured to be visible using medical imaging techniques. The stent can be manufactured from materials that are bioabsorbable and / or include the ability to release pharmacologically active substances over time.
Owner:MANLI INT
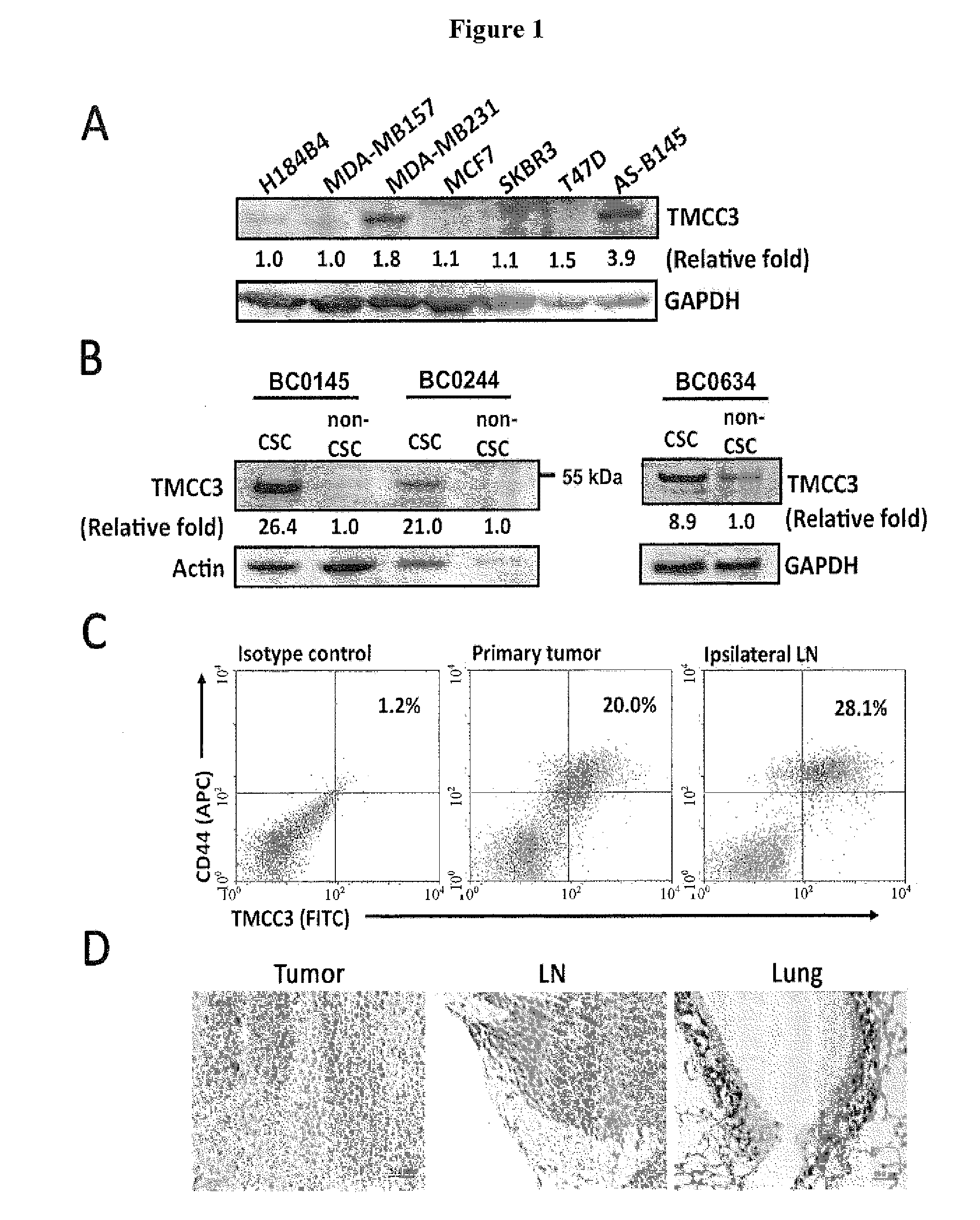
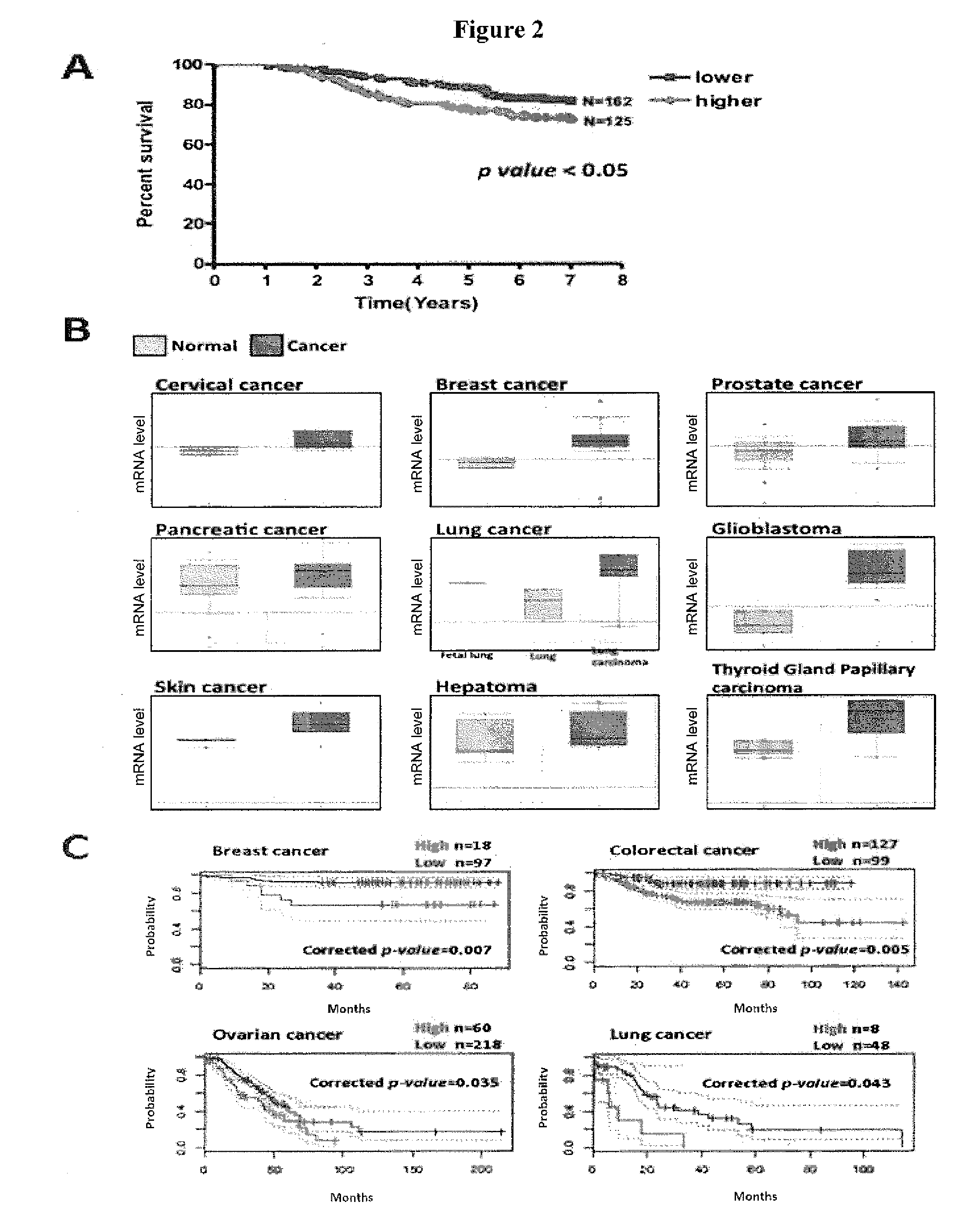
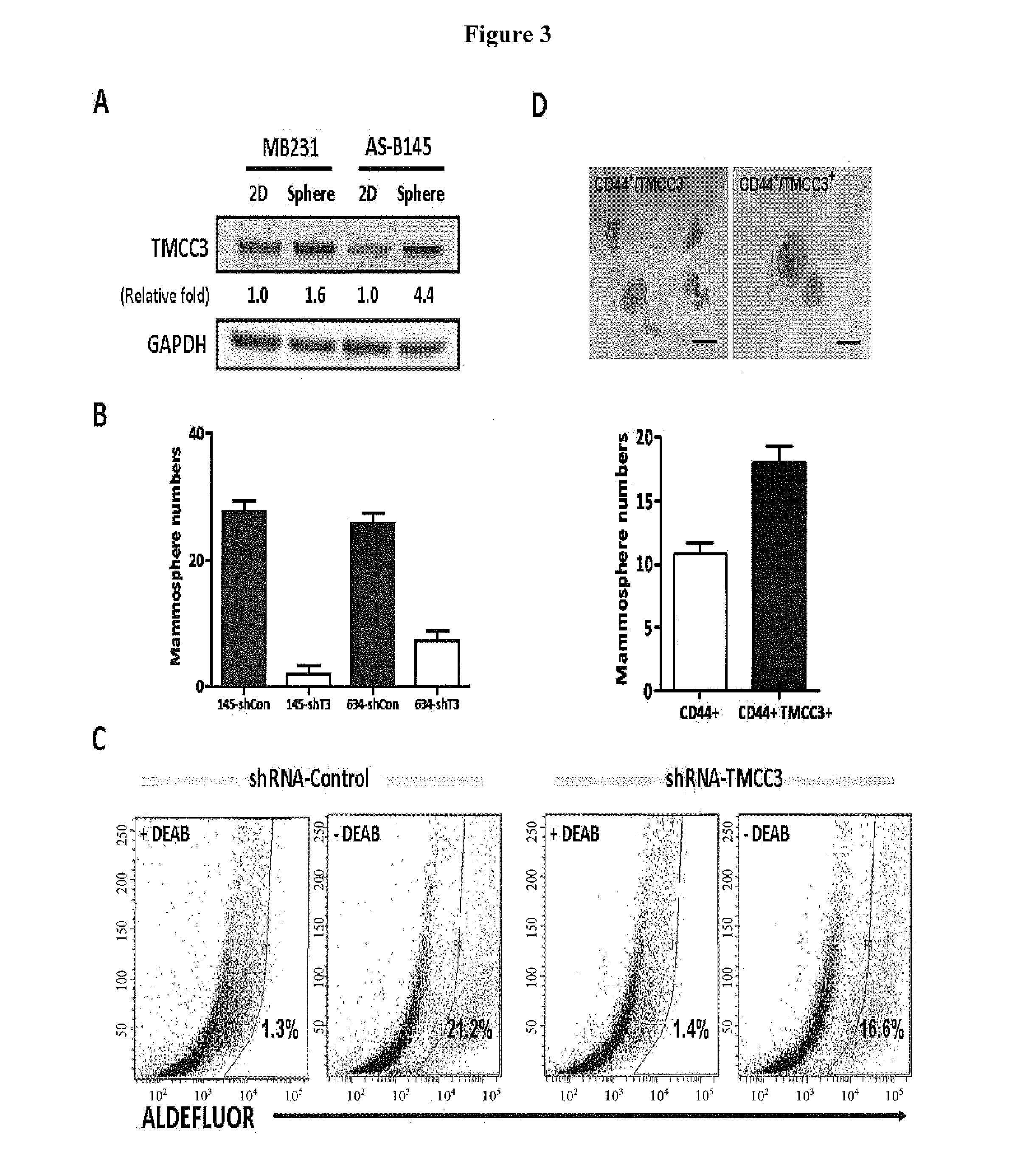
Methods for suppressing cancer by inhibition of tmcc3
ActiveUS20140363372A1Designing can be facilitatedWell representedMicrobiological testing/measurementImmunoglobulins against cell receptors/antigens/surface-determinantsEpitopeExtracellular Structure
A method for treating cancer includes administering to a subject in need thereof an antibody against a transmembrane and coiled-coil domains protein 3 (TMCC3), wherein the antibody binds to an epitope in an extracellular domain of TMCC3. The antibody binds to an epitope in an intercoil domain of TMCC3. A method of diagnosing or assessing a cancer condition includes assessing a level of expression or activity of a transmembrane and coiled-coil domains protein 3 (TMCC3) in a sample, wherein an increase in the level of expression of activity of TMCC3 as compared to a standard indicates the presence of cancer stem cells in the sample.
Owner:DEV CENT FOR BIOTECHNOLOGY +2
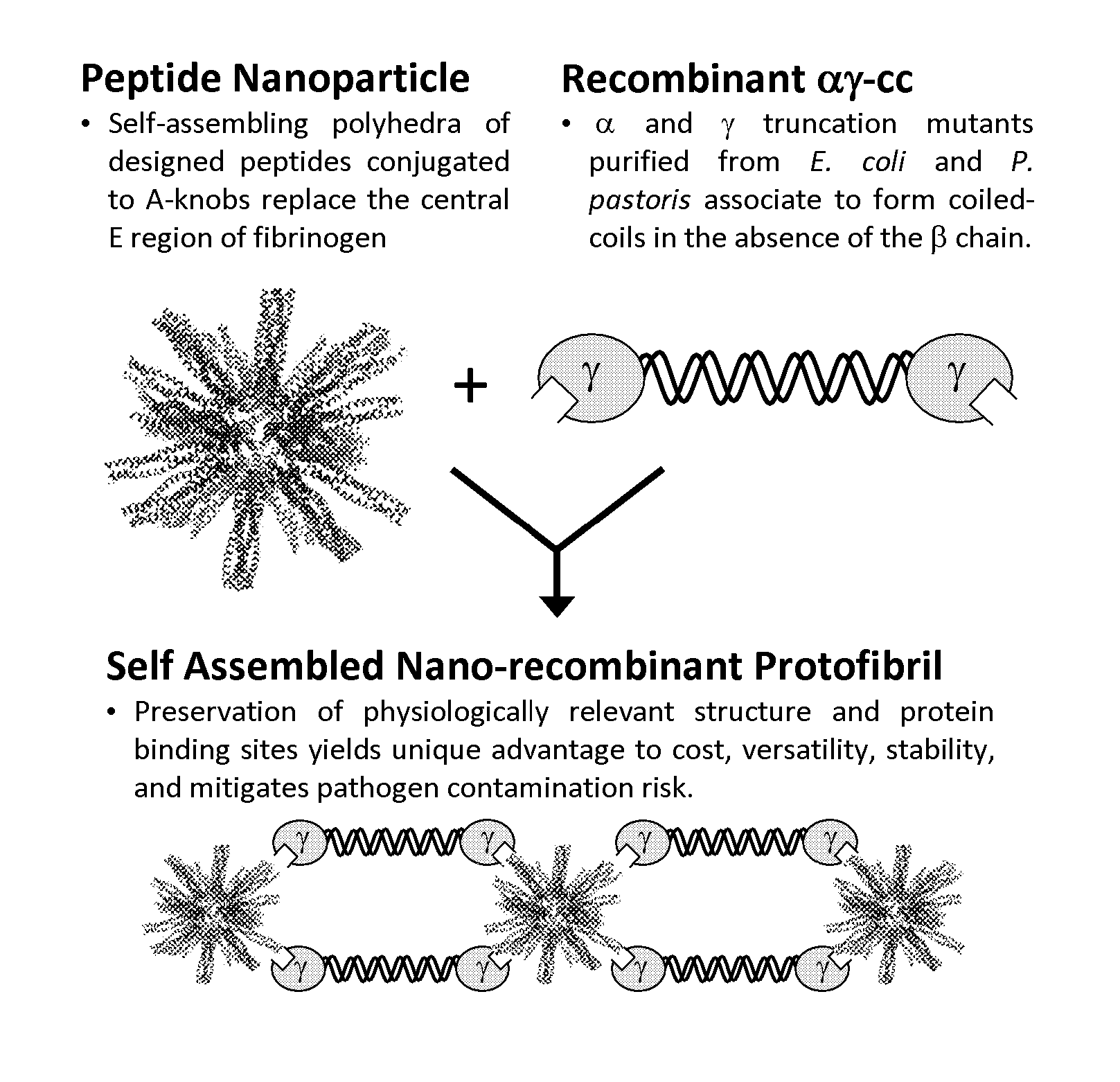
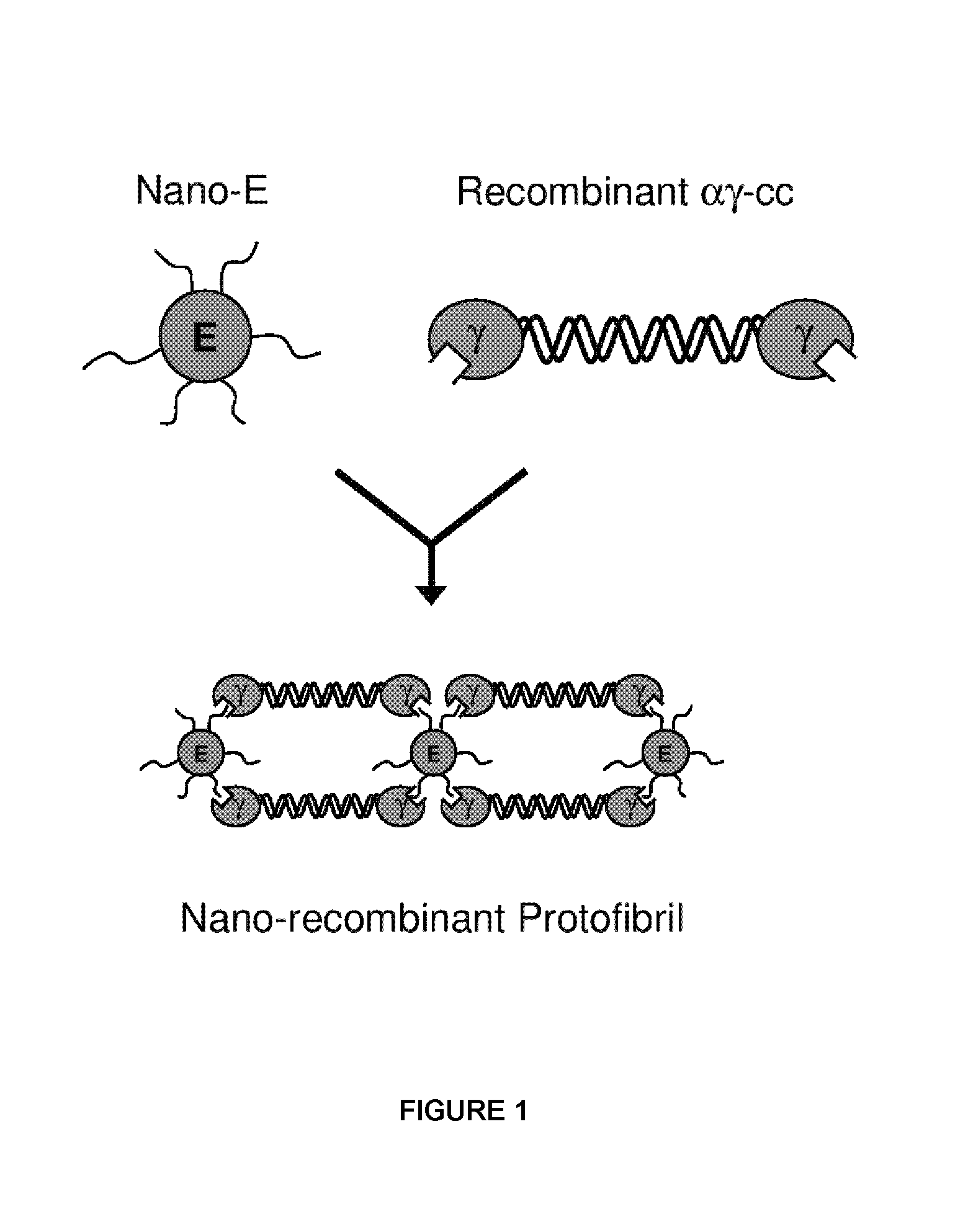
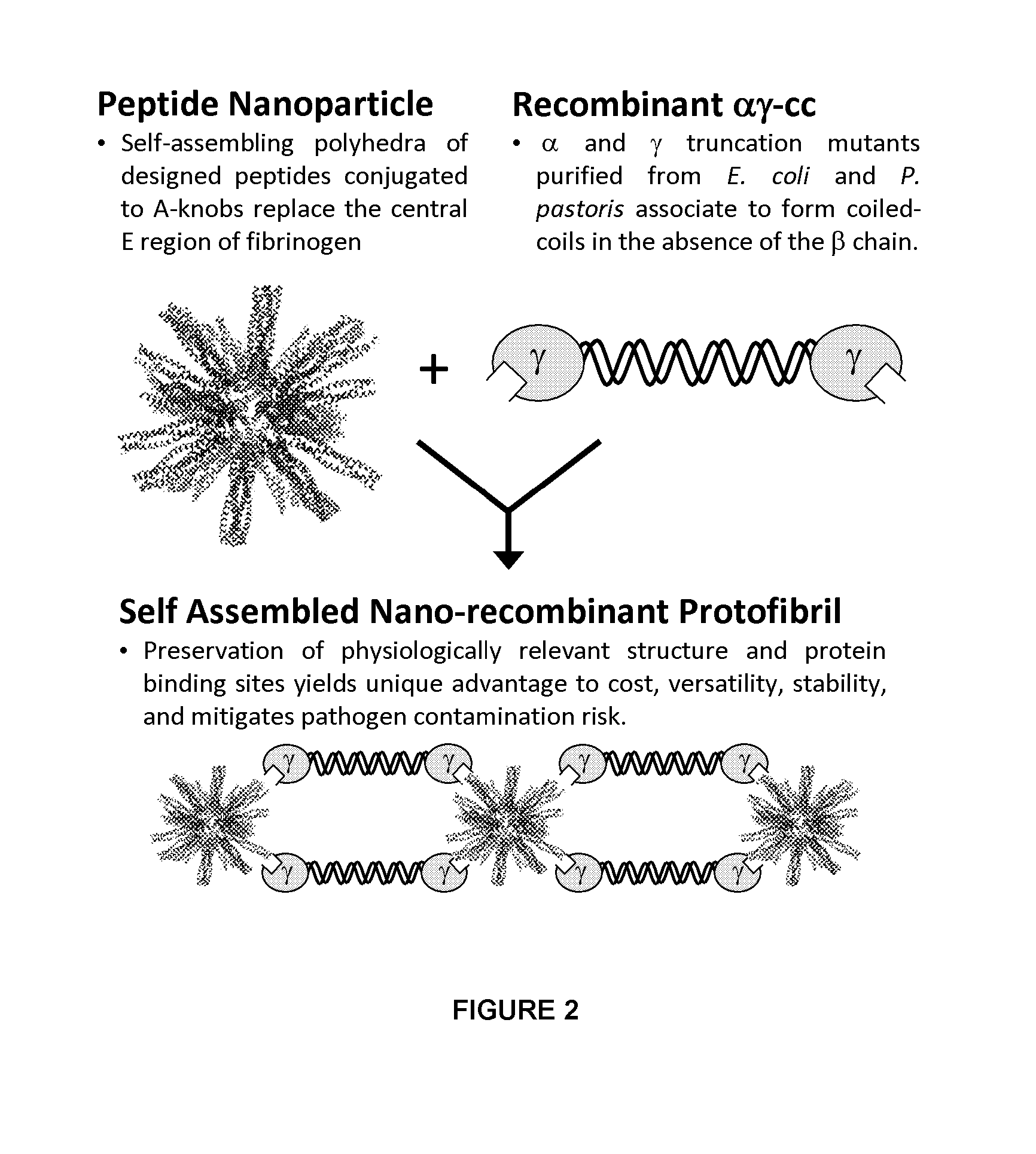
Nano-recombinant fibrinogen for fibrin sealants
A fibrin-based hemostatic agent suitable for both civilian and military use is disclosed. The hemostatic agent comprises (i) nanoparticles to which a plurality of Knob-A recognition sequences are attached, and (ii) coiled-coils of recombinantly-produced human fibrinogen α and chains and the γ chain globular domain. A delivery system for the hemostatic agent also is disclosed, which additionally comprises means for delivering (i) and (ii) to a wound site. The delivery means may be a CO2 canister or a shaker jet.
Owner:BAE SYST INFORMATION & ELECTRONICS SYST INTERGRATION INC
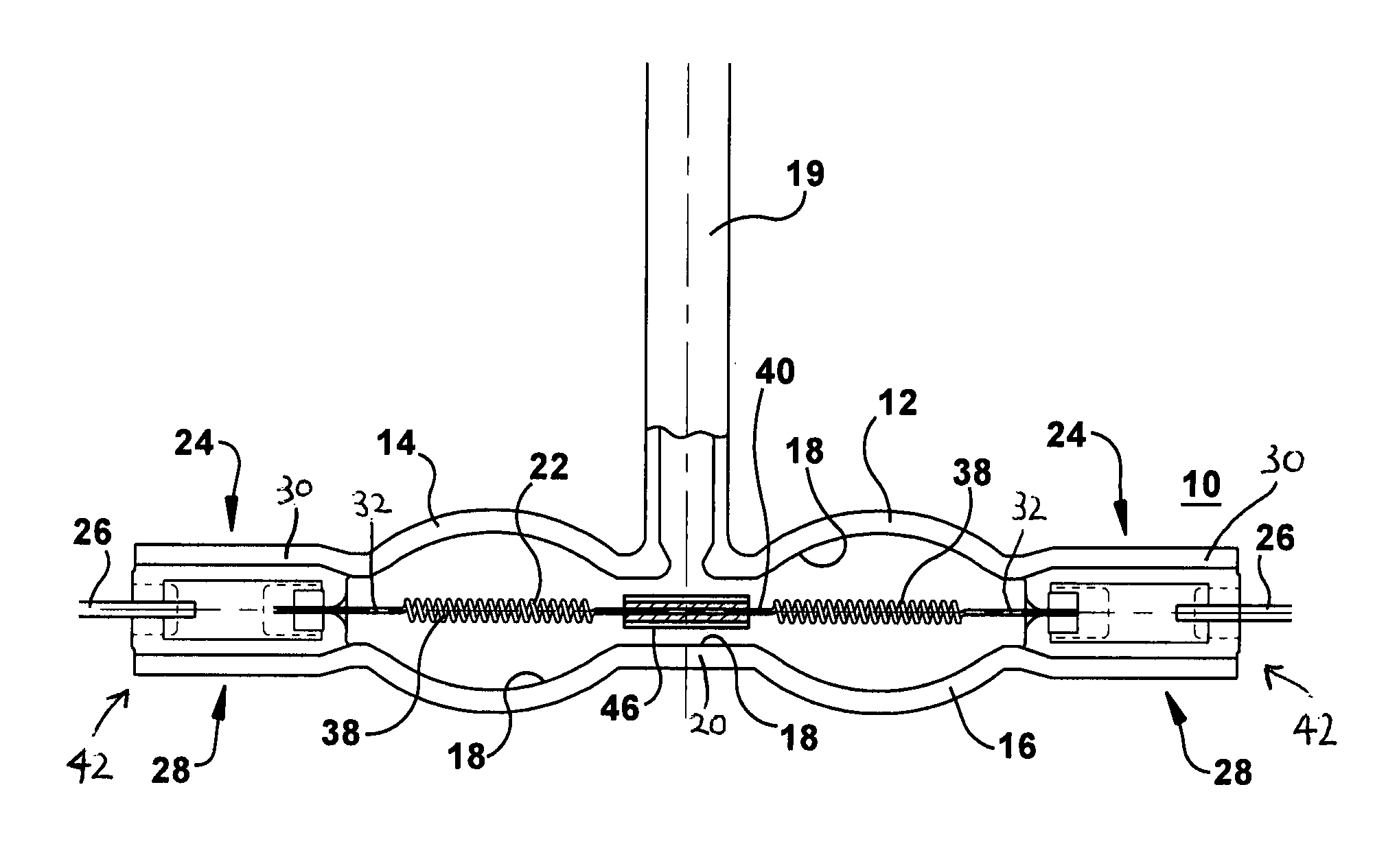
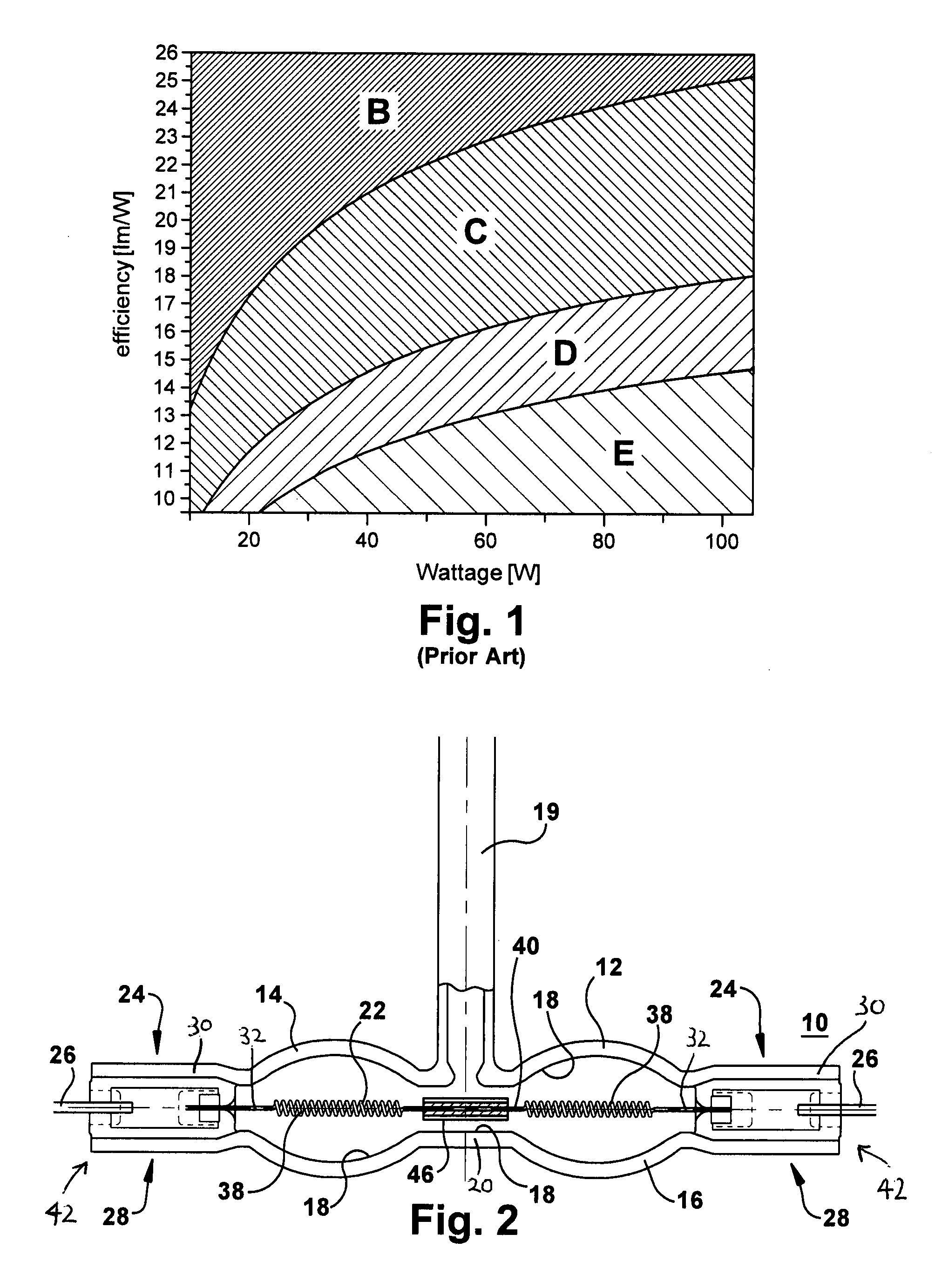
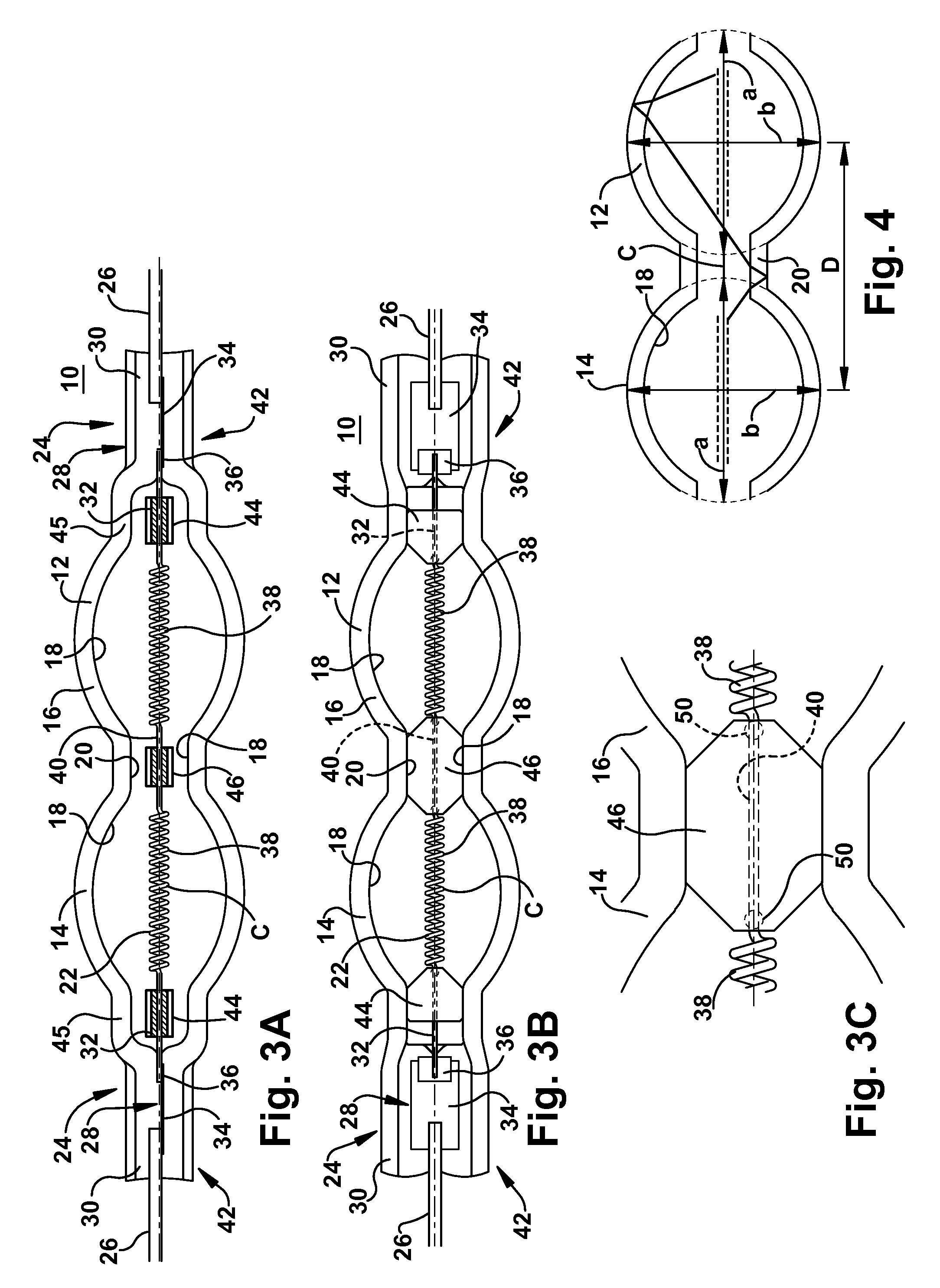
Efficient halogen lamp
InactiveUS20120319576A1High melting pointPrevent high current arcingIncadescent body mountings/supportLamp incadescent bodiesCoiled coilExtremity Part
A lamp includes a light transmissive envelope comprising two spaced apart elliptical portions that together form a hollow interior. The envelope has sealed end portions. Leads are in electrical contact with the filament near the end portions of the envelope for providing power to the lamp. There is a central portion of the envelope that spaces apart the elliptical portions. An electrically conductive filament is disposed in the interior of the envelope. The filament includes coiled-coil portions disposed in the elliptical portions in a coiled-coil shape and a single coil interval portion disposed between the coiled-coil portions at the central portion of the envelope. At least one filament support positions the filament near a center of the envelope. Gas is contained in the interior of the envelope.
Owner:GENERAL ELECTRIC CO
Chimeric antigen receptor
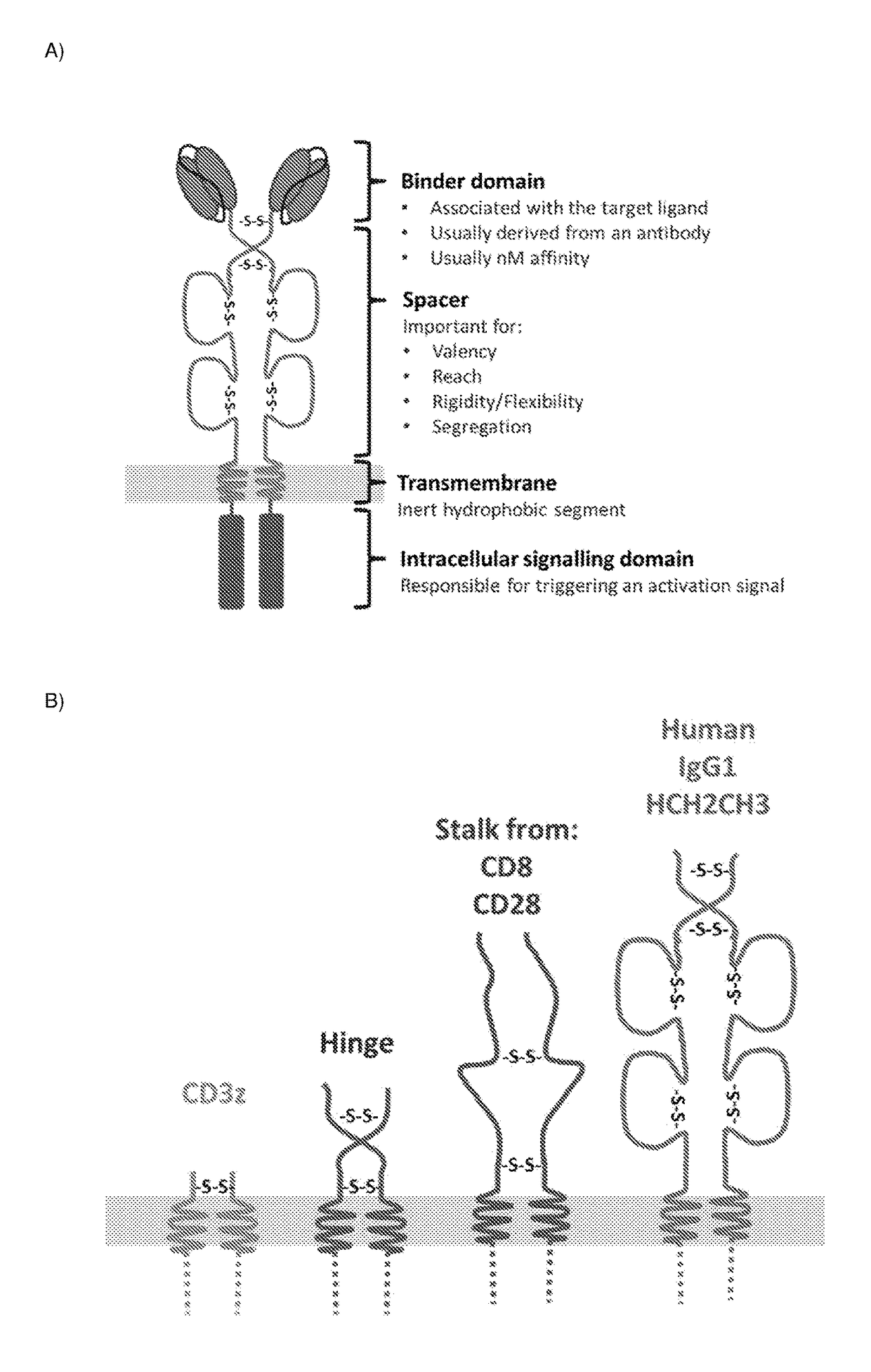
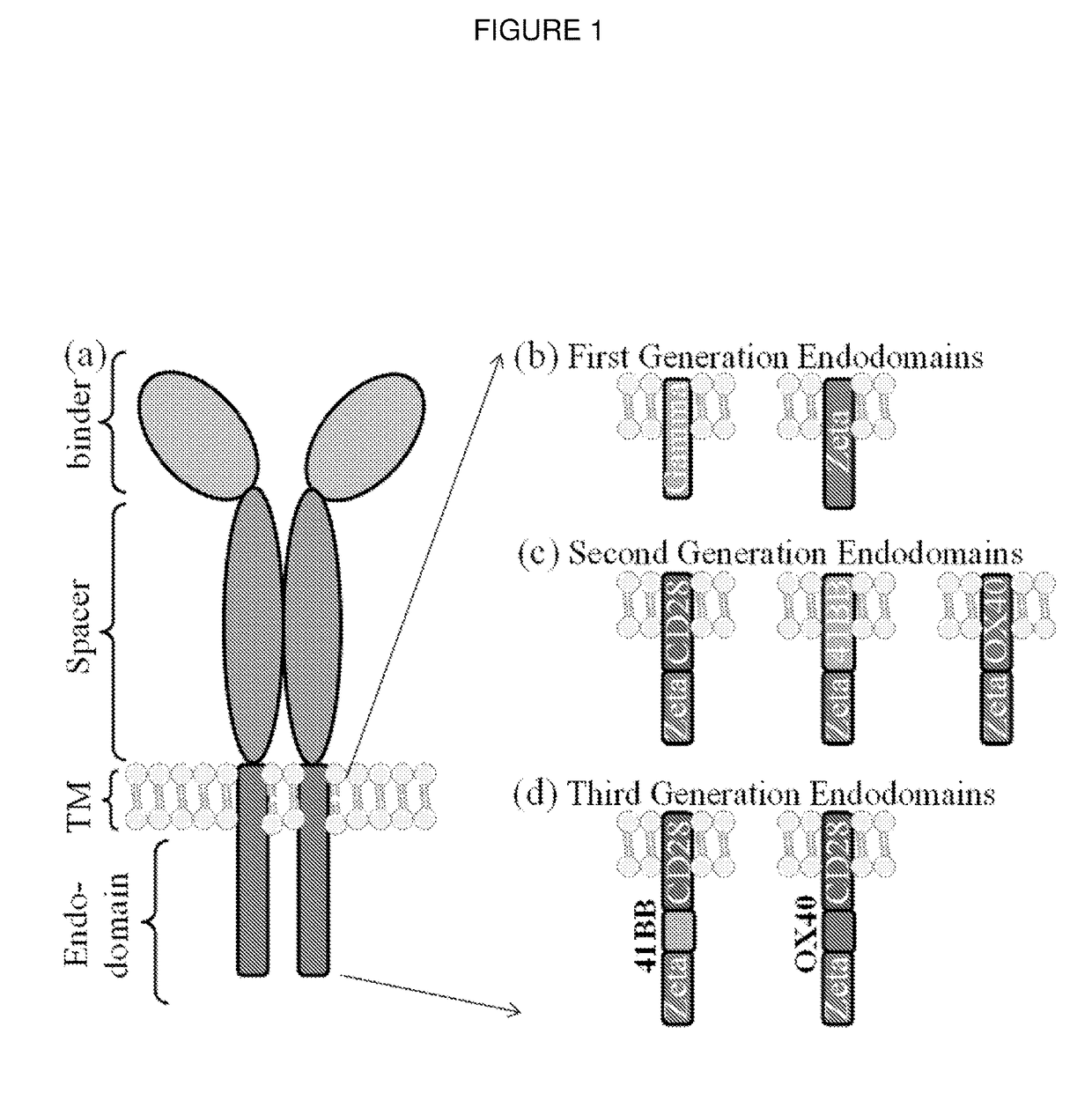
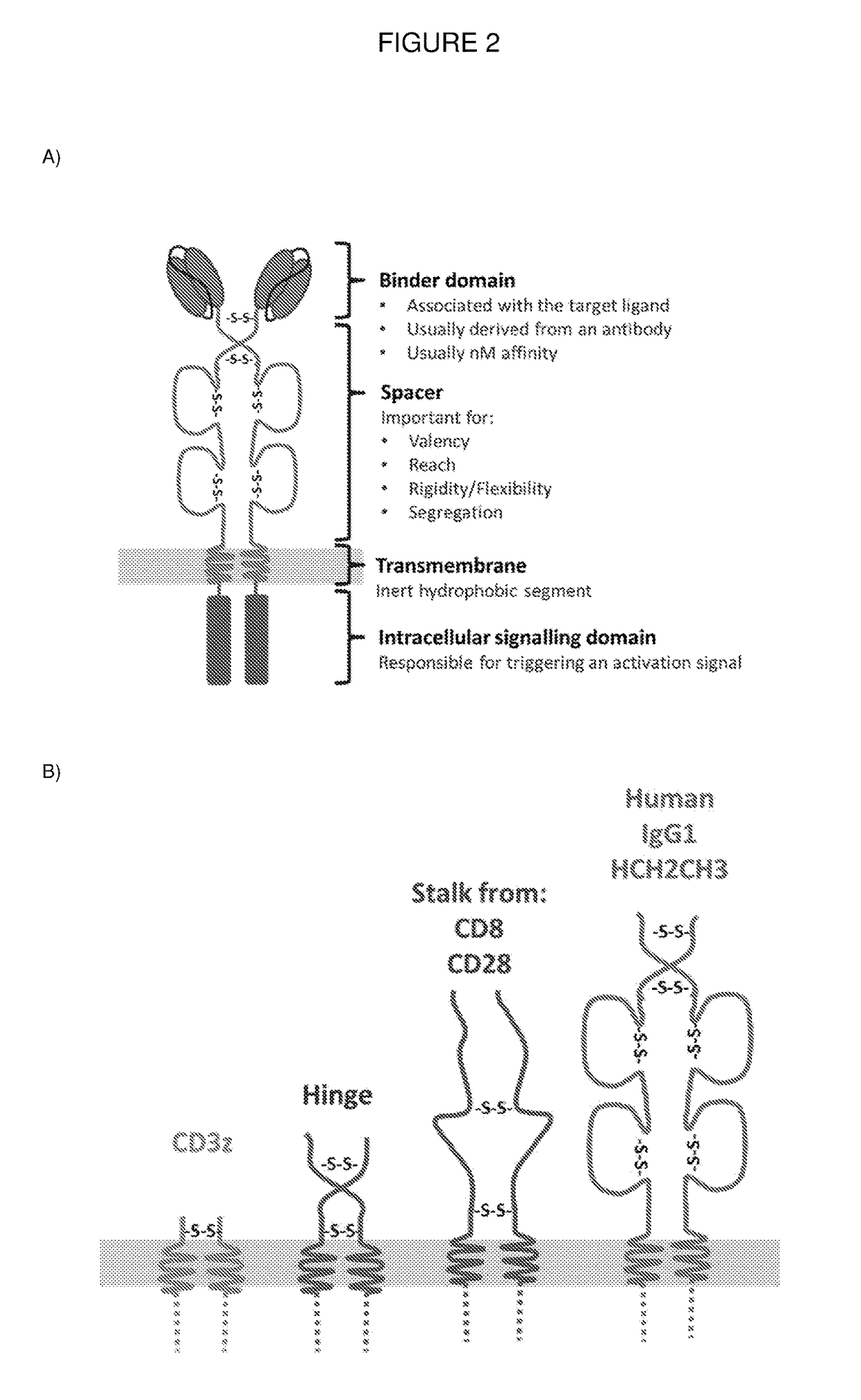
ActiveUS20180050065A1High transduction efficiencyGood flexibilityPolypeptide with localisation/targeting motifCell receptors/surface-antigens/surface-determinantsBinding domainAntigen binding
The present invention provides a chimeric antigen-receptor (CAR)-forming polypeptide comprising: (i) an antigen-binding domain; (ii) a coiled-coil spacer domain; (iii) a transmembrane domain; and (iv) an endodomain. The invention also provides a multimeric CAR formed by association of a plurality of CAR-forming polypeptides by virtue of association of their coiled-coil spacer domains.
Owner:AUTOLUS LIMIED
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com