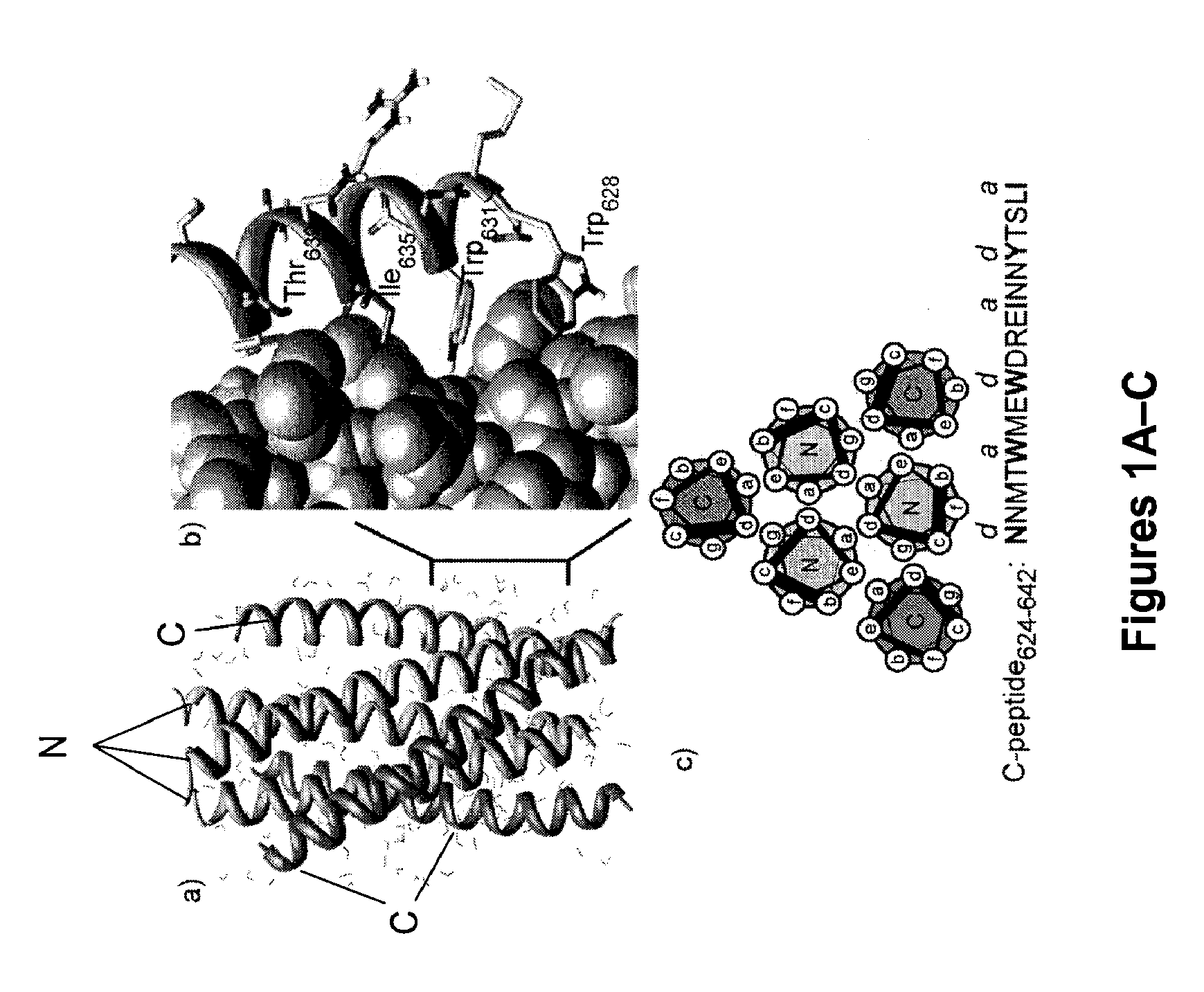
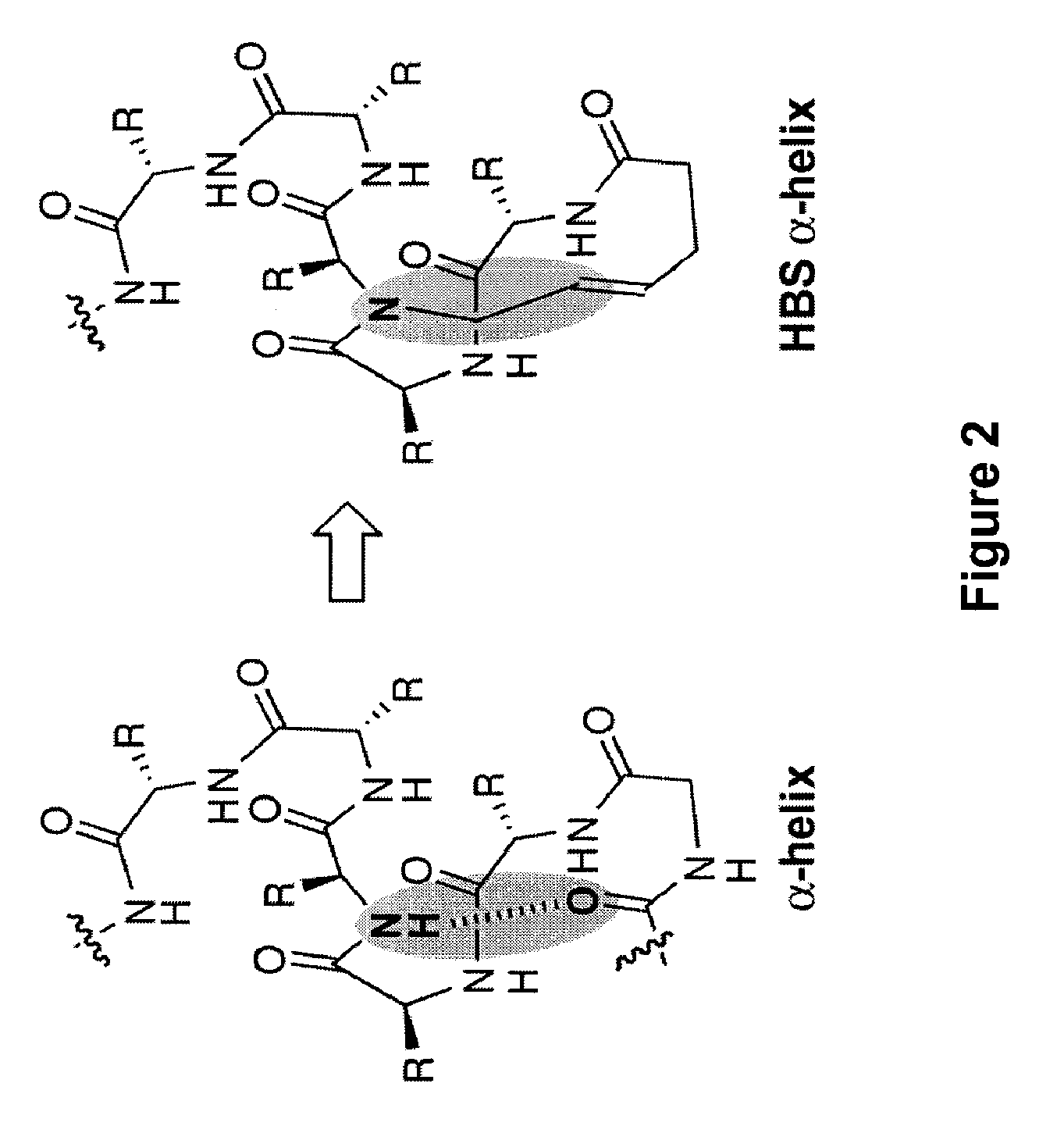
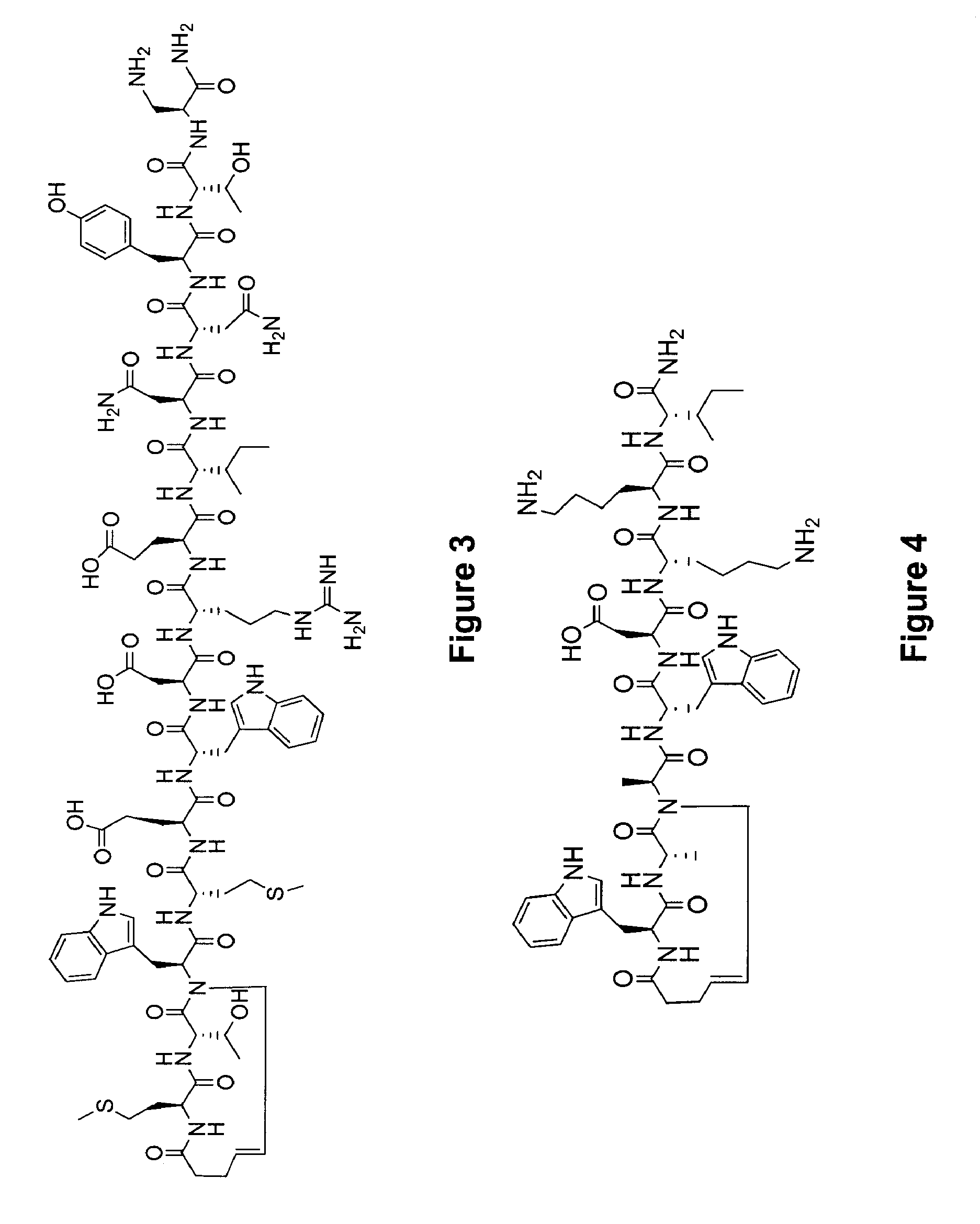
Control of viral-host membrane fusion with hydrogen bond surrogate-based artificial helices
a technology of surrogate-based artificial helices and viralhosts, which is applied in the direction of drug compositions, peptide sources, peptide/protein ingredients, etc., can solve the problem that not all patients are responsive to existing therapies, and achieve the effect of inhibiting viral infectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Synthesis of Peptides 1-11 and Characterization of Peptides 1-12
[0101]Peptides 1-11 were synthesized as shown in Scheme 1. Peptides 1 (i.e., AcMTWMEWDREINNYT-NH2 (SEQ ID NO: 14)), 10 (i.e., AcMTWEEWDKKIEEYTKKI—NH2 (SEQ ID NO: 15)), and 11 (i.e., peptide IZN17, AcIKKEIEAIKKEQEAIKKKIEAIEKLLQLTVWGIKQLQARIL-NH2 (SEQ ID NO: 16)), and resin-bound bis-olefins (17) were synthesized by conventional Fmoc solid phase chemistry on Rink amide HMBA resin (NovaBiochem), 0.05-0.15 mmol scale, with appropriate substitutions of N(allyl)-dipeptides 13-16 and 4-pentenoic acid. In each coupling step, the Fmoc group was removed by treatment with 20% piperidine in NMP (2×20 min). The next Fmoc amino acid (4 equiv.) in the sequence was activated with HBTU (3.6 equiv.) in a 5% DIPEA / NMP solution for 15 minutes, added to the resin bearing the free amine. The resulting mixture was shaken for 60 minutes. The coupling efficiency for each step was monitored by ninhydrin test. After the peptide was assembled on t...
example 2
Circular Dichroism Spectroscopy of Peptides 1-10
[0106]CD spectra were recorded on an AVIV 202SF CD spectrometer equipped with a temperature controller using 1 mm length cells and a scan speed of 5 nm / min. The spectra were averaged over 10 scans with the baseline subtracted from analogous conditions as that for the samples. The samples were prepared in 0.1× phosphate buffered saline (13.7 mM NaCl, 1 mM phosphate, 0.27 mM KCl, pH 7.4), containing 10% trifluoroethanol, with the final peptide concentration of 50-100 μM. The concentrations of unfolded peptides were determined by the UV absorption of the tyrosine residue at 276 nm in 6.0 M guanidinium hydrochloride aqueous solution. The helix content of each peptide was determined from the mean residue CD at 222 nm, [θ]222 (deg cm2 dmol−1) corrected for the number of amino acids. Percent helicity was calculated from the ratio [θ]222 / [θ]max, where [θ]max=(−44000+250T)(1−k / n), with k=4.0 and n=number of residues. For details on θmax calcula...
example 3
Affinity of Peptide 12 for IZN17
[0107]The relative affinity of peptides 1-10 for IZN17 (i.e., peptide 11) was determined using a fluorescence polarization-based competitive binding assay (Eckert & Kim, “Design of Potent Inhibitors of HIV-1 Entry from the gp41 N-Peptide Region,”Proc. Nat'l Acad. Sci. U.S.A. 98:11187-92 (2001); Stephens et al., “Inhibiting HIV Fusion with a β-Peptide Foldamer,”J. Am. Chem. Soc. 127:13126-7 (2005), which are hereby incorporated by reference in their entirety) with fluorescein-labeled peptide 12, shown in FIG. 13. FIG. 14 is a graph showing the saturation binding curve of fluorescein-labeled peptide 12 with IZN17 in PBS buffer at 25° C.
[0108]All samples were prepared in 96-well plates in 1× phosphate buffered saline (137 mM NaCl, 10 mM phosphate, 2.7 mM KCl, pH 7.4) with 0.1% pluronic F-68 (Sigma). A solution of 25 μM IZN17 and 15 nM fluorescein-labeled peptide 12 was incubated at 25° C. After 1 hour, appropriate concentrations (10 nM to 500 μM) of the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com