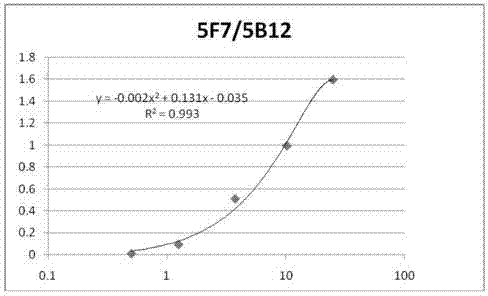
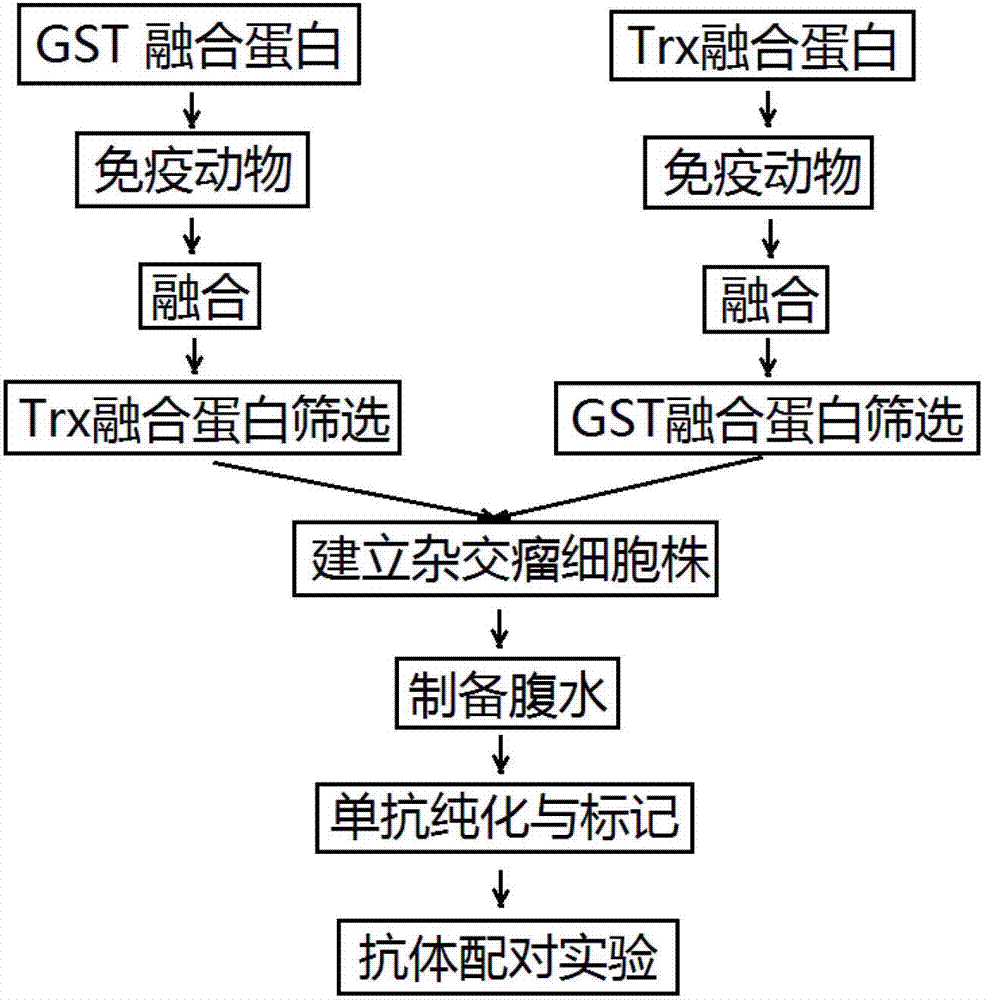
Anti-C-peptide monoclonal antibody and use thereof in detection of C-peptide content
A monoclonal antibody and application technology, applied in the preparation of reagents or drugs for detecting or diagnosing C-peptide, the field of monoclonal antibodies, can solve the problems of inappropriate immunization antigen or screening antigen, poor stability of C-peptide, easy degradation, etc., to avoid C-peptide is unstable, facilitates purification, increases solubility and stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Example 1 Preparation of C-peptide-GST and C-peptide-Trx fusion proteins
[0019] 1) Experimental materials
[0020] Expression vector: pGEX-4T-1 was purchased from GE healthcare, pET32 was purchased from invitrogen; expression host bacteria: Escherichia coli BL21(DE3), purchased from Beijing Tiangen Biotechnology Co., Ltd.; yeast powder and peptone: both products of Oxide Company;
[0021] 2) Experimental method
[0022] The C-peptide gene sequence (the nucleotide sequence is shown in SEQ ID No.1; the encoded amino acid sequence is shown in SEQ ID No.2) has a BamHI site at the 5' end and an XhoI site at the 3' end , inserted into the pMD-18-T vector, the target gene was double-digested with BamHI and XhoI and then inserted into the pGEX-4T-1 and pET32a vectors treated in the same way to construct the GST-C peptide expression vector pGEX- C peptide and Trx-C peptide expression vector pET32a-C peptide, transfer the plasmid into Escherichia coli BL21 (DE3) competent ce...
Embodiment 2
[0023] Example 2 Preparation of anti-C peptide paired monoclonal antibodies
[0024] 1) Experimental materials
[0025] SP 2 / 0 was purchased from the Cell Center of Shanghai Institute of Biochemistry; Balb / c mice were purchased from the Experimental Animal Center of the Chinese Academy of Medical Sciences; fetal bovine serum: the product of Hangzhou Sijiqing Company; DMEM, HAT and HT are all products of Invitrogen Company; 50% PEG1450 The solution is the product of Sigma Company; other drugs are of analytical grade.
[0026] 2) Experimental method
[0027] 2.1 Immunization of mice
[0028] Select 6-8 female Balb / c mice aged 6-8 weeks to immunize with C-peptide-GST and C-peptide-Trx fusion proteins expressed in Escherichia coli respectively. Immunizations were performed 3 times before cell fusion. First immunization: Take 50 μg / mouse of antigen emulsified with an equal volume of Freund’s complete adjuvant and inject it subcutaneously on the back of the mouse, and inject 0.2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com