Coiled coil and/or tether containing protein complexes and uses thereof
a protein complex and tether technology, applied in the field of new engineered proteins and multispecific protein complexes, can solve the problems of elusive multispecific antibody building technologies, and low yield of heterodimer formation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Construction of Vectors for the Expression of Coiled Coil Containing Antibodies
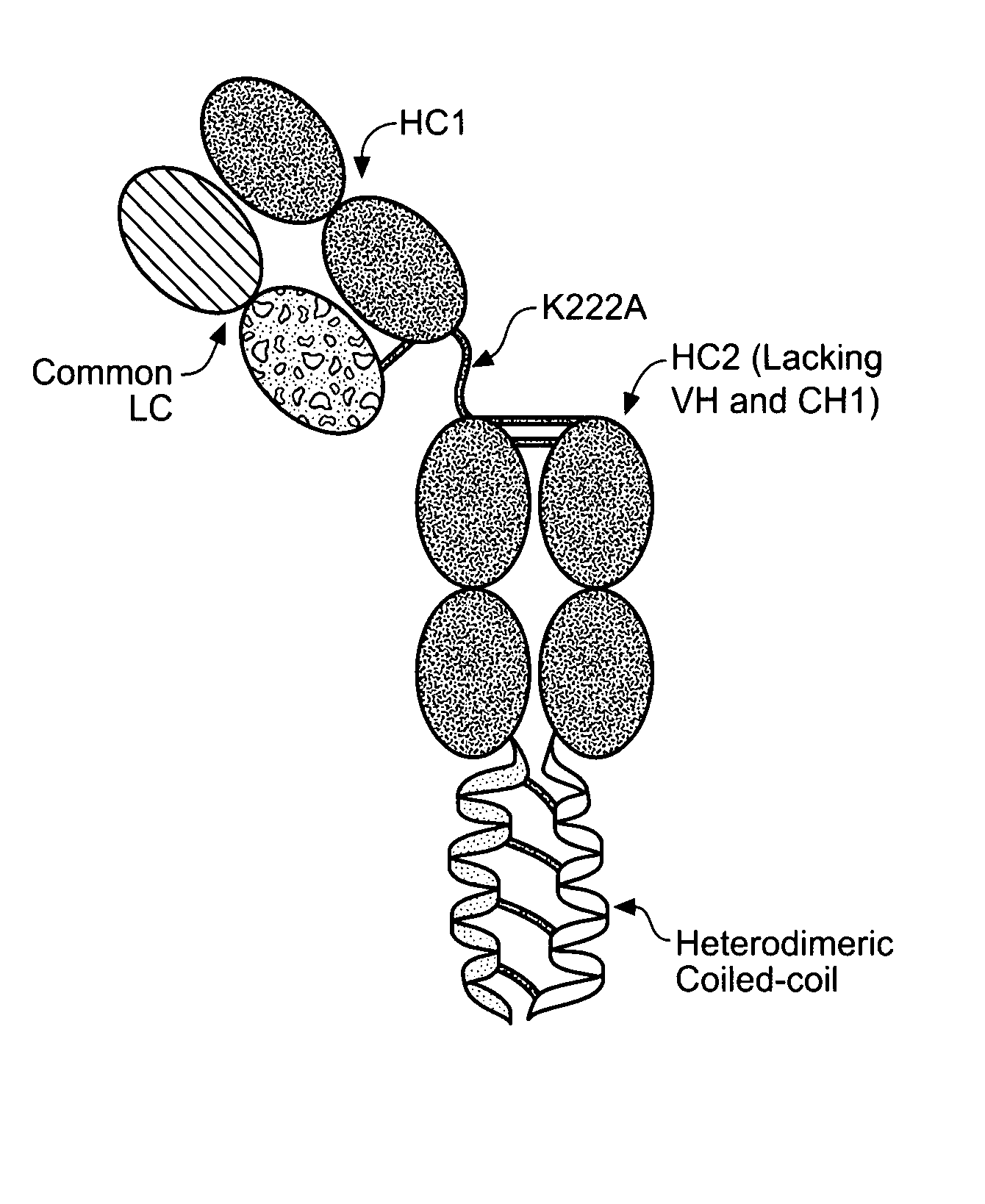
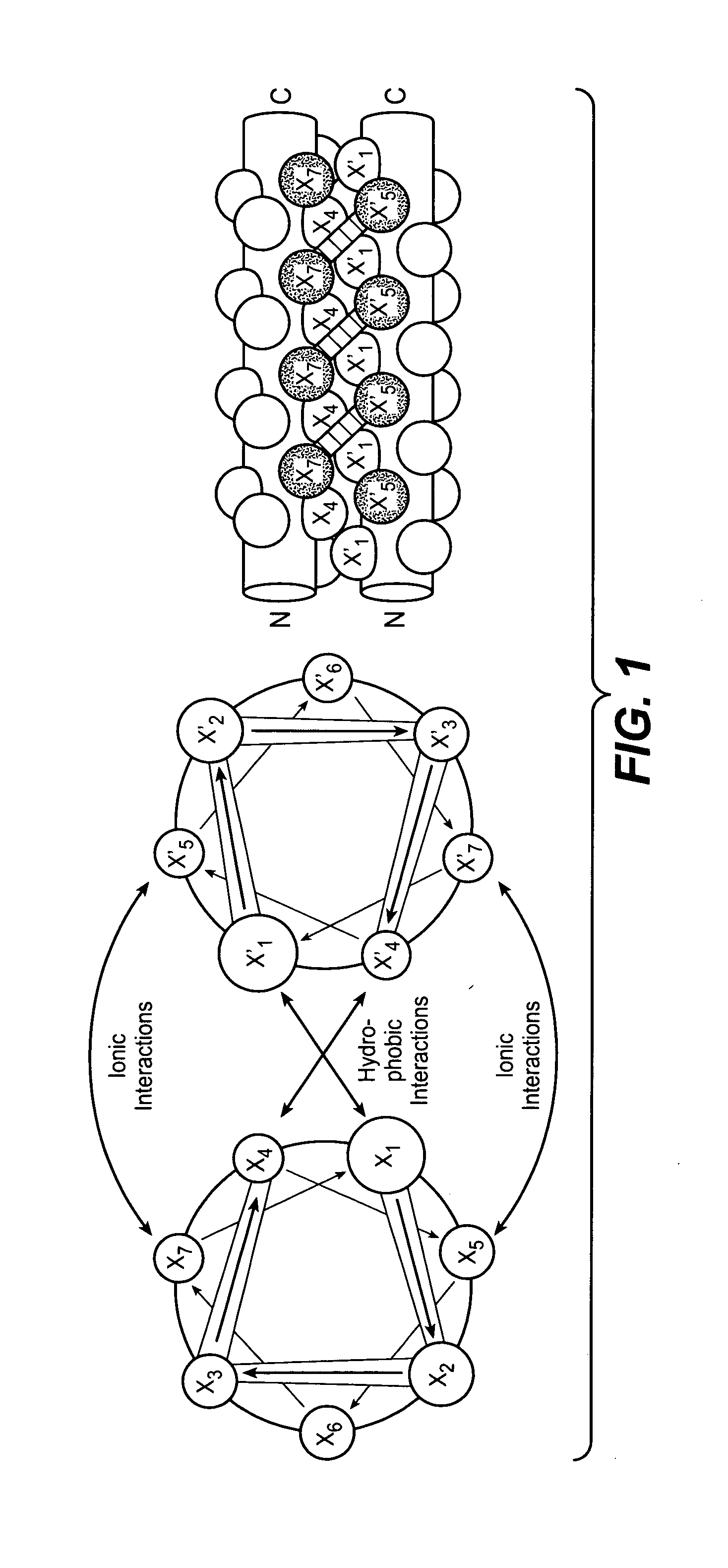
[0368]The coiled coil heterodimerization domains described herein can be linked to a constant chain (e.g., the C-terminus of the HC) of any antibody. Numerous antibody sequences that can be used to construct coiled coil containing antibodies are known in the art and techniques required to manipulate DNA sequences are also well known in the art. An exemplary method for constructing coiled coil containing antibodies is described below.
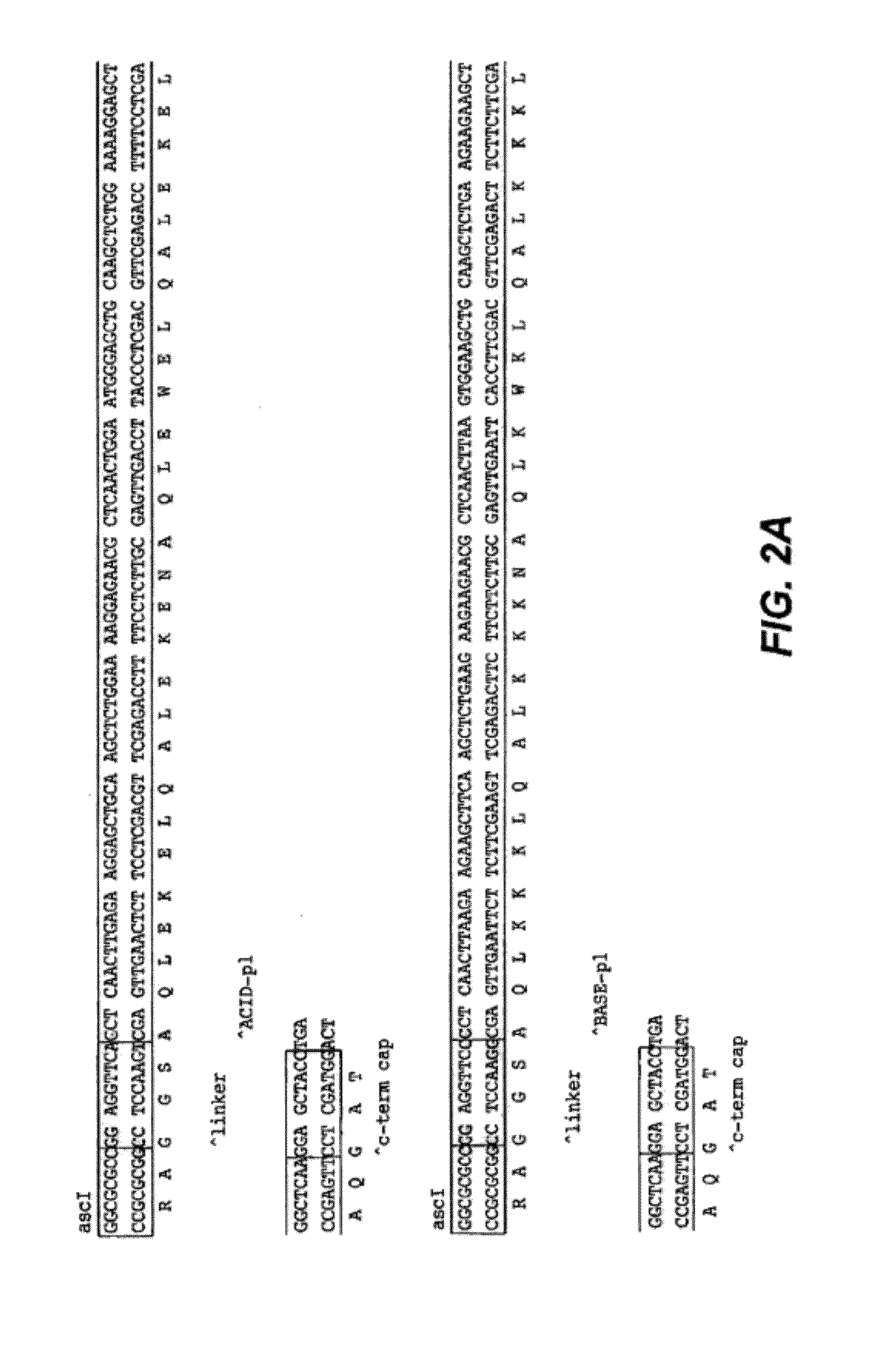
[0369]The HC backbone for the generation of antibodies containing a coiled coil was constructed as follows. Sense and anti-sense oligonucleotides were designed and synthesized to encode either the ACID.p1 (GGSAQLEKELQALEKENAQLEWELQALEKELAQGAT; SEQ ID NO:33) or BASE.p1 (GGSAQLKKKLQALKKKNAQLKWKLQALKKKLAQGAT; SEQ ID NO:34) coiled coil domain sequence with 5′ AscI and 3′ XbaI overhangs. The oligonucleotides were annealed, phosphorylated, and ligated into a digested and dephosphory...
example 2
Purification of Coiled Coil Containing Antibodies
[0375]An exemplary schema that can be used to purify coiled coil containing antibodies is shown below.
[0376]In particular, antibodies were purified from conditioned media using mAbSure Select resin from GE Healthcare (Sweden) overnight at 4° C. The column was washed with two column volumes (CV) of PBS (phosphate buffered saline), followed by 10 CV of PBS+0.1% Triton X114 detergent, followed by 10 CV potassium phosphate buffer. The columns were eluted with 10 mM Acetic Acid (pH 2.9) and immediately diluted with Arginine (100 mM final concentration) and Tris (200 mM final concentration), pH 8.0. Coiled coils were removed from antibodies upon treatment with a 1:500 (weight:weight) ratio of Lys-C endopeptidase (Wako Pure Chemical Laboratories) at 37° C. for 1-5 hours. Cleaved samples were loaded back over an mAbSure resin column to separate cleaved coiled-coils from antibodies and eluted as above. Antibody concentrations were adjusted to ...
example 3
Cleavage of Coiled Coil Containing Antibodies
[0381]The various coiled coil containing antibodies were subjected to cleavage experiments to show that the coiled coil (and tether, if present) could be cleaved from the antibody sequence while leaving the antibody sequence intact. In particular, FIGS. 13A and B show that the coiled coil was cleaved from an exemplary α-FcεR1 / α-FcγR2b antibody using Lys-C endopeptidase and that the antibody remained intact. The theoretical mass for the antibody with the coiled coil is within the margin of error of the mass experimentally observed by mass spectrometry. Similary, the theoretical mass for the engineered antibody without the coiled coil is within the margin of error of that experimentally observed by mass spectrometry showing that Lys-C cleaved the coiled coil from the antibody.
[0382]Mass spectrometry results also demonstrated that Lys-C endopeptidase did not cleave the LC or HC of the exemplary α-FcER1 / α-FcγR2b antibody (FIGS. 14A and B). In...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hydrophobic | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
| soluble | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com