Compound containing Lafutidine and preparation containing Lafutidine
A technology of lafutidine and its composition, which is applied in the field of lafutidine preparations, can solve the problems of poor absorption and poor water solubility of lafutidine, and achieve excellent results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
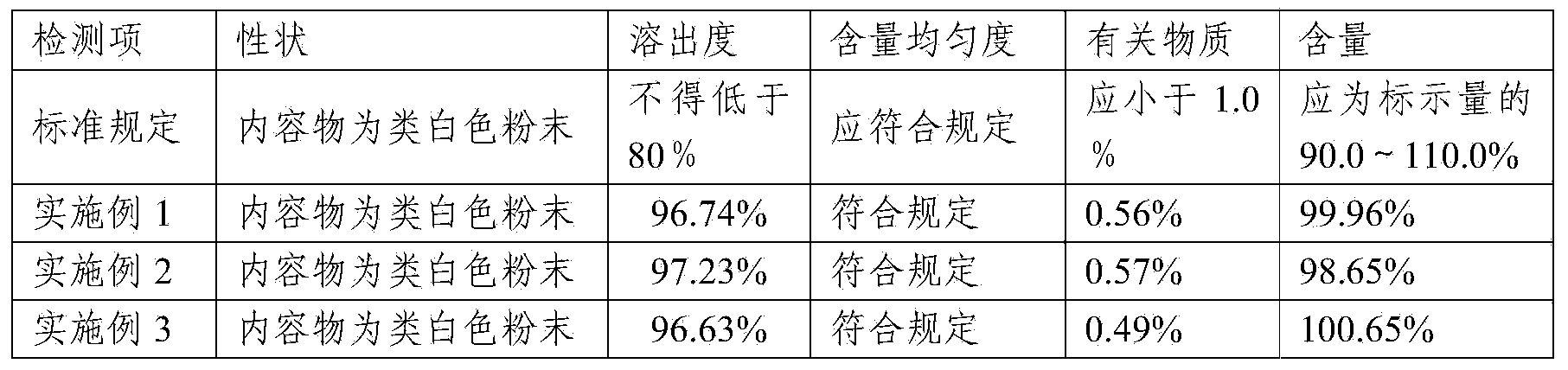
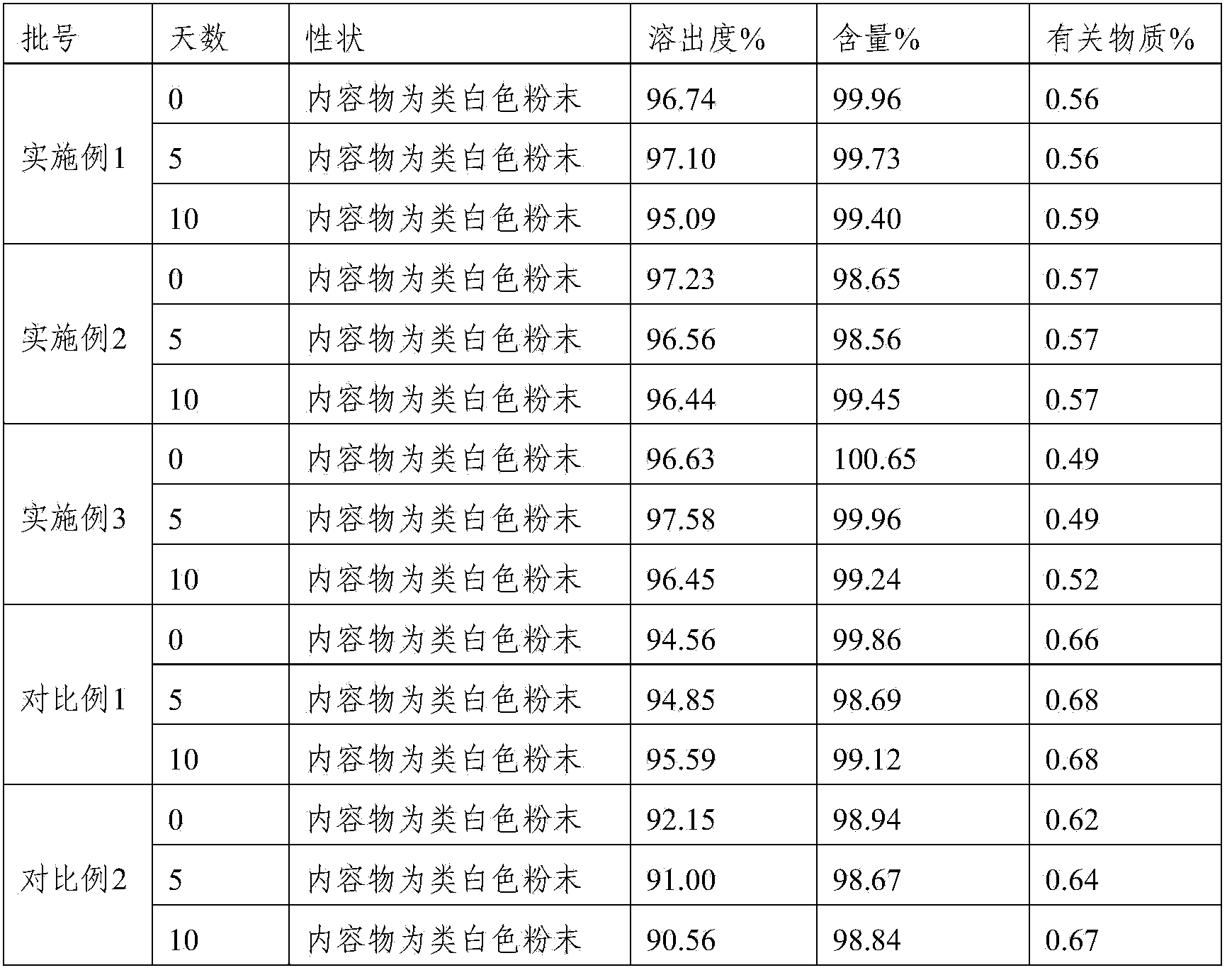
Embodiment 1
[0032] Embodiment 1: lafutidine capsules
[0033] 1. Composition: Lafutidine 5kg, lactose 106kg, Tween-803kg, hydroxypropyl cellulose 4kg, talcum powder 55kg.
[0034] 2. Preparation method:
[0035] 1) Pulverize lafutidine, pass through a 100-mesh sieve, and set aside;
[0036] 2) Grind talcum powder, lactose, and hydroxypropyl cellulose separately, and pass through an 80-mesh sieve;
[0037]3) Weigh each component according to the composition, mix the main drug and auxiliary materials for 15-20 minutes by equal amount addition method, and fill 3# capsules.
Embodiment 2
[0038] Embodiment 2: lafutidine capsules
[0039] 1. Composition: Lafutidine 5kg, lactose 120kg, Tween-803kg, hydroxypropyl cellulose 5kg, talcum powder 60kg.
[0040] 2. Preparation method:
[0041] 1) Pulverize lafutidine, pass through a 100-mesh sieve, and set aside;
[0042] 2) Grind talcum powder, lactose, and hydroxypropyl cellulose separately, and pass through an 80-mesh sieve;
[0043] 3) Weigh each component according to the composition, mix the main drug and auxiliary materials for 15-20 minutes by equal amount addition method, and fill 3# capsules.
Embodiment 3
[0044] Embodiment 3: lafutidine capsules
[0045] 1. Composition: Lafutidine 5kg, lactose 85kg, Tween-802kg, hydroxypropyl cellulose 3kg, talc 50kg.
[0046] 2. Preparation method:
[0047] 1) Pulverize lafutidine, pass through a 100-mesh sieve, and set aside;
[0048] 2) Grind talcum powder, lactose, and hydroxypropyl cellulose separately, and pass through an 80-mesh sieve;
[0049] 3) Weigh each component according to the composition, mix the main drug and auxiliary materials for 15-20 minutes by equal amount addition method, and fill 3# capsules.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com